Integrative Approach for the Identification and Delimitation of Orthops Species (Heteroptera, Miridae, and Mirinae) in the Palearctic
Abstract
Orthops is a widely distributed plant bug genus comprising 35 species. Its nominotypical subgenus includes seven species mostly known from the Palearctic, and four of them are widely distributed. Most of them live in sympatry having only little morphological differences. The species limits have never been tested using the molecular data. The aim of this work is to test whether currently defined species represent monophyletic lineages and to find their interrelationships using an integrative approach. Morphological studies on external characters and male and female genitalia were performed. The molecular studies were based on the mitochondrial (cytochrome c oxidase subunit I [COI] and 12S ribosomal RNA [rRNA]) and nuclear (internal transcribed spacer I [ITS1] and calcium ATPase [Ca-ATPase]) markers and included comparison of the intra- and interspecific distances, species delimitation (ABGD, BPP, bGMYC, PTP, and bPTP), and phylogenetic analyses. All markers showed interspecific differences, and COI was the most variable. It was found that all species differed from each other morphologically, and the most reliable character complexes were parameres and female genitalia. In most analyses, Orthops kalmii and O. campestris were monophyletic. Orthops basalis formed a clade in most phylogenetic trees. Most of the species delimitation analyses confirmed the status of those three species. Orthops scutellatus was split into two clades, Palearctic and North American, which was also confirmed by the species delimitation analyses. Those two groups differed in parameres. Orthops campestris and O. scutellatus form a clade in all analyses, and O. basalis forms a clade with O. kalmii in most analyses.
1. Introduction
The Palearctic is the largest biogeographic realm with many biomes and climatic zones. Although the Palearctic insect fauna is the best studied in comparison to other regions, most species are diagnosed using only morphological characters, which is insufficient for testing the intraspecific variation and cryptic species. The molecular-based taxonomic studies allow for the further research of population structure, phylogeography, and biodiversity assessment.
Molecular studies of species limits are especially important for the widely distributed and relatively common species because they are usually relatively abundant and can shape the ecosystems. Some species are extremely variable externally and might have a complex population structure [1]. Therefore, an integrative approach that combines both morphology and molecular data is needed to disentangle the taxonomy of such taxa. However, studies on the widely distributed species across the Palearctic are rare.
Miridae or plant bugs are among the largest hemimetabolous insect families, comprising more than 11,000 described species. This family is diverse in all continents, except for Antarctica [2]. In the Palearctic, there are ~2800 mirid species [3]. Within the plant bugs, the subfamily Mirinae is the most diverse and comprises many widely distributed species, including trans-Palearctic, trans-Holarctic, and those inhabiting three or more continents [1, 4–6]. Lygus complex within Miridae is distributed across multiple continents and comprises ~50 genera, which are very similar to each other, and many of them are taxonomically challenging [1, 5].
Orthops is a genus within the Lygus complex [1, 5], and it is distributed across the Palearctic, as well as Africa and North America. The genus currently comprises 35 species, and they are usually characterized as medium-sized herbivorous bugs (3–6 mm) that live on plants. Orthops includes two subgenera: Orthops and Montanorthops [3, 7]. Species of the subgenus Montanorthops are known from one or few specimens. The subgenus Orthops comprises seven species: O. basalis (Costa, 1853), O. campestris (Linnaeus, 1758), O. ferrugineus (Reuter, 1906), O. frenatus (Horváth, 1894), O. kalmii (Linnaeus, 1758), O. scutellatus, and O. vitticeps (Reuter, 1906) [3, 8, 9]. They are mostly recorded from the plant family Apiaceae [3, 7, 10]. Within this subgenus O. basalis, O. campestris, and O. kalmii are widely distributed and known from the Western Europe to Eastern Siberia, O. frenatus inhabits mostly Caucasus and Central Asia, and O. scutellatus is known from the large areas in the Eastern and Western Siberia, East Asia, and the Nearctic [3]. Orthops vitticeps and O. ferrugineus are found in Sichuan (China) [3]. Although a number of the keys to species and comparisons were provided, they were regional in focus, and none of them included all the species from this subgenus [8, 10–16].
In this study, we concentrate on the subgenus Orthops. According to the previous works, there are few morphological differences between the species. The four widely distributed species differ mainly in the coloration of the frons and the structure of the parameres. It remains unclear whether these characters are species-specific or represent intraspecific variability. So far, there have been no comparative studies of the aedeagus and female genitalia published for the subgenus Orthops species, although those structures can be important for the species discrimination within Mirinae [5, 17, 18]. Although the cytochrome c oxidase subunit I (COI) region sequences for the subgenus Orthops were obtained during the regional barcoding projects Raupach et al. [19, 20], the species and their interrelationships have never been properly tested using the molecular data in a phylogenetic context.
The aim of the current study is to test the monophyly of the subgenus Orthops, species limits in the subgenus Orthops using the morphological and molecular data, and the phylogenetic relationships between them to clarify their status as independent lineages.
2. Material and Methods
2.1. Taxa and Specimens for the Morphological Studies
Initially, the morphological specimens were sorted based on the keys published in Kerzhner and Jaczewski [13] and Vinokurov and Kanyukova [10]. We examined 3008 specimens, including 322 of O. basalis, 160 of O. campestris, 15 of O. ferrugineus, 695 of O. frenatus, 769 of O. kalmii, 1042 of O. scutellatus, and 5 of O. vitticeps. All specimens are preserved at the Zoological Institute of the Russian Academy of Sciences, St Petersburg, Russia (ZIN). A label with the unique specimen identifier (USI) was attached to each examined specimen. The data on the specimens, that is, locality, collecting event, and host plant records, were entered in the Arthropod Easy Capture Locality Database (https://research.amnh.org/pbi/locality/). More than seven specimens of males and females each from different series were dissected for the widely distributed species to assess the intraspecific variation and to assure that the characters were stable within each species.
2.2. Taxa and Specimens for the Molecular Studies
Sequences were obtained from 39 specimens of four widely distributed species of the subgenus Orthops (Figure 1). Additionally, we downloaded 55 COI sequences from GenBank (see Supporting Information 1: Table SI1 for the full list of vouchers, sequences, and GenBank accession numbers used in this study). The localities cover Europe, Asia, and North America, but most specimens were collected from different localities in Russia. The molecular data for O. ferrugineus, O. frenatus, and O. vitticeps were not included.
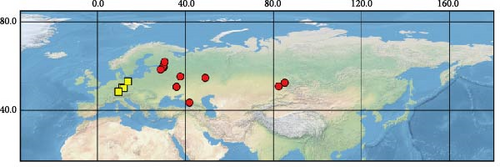
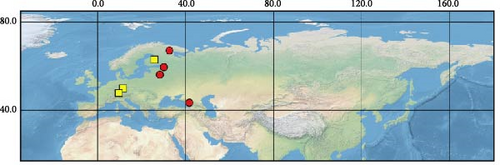
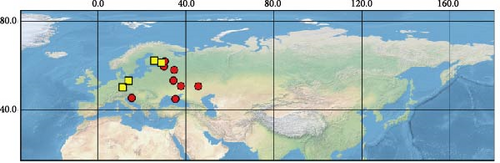
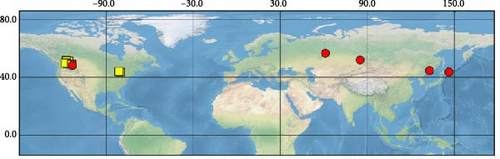
For this study, we also obtained sequences of O. mutans (Stål 1858). This species is not assigned to any subgenus [3] but bears the characters which makes them closer to Montanorthops (Discussion). The COI sequences of Orthops palus [21], known from Africa, were downloaded from GenBank. Sixteen species from other Lygus complex genera were included to test the monophyly of the subgenus; they are Agnocoris rubicundus (Fallén, 1807), Apolygus malaisei (Lindberg, 1925), Cyphodemidea saundersi (Reuter, 1896), Eolygus rubrolineatus (Matsumura, 1913), Henrylygus nubilus (Van Duzee, 1914), Knightomiris distinctus (Knight, 1917), Liocoris tripustulatus (Fabricius, 1781), Lygidea annexa (Uhler, 1872), Lygidolon hecate, Linnavuori, 1970, Lygocoris pabulinus (Linnaeus, 1761), Lygus rugulipennis Poppius, 1911, Nonlygus nubilatus (Knight, 1917), Pachylygus nigrescens (Kerzhner, 1977), Peltidolygus scutellatus (Yasunaga, Schwartz and Chérot, 2002), Pinalitus rubricatus (Fallén, 1807), and Proba sp. Distant. The sequences of Stenodema trispinosa Reuter, 1904, from the tribe Stenodemini (Mirinae), were also added to the analysis. The sequences of A. rubicundus, A. malaisei, L. pabulinus, L. rugulipennis, L. tripustulatus, O. mutans, P. rubricatus, and S. trinspinosa were obtained in this study; others were downloaded from GenBank.
2.3. Dissection and Terminology
The genital capsule or the entire abdomen was removed and, in most cases, boiled in 10% potassium hydroxide (KOH) until the sclerites were soft enough for dissection. Afterward, the abdomen or genital capsule was washed in clean water and then dissected in glycerol. To inflate the aedeagus, the abdomen was boiled in 40% lactic acid and then washed and dissected in 96% ethanol (see Methodology in [5]). The genitalia of at least 10 males and females each were examined to assure that the characters used for the diagnoses are stable within the species. The drawings were completed using a Leica DM2500 microscope with the drawing device attached. The terminology for the male genitalia follows Kerzhner and Konstantinov [22]. The terminology for the female genitalia follows Davis [23]. The subdivision of Russia follows Kerzhner and Josifov [3].
2.4. Imaging
The photographs of the habitus, parameres, and genital capsule were completed using the Canon EOS 5D Mark IV camera. The images were taken as stacks and combined using the Helicon Focus software. The scanning electron microscope (SEM) images were taken using Hitachi TM-1000 SEM. The specimens were uncoated.
2.5. Measurements
The measurements were taken with the Leningrad Optical Mechanical Association (LOMO) microscope using a graticule and ×10 eyepiece. The measurements are provided in Tables 1 and 2. Scale bars for habitus images equal to 1 mm, and the scale bars for genitalia structures equal to 0.1 mm. All measurements provided in the Taxonomy section are in millimeters.
| Species | Statistical indicator | Length | Width | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body | Cun-Clyp | Pronotum | AntSeg1 | AntSeg2 | Head | Pronotum | InterOcDi | ||
| O. basalis | |||||||||
| ♂ (N = 10) | Mean | 4.70 | 3.02 | 0.70 | 0.42 | 1.19 | 0.90 | 1.60 | 0.38 |
| SD | 0.30 | 0.19 | 0.08 | 0.04 | 0.06 | 0.02 | 0.08 | 0.02 | |
| Range | 1.00 | 0.60 | 0.27 | 0.10 | 0.15 | 0.08 | 0.26 | 0.08 | |
| Min | 4.20 | 2.70 | 0.63 | 0.38 | 1.13 | 0.87 | 1.44 | 0.36 | |
| Max | 5.20 | 3.30 | 0.90 | 0.49 | 1.28 | 0.95 | 1.69 | 0.44 | |
| ♀ (N = 10) | Mean | 4.59 | 3.17 | 0.72 | 0.39 | 1.11 | 0.92 | 1.66 | 0.43 |
| SD | 0.29 | 0.11 | 0.04 | 0.04 | 0.05 | 0.02 | 0.04 | 0.02 | |
| Range | 0.80 | 0.30 | 0.10 | 0.10 | 0.13 | 0.05 | 0.13 | 0.05 | |
| Min | 4.20 | 3.00 | 0.67 | 0.33 | 1.03 | 0.90 | 1.59 | 0.41 | |
| Max | 5.00 | 3.30 | 0.77 | 0.44 | 1.15 | 0.95 | 1.72 | 0.46 | |
| O. campestris | |||||||||
| ♂ (N = 10) | Mean | 4.07 | 2.85 | 0.66 | 0.34 | 1.07 | 0.89 | 1.52 | 0.39 |
| SD | 0.33 | 0.21 | 0.04 | 0.04 | 0.07 | 0.02 | 0.06 | 0.01 | |
| Range | 0.90 | 0.60 | 0.13 | 0.15 | 0.21 | 0.08 | 0.23 | 0.03 | |
| Min | 3.70 | 2.60 | 0.58 | 0.28 | 1.00 | 0.87 | 1.46 | 0.38 | |
| Max | 4.60 | 3.20 | 0.71 | 0.44 | 1.21 | 0.95 | 1.69 | 0.41 | |
| ♀ (N = 10) | Mean | 4.23 | 3.00 | 0.68 | 0.35 | 1.00 | 0.91 | 1.63 | 0.42 |
| SD | 0.36 | 0.27 | 0.05 | 0.03 | 0.06 | 0.02 | 0.07 | 0.01 | |
| Range | 1.10 | 0.90 | 0.19 | 0.08 | 0.23 | 0.05 | 0.23 | 0.03 | |
| Min | 3.50 | 2.50 | 0.56 | 0.31 | 0.90 | 0.87 | 1.51 | 0.41 | |
| Max | 4.60 | 3.40 | 0.75 | 0.38 | 1.13 | 0.92 | 1.74 | 0.44 | |
| O. ferrugineus | |||||||||
| ♂ (N = 2) | M1 | 5.40 | 3.50 | 0.71 | — | — | 0.87 | 1.74 | 0.33 |
| M2 | 5.20 | 4.20 | 0.65 | 0.38 | 1.28 | 0.85 | 1.56 | 0.36 | |
| ♀ (N = 10) | Mean | 4.59 | 3.70 | 0.66 | 0.28 | 1.07 | 0.88 | 1.64 | 0.38 |
| SD | 0.28 | 0.35 | 0.04 | 0.11 | 0.08 | 0.03 | 0.05 | 0.02 | |
| Range | 1.00 | 1.30 | 0.10 | 0.44 | 0.23 | 0.08 | 0.21 | 0.05 | |
| Min | 4.10 | 3.00 | 0.60 | 0.00 | 1.00 | 0.85 | 1.54 | 0.36 | |
| Max | 5.10 | 4.30 | 0.71 | 0.44 | 1.23 | 0.92 | 1.74 | 0.41 | |
| O. frenatus | |||||||||
| ♂ (N = 10) | Mean | 4.36 | 3.53 | 0.64 | 0.34 | 1.01 | 0.87 | 1.42 | 0.36 |
| SD | 0.10 | 0.32 | 0.03 | 0.12 | 0.36 | 0.03 | 0.05 | 0.02 | |
| Range | 0.30 | 1.10 | 0.08 | 0.41 | 1.15 | 0.08 | 0.15 | 0.05 | |
| Min | 4.20 | 2.80 | 0.60 | 0.00 | 0.00 | 0.82 | 1.36 | 0.33 | |
| Max | 4.50 | 3.90 | 0.69 | 0.41 | 1.15 | 0.90 | 1.51 | 0.38 | |
| ♀ (N = 10) | Mean | 4.37 | 3.67 | 0.64 | 0.33 | 1.00 | 0.86 | 1.49 | 0.38 |
| SD | 0.14 | 0.19 | 0.03 | 0.06 | 0.03 | 0.03 | 0.09 | 0.02 | |
| Range | 0.40 | 0.60 | 0.08 | 0.13 | 0.10 | 0.08 | 0.23 | 0.05 | |
| Min | 4.20 | 3.40 | 0.60 | 0.26 | 0.95 | 0.82 | 1.38 | 0.36 | |
| Max | 4.60 | 4.00 | 0.69 | 0.38 | 1.05 | 0.90 | 1.62 | 0.41 | |
- Abbreviation: SD, standard deviation.
| Species | Statistical indicator/specimen number | Length | Width | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body | Cun-Clyp | Pronotum | AntSeg1 | AntSeg2 | Head | Pronotum | InterOcDi | ||
| O. kalmii | |||||||||
| ♂ (N = 10) | Mean | 4.28 | 2.80 | 0.64 | 0.36 | 1.19 | 0.93 | 1.50 | 0.36 |
| SD | 0.20 | 0.12 | 0.04 | 0.02 | 0.12 | 0.07 | 0.08 | 0.02 | |
| Range | 0.60 | 0.40 | 0.13 | 0.05 | 0.38 | 0.23 | 0.28 | 0.05 | |
| Min | 3.90 | 2.60 | 0.58 | 0.33 | 1.05 | 0.85 | 1.38 | 0.33 | |
| Max | 4.50 | 3.00 | 0.71 | 0.38 | 1.44 | 1.08 | 1.67 | 0.38 | |
| ♀ (N = 10) | Mean | 4.35 | 2.99 | 0.67 | 0.35 | 1.08 | 0.95 | 1.64 | 0.42 |
| SD | 0.13 | 0.17 | 0.02 | 0.02 | 0.05 | 0.02 | 0.04 | 0.02 | |
| Range | 0.40 | 0.50 | 0.06 | 0.08 | 0.13 | 0.08 | 0.13 | 0.08 | |
| Min | 4.20 | 2.80 | 0.65 | 0.31 | 1.03 | 0.90 | 1.59 | 0.36 | |
| Max | 4.60 | 3.30 | 0.71 | 0.38 | 1.15 | 0.97 | 1.72 | 0.44 | |
| O. scutellatus (Palearctic) | |||||||||
| ♂ (N = 10) | Mean | 4.92 | 3.13 | 0.68 | 0.38 | 1.16 | 0.91 | 1.62 | 0.37 |
| SD | 0.18 | 0.11 | 0.04 | 0.02 | 0.05 | 0.01 | 0.04 | 0.02 | |
| Range | 0.60 | 0.30 | 0.15 | 0.08 | 0.18 | 0.03 | 0.13 | 0.05 | |
| Min | 4.60 | 3.00 | 0.60 | 0.33 | 1.03 | 0.90 | 1.54 | 0.33 | |
| Max | 5.20 | 3.30 | 0.75 | 0.41 | 1.21 | 0.92 | 1.67 | 0.38 | |
| ♀ (N = 10) | Mean | 4.48 | 2.97 | 0.70 | 0.34 | 1.11 | 0.90 | 1.65 | 0.41 |
| SD | 0.29 | 0.37 | 0.03 | 0.03 | 0.05 | 0.02 | 0.06 | 0.01 | |
| Range | 0.80 | 1.30 | 0.08 | 0.08 | 0.15 | 0.05 | 0.15 | 0.05 | |
| Min | 4.10 | 2.20 | 0.65 | 0.31 | 1.03 | 0.87 | 1.59 | 0.38 | |
| Max | 4.90 | 3.50 | 0.73 | 0.38 | 1.18 | 0.92 | 1.74 | 0.44 | |
| O. scutellatus (Nearctic) | |||||||||
| ♂ (N = 6) | M1 | 4.40 | 3.50 | 0.65 | 0.38 | 1.08 | 0.90 | 1.56 | 0.38 |
| M2 | 4.60 | 3.70 | 0.69 | 0.36 | 1.00 | 0.87 | 1.62 | 0.38 | |
| M3 | 4.60 | 3.70 | 0.67 | 0.38 | 1.05 | 0.85 | 1.54 | 0.38 | |
| M4 | 4.40 | 3.40 | 0.65 | 0.38 | 1.05 | 0.82 | 1.51 | 0.36 | |
| M5 | 4.20 | 3.50 | 0.58 | 0.33 | 0.00 | 0.90 | 1.46 | 0.38 | |
| M6 | 4.20 | 3.70 | 0.60 | 0.28 | 1.03 | 0.90 | 1.54 | 0.38 | |
| ♀ (N = 4) | F1 | 4.80 | 3.60 | 0.63 | 0.38 | 1.05 | 0.90 | 1.62 | 0.41 |
| F2 | 4.60 | 3.70 | 0.60 | 0.38 | 1.03 | 0.90 | 1.62 | 0.38 | |
| F3 | 4.60 | 3.70 | 0.60 | 0.44 | 1.03 | 0.92 | 1.64 | 0.38 | |
| F4 | 4.50 | 3.50 | 0.58 | 0.33 | 0.97 | 0.90 | 1.59 | 0.38 | |
| O. vitticeps | |||||||||
| ♀ (N = 5) | F1 | 4.00 | 2.70 | 0.63 | 0.31 | 1.03 | 0.79 | 1.33 | 0.38 |
| F2 | 4.20 | 2.70 | 0.65 | 0.33 | 1.03 | 0.77 | 1.41 | 0.31 | |
| F3 | 4.50 | 2.80 | 0.71 | 0.31 | 1.00 | 0.77 | 1.62 | 0.33 | |
| F4 | 4.30 | 3.00 | 0.71 | 0.33 | 1.00 | 0.85 | 1.59 | 0.41 | |
| F5 | 4.30 | 3.10 | 0.69 | 0.31 | 1.03 | 0.85 | 1.54 | 0.38 | |
- Abbreviation: SD, standard deviation.
2.6. DNA Extraction, Amplification, and Sequencing
Total DNA was extracted from the abdomen of dry and ethanol-stored specimens. The abdomens remained and stored in glycerol for the subsequent taxonomic identifications. Blood and Tissue Kit (Qiagen) and Extract DNA Blood and Cells Kit (Evrogen) were used for the extractions. We mostly followed the standard protocols for those kits. The tissues were left overnight in the mixture lysis and proteinase K in the water bath. Many specimens were dry or relatively old, that is, collected more than 5 years ago; therefore, the amount of the elution buffer was decreased to 25 or 50 μL to increase the yielded DNA concentration.
Two mitochondrial markers, that is, COI and 12S ribosomal RNA (rRNA), and two nuclear markers, that is, internal transcribed spacer I (ITS1) and calcium ATPase (Ca-ATPase), were used. The primers for COI were taken from Vishnevskaya, Saifitdinova, and Lukhtanov [24], for 12S rRNA from Kocher et al. [25], for ITS1 from Hinomoto et al. [26], and for Ca-ATPase from Pazhenkova and Lukhtanov [27]. All polymerase chain reaction (PCR) reactions included 35 cycles. The PCR protocols are provided in Table 3. The PCR products were visualized in a 1.5% agarose gel, stained with ethidium bromide, and visualized under an ultraviolet (UV) transilluminator. The PCR products were cleaned and sequenced in Evrogen (https://evrogen.ru/). The products from both COI primers pairs were between 541 and 771 bp in length, and they largely overlapped. The products of 12S rRNA were between 240 and 325 bp. The products of ITS1 were between 653 and 817 bp. The products of Ca-ATPase were between 270 and 416 bp.
| Marker | Primer name | Primer sequence | Annealing temperature (°C) | Extension and final extension temperature (°C) | Reference for primers |
|---|---|---|---|---|---|
| COI | COIF1 | CCACAAATCATAAAGATATTGGAAC | 45 or 42 | 68 | Vishnevskaya et al. [24] |
| COIR1 | TGATGAGCTCATACAATAAATCCTA | ||||
| 12S rRNA | 12Sai | AAACTAGGATTAGATACCCTATTAT | 48 | 68 | Kocher et al. [25] |
| 12Sbi | AAGAGCGACGGGCGATGTGT | ||||
| ITS1 | ITS1F | GTCGCTACTACCGATTGAATGG | 52 or 54 or 56 | 72 | Hinomoto et al. [26] |
| ITS1R | GTGTCCTGCAGTTCACATGG | ||||
| Ca-ATPase | CATF | GAATACGARCCBGAAATGGGWAARGT | 52 or 54 | 72 | Pazhenkova and Lukhtanov [27] |
| CATR | CDCCRTGRGCGGGGTCGTTRAAGTG | ||||
- Abbreviations: Ca-ATPase, calcium ATPase; COI, cytochrome c oxidase subunit I; ITS1, internal transcribed spacer I; rRNA, ribosomal RNA.
Raw sequences were manually checked in Geneious v. 11, and contigs were created in the same software. They were tested for contamination using the Basic Local Alignment Search Tool (BLAST) algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The alignments were performed in Geneious v. 11 using the Geneious algorithm and were manually checked using this software. They were trimmed at both ends in cases if the data were absent for more than half of the sequences.
The sequences were uploaded to GenBank (https://www.ncbi.nlm.nih.gov/genbank/); the accession numbers are OQ785663–OQ785705 for COI, OQ732795–OQ732824 for 12S, OQ732778–OQ732794 for ITS1, and OQ798024–OQ798038 for Ca-ATPase (Supporting Information 1: Table SI1).
2.7. Phylogenetic Analysis
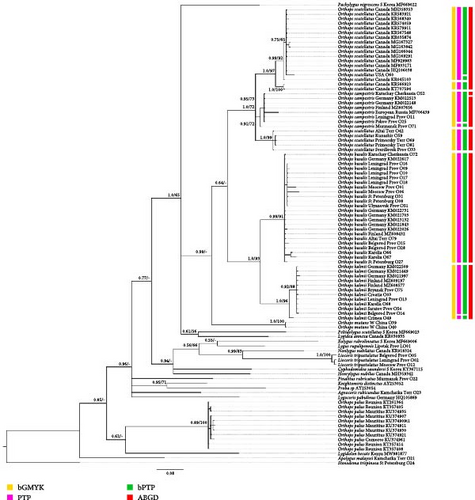
For the phylogenetic analysis of Palearctic Orthops species, the following datasets were used. Dataset 1 includes COI sequences for 101 specimens, 46 of them are obtained in the current study, and 55 were downloaded from GenBank. The ingroup, comprising representatives of the subgenus Orthops, consists of 67 sequences. The remaining 34 sequences belong to the outgroup taxa, which are A. rubicundus, Apolygus malaysei, C. saundersi, Eolygus rubrolineatus, H. nubilus, K. distinctus, Liocoris tripustulatus, L. annexa, Lygidolon hecate, L. pabulinus, L. rugulipennis, N. nubilatus, Orthops mutans, Orthops palus, Pachylygus nigrescens, Peltidolygus scutellatus, P. rubricatus, Proba sp., and S. trispinosa (Supporting Information 2: Table SI3). The tree was rooted to S. trispinosa, because it is the most distantly related and belongs to another Mirinae tribe, the Stenodemini. Dataset 2 includes COI sequences for 58 Orthops specimens; Pachylygus nigrescens was chosen as a root, as the present and previous studies demonstrate that it is one of the most closely related genera to Orthops (Figure 2 [28]).
In the other datasets, only representatives of the subgenus Orthops, O. mutans, and Liocoris tripustulatus were included with L. tripustulatus chosen as a root. All sequences for those datasets were obtained during the current study. Dataset 3 includes 12S rRNA sequences for 30 specimens. Dataset 4 includes Ca-ATPase sequences for 15 specimens. Dataset 5 includes ITS1 sequences for 17 specimens. Dataset 6 includes COI, and 12S rRNA sequences for 46 specimens. Dataset 7 includes COI, 12S rRNA, Ca-ATPase, and ITS1 for 46 specimens.
Maximum likelihood analysis was performed using RAxML v. 8.2.12 [29] with 10,000 bootstrap replicates. Bayesian inference also was performed using MrBayes v. 3.2.7 software [30] with a 10 million chain length, and posterior probabilities were used for the node support (PP). The burn-in was set at 25% as per standard protocol. The log files were checked to assure that the standard deviation of split frequencies reached 0.01. The nucleotide substitution models for each codon position were found using MrModeltest 2.4 [31]. For all calculations, the server Dell PowerEdge R7525 (Dell Inc., USA) was utilized. The trees resulting from the analyses were visualized using the FigTree software (http://tree.bio.ed.ac.uk/software/figtree/).
2.8. Species Delimitation
The datasets mostly correspond to those used for the phylogenetic analysis. For Dataset 1 (COI only), most of the outgroup taxa were removed, because their sequences could affect the results of the subgenus Orthops species delimitation. However, Orthops mutans was left because it is a sister group to the subgenus Orthops, and the tree was rooted to Pachylygus nigrescens because it is closely related to the group of interest in the phylogenies (Figure 2, Supporting Information 3: Figures SI1 and SI2).
Four species delimitation analyses for Palearctic representatives of Orthops were performed on all markers separately (ABGD, bGMYK, PTP, and bPTP). Then the analyses were conducted for two combined datasets (BPP, bGMYK, PTP, and bPTP).
The Automatic Barcode Gap Discovery (ABGD) algorithm, which utilizes a sequence alignment for the data and searches for a gap in the distribution of pairwise sequences, was implemented [32]. The ABGD analyses were conducted through the online tool (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html). The P range was chosen as 0.001–0.01. The matrix of pairwise distances was estimated using the Kimura (K80) model.
For the bGMYC, PTP, and bPTP analyses, the datasets were trimmed of ambiguous reads at the end, and all identical sequences were removed as zero-length branches can affect the results [33]. Bayesian inference analysis in BEAST 2.5 was used to obtain 100 trees, and analyses were performed on these trees to overcome the problems with the phylogenetic uncertainty occurring when the species delimitation is applied for the single tree [33, 34]. After each analysis, the log file was uploaded to Tracer 1.7.1 [35] to check whether all parameters had effective sampling size exceeding 200, which is considered adequate for convergence (https://beast.community/analysing_beast_output, accessed May 25, 2023). Burn-in was set at 10%, which is a default setting. LogCombiner was used to obtain the .tre file with the 100 trees for each analysis.
Bayesian and maximum likelihood implementations of the Poisson tree processes model (bPTP and PTP) [36] were used, using the scripts in Python (https://github.com/zhangjiajie/PTP). The PTP method utilizes branch length information from a phylogeny to estimate the average expected number of substitutions per site between two branching events. It then implements two Poisson processes (intra- and interspecific branching events) and clusters the phylogenetic tree based on the results. The Bayesian version of the PTP model for species delimitation uses the number of substitutions estimated from branch lengths in the maximum likelihood tree [36]. The parameters for the run were 100,000 Markov chain Monte Carlo (MCMC) generations. The analyses were performed using the thinning interval of 100, 25% of burn-in, and the outgroups were removed to improve the delimitation results. The run was performed with 100,000 MCMC in most cases. The analysis for the dataset with all markers was performed with different MCMC numbers (10,000, 500,000, and 1,000,000).
Bayesian implementation of the Generalized Mixed Yule Coalescent approach (bGMYC) [33, 37] was also conducted for each dataset. The GMYC model aims to distinguish between coalescent and speciation events in the branching patterns of an ultrametric gene tree by identifying the threshold at which these events occur [38]. However, the accuracy of this calculation is heavily influenced by phylogenetic uncertainty, taxon sampling, and ultrametrization of the tree. The bGMYC implementation addresses these limitations by allowing the use of posterior distributions of trees as input instead of a single tree, thereby mitigating these issues [33]. The bGMYC analysis was performed in R with the bGMYC package, and parameters were set according to the developer’s instructions (http://nreid.github.io/assets/bGMYC_instructions_14.03.12.txt): MCMC = 50,000, burnin = 40,000, and thinning = 100. As bGMYC provides the list of all possible species, the set of species with the maximum mean support values was chosen.
The Bayesian Phylogenetics and Phylogeography (BPP) method, based on a multispecies coalescent model, was used to delimit species. This method eliminates the need for a user-specified guide tree in species delimitation and incorporates phylogenetic uncertainty in a Bayesian framework [39]. Two combined datasets (12S rRNA + COI and 12S rRNA + COI + Ca-ATPase + ITS1) were analyzed. Our analyses is based on eight populations: O. basalis, O. campestris, O. kalmii, O. kalmii (O49), O. mutans, Palearctic O. scutellatus, Nearctic O. scutellatus (O60), and L. tripustulatus. We grouped the specimens based on the results of the phylogenetic analysis; that is, the smallest groups of species appearing in most of the phylogenetic analyses as clades were chosen. Additionally, specimen of O. kalmii O49 was also treated separately, because it formed a sister group relationship with other specimens of this species in the trees based on COI only and all markers with medium to high supports. The analysis [39], which involves delimitation and construction of species trees, was performed with nsamples = 100,000, sampfeq = 5, burnin = 25,000, and default settings for all other parameters. The analysis was run using the tool BPP 3.1 [40].
3. Results
3.1. Morphological Characters, Key, and Diagnoses
The traditional features for identifying Orthops species were the following: the coloration of hemelytron, the body size, the shape of parameres, the coloration pattern on the frons, the vertex width/eye diameter ratio, the absence or presence of the longitudinal stripe on tibiae, and the length of the antennal segment III [13, 14, 16].
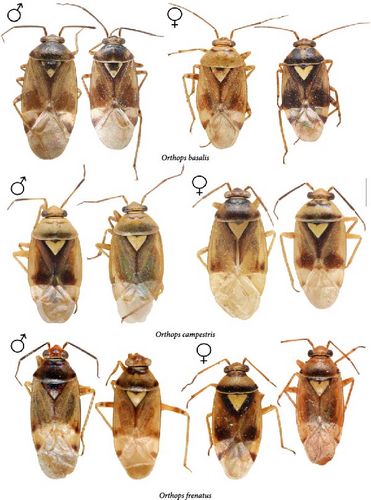
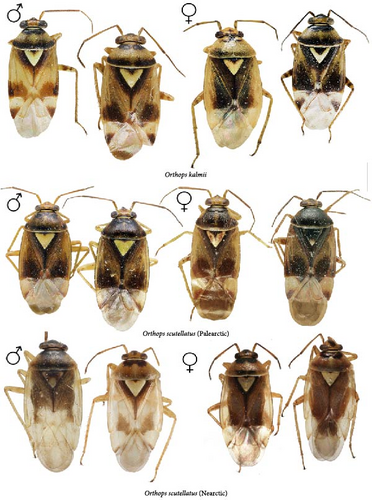
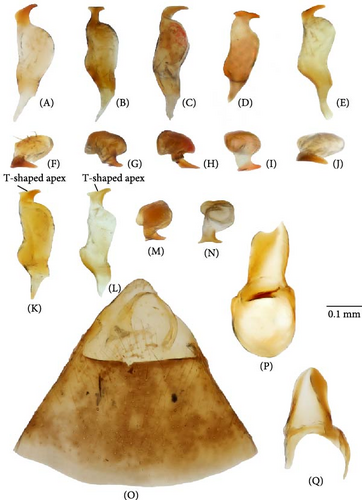
All those characters were checked, and we also examined the differences in the structure of the aedeagus and the female genitalia for the first time. The hemelytron coloration can be helpful for the species differentiation in some cases, but it should not be used alone in case of the widely distributed species. Although O. basalis and O. kalmii are usually dark brown to black, there is considerable variation in color in those two species, and some representatives can be as pale as O. campestris and O. scutellatus (Figures 3 and 4). Similarly, the shape of marking on the frons can be misleading. The paired markings on the frons were diagnostic for O. basalis, but this condition was found in other species too (Figure 5G,Q), and some specimens of O. basalis have a single marking.
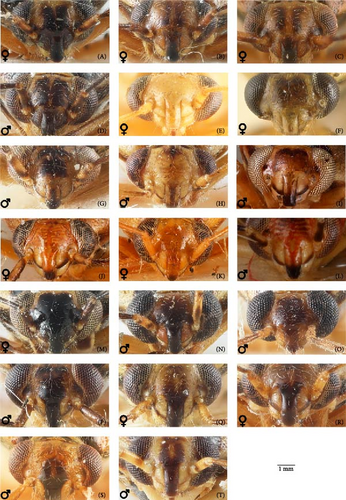
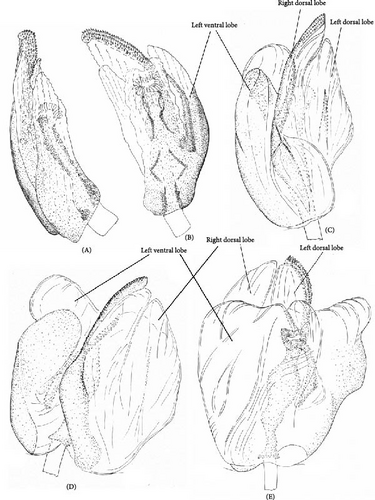
The most reliable character complex for the species discrimination is the shape of both parameres (Figures 6 and 7). However, in some cases, the differences are very small and should be used if several specimens of the different species can be compared. The aedeagus structure is less variable and is identical in O. campestris, O. kalmii, and O. scutellatus. The genital capsule and theca are identical in all species, and the illustrations for those structures were provided, which can be useful for the further studies of Orthops and closely related to Mirini genera (Figure 8M–O). The most species, except for the O. basalis and O. kalmii, can be identified using the female genitalia, that is, dorsal labiate plate and posterior wall of bursa copulatrix characters.
3.2. Subgenus Orthops
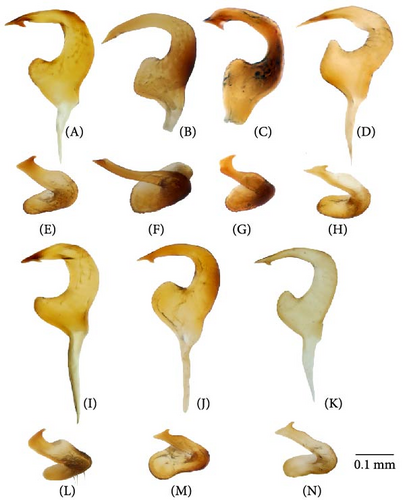
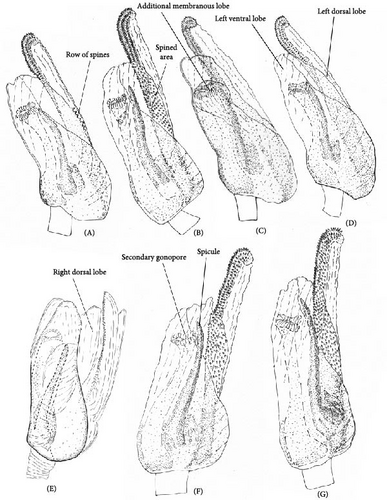
The body color is variable, usually yellow, green, or pale brown with brown to black markings on the head, pronotum, and posterior part of the corium, and sometimes reddish (Figures 3–5, 9). Spines on the tibiae are dark brown to black. The pronotum, scutellum, and hemelytron shallowly and rarely punctate (Figures 3, 4, 9). The setae are longer than the collar length (Figure 10O,P). The left paramere is C-shaped, and the basal lobe is prominent without spines (Figure 6). The hypophysis of the left paramere is shown in the posterior view with single angulate outgrowth (Figure 6). The right paramere is usually with hooked, straight, or T-shaped hypophysis in dorsal view (Figure 7; Figure 3b in [41]). The parameres are subequal in length (Figures 6 and 7). The theca has a single rounded outgrowth on the right-hand side (Figure 7). The vesica has three lobes (Figure 8C–E). The left ventral lobe features a spicule and a sclerotized area around the spicule base, with the ventral side of this lobe being sclerotized (Figures 8, 11). The right dorsal lobe has an elongated sclerotized area with teeth rows in the apical half. The left dorsal lobe lacks sclerotization (Figure 8). The spicule in the vesica reaches secondary gonopore in repose or slightly surpasses it (Figure 11). The dorsal labiate plate is visibly sclerotized with toothed lateral structures (Figure 12A–C, G–I, O–Q). The sclerotized ring is oval and two to three times wider than long (Figure 12A–C, G–I, O–Q). The posterior wall has interramal lobes separated by a distance subequal to the interramal lobe width (Figure 12D–F, J–N).


3.3. Key to Species of the Subgenus Orthops
- 1.
The frons yellow to dark brown, often with various brown to black markings (Figure 5). Hemelytron with dark markings (Figures 3, 4, and 9)…………………2
- -
The frons red or reddish brown (Figure 9). Hemelytron uniformly yellowish brown with reddish tinge to rusty red (Figure 9)………………… О. ferrugineus
- 2.
The basal lobe of the left paramere rounded, not curved (Figures 6A,I); hypophysis of the right paramere r-shaped (Figure 7A,E); area between the sclerotized rings on the dorsal labiate plate with three separate thin sclerites (Figure 12A)………………… 3
- -
The basal lobe of the left paramere subquadrangular (Figure 6B), subrectangular (Figure 6J), or acute and curved (Figure 6D,K); hypophysis of the right paramere T-shaped (Figure 7B,D,K,L) or straight [41]; area between the sclerotized rings on the dorsal labiate plate with sickle-shaped membranous sclerite (Figure 12B), membranous swelling (Figure 12H), or without any structures (Figure 12I)………………… 4
- 3.
The right paramere twice as long as wide (Figure 7A). The left dorsal lobe with row of spines at the base of the elongate sclerotized area (Figure 11A). The frons usually with paired markings (Figure 5A–D)………………… О. basalis
- -
The right paramere three times as long as wide (Figure 7E). The left dorsal lobe without spined area and without rows of teeth at the base of the elongate sclerotized area (Figure 11D). The frons often with single marking of variable shape (Figure 5M–P)………………… O. kalmii
- 4.
The left paramere with subrectangular basal lobe (Figure 6B). Hypophysis of the right paramere T-shaped (Figure 7B), with apex ca. 0.3× as wide as the left paramere medially. The apex of the left paramere not curved in posterior view (Figure 6F). The dorsal labiate plate with distinct sickle-shaped sclerite between sclerotized rings (Figure 12B); membranous oval structures present (Figure 12B). Dark marking on scutellum basally small (Figure 3)………………… O. campestris
- -
The left paramere with the basal lobe subquadrangular or acute and curved (Figure 6A,C). Hypophysis of the right paramere straight or T-shaped, more than 0.5× as wide as the right paramere body. The apex of the left paramere in posterior view curved (Figure 6E,G,H,L–N). The dorsal labiate plate with three separated thin sclerites (Figure 12A), without any structures between the sclerotized rings (Figure 12A) or with indistinct curved areas (Figure 12G). Membranous oval structures absent………………… 5
- 5.
The right paramere hypophysis straight [41]; the dorsal labiate plate without any sclerotized areas between the sclerotized rings (Figure 12A) and the posterior wall of the bursa copulatrix without sclerotized area between the interramal lobes (Figure 12N); coloration more or less contrasting (Figure 9), markings on the dorsum brown to black.………………… O. vitticeps
- -
The right paramere hypophysis T-shaped (Figure 7K,L); the dorsal labiate plate with swollen membranous structure between the sclerotized rings (Figure 12H) or the posterior wall of the bursa copulatrix with acute sclerotized area between the interramal lobes (Figure 12L).………………… 6
- 6.
The right dorsal lobe in the vesica with elongate sclerotized area narrow with small spines, not differentiated into rows (Figure 11E); the posterior wall of the bursa copulatrix with acute sclerotized area between the interramal lobes (Figure 12J); the lateral sclerite near the sclerotized ring absent (Figure 12G).………………… O. frenatus
- -
The right dorsal lobe in the vesica with elongate sclerotized area wide with large spines placed at least in three distinct rows (Figure 11F,G); the posterior wall of the bursa copulatrix without sclerotized area between the interramal lobes (Figure 12L); the lateral sclerite near the sclerotized ring present (Figure 12H).………………… 7 (O. scutellatus).
- 7.
The left paramere with quadrangular basal lobe (Figure 6J). The right paramere apically T-shaped; its right apex only slightly protruding (Figure 7K).………………… O. scutellatus (Palaearctic)
- -
The left paramere with acute and curved basal lobe (Figure 6K) [42]. The right paramere T-shaped apically; its right apex distinctly protruding (Figure 7L) [42].………………… O. scutellatus (Nearctic).
Orthops basalis (Costa, A., 1853)
Figures 1A, 3, 5A–D, 6A,E, 7A,F,O,P, 8C–E, 10A,K,O, 11A, and 12A,D,P.
Phytocoris basalis Costa, A., 1853: 62 (original description).
Orthops basalis [43]: 175 (catalogue); [44]: 43 (diagnosis, hosts); [45]: 385 (new synonymy, discussion); [13]: 945 (diagnosis, distribution); [14]: 214 (description, life history information, distribution); [15]: 416 (description, distribution); [9]: 854 (catalogue); [10]: 92–93 (figures of male genitalia); [3]: 132 (catalogue); [8]: 223–225 (description, figures of male genitalia); [16]: 58, 61 (diagnosis, figures of male genitalia). For more references, see Carvalho [43], Kerzhner and Josifov [3], and Schuh [9].
Diagnosis. Body size in males 4.30–5.20, in females 4.20–5.00. This species can be distinguished from its congeners in the following set of characters: coloration yellow or green with contrasting brown to black markings (Figure 3); the frons usually with two separate markings (Figure 5A–D), the femora yellow with two brown rings apically (Figure 3); the left dorsal lobe of the vesica with row of spines and without spined area (Figure 11A); the left paramere at least twice as long as wide, with rounded basal lobe (Figure 6A); the dorsal labiate plate with three separate thin structures medially; the posterior wall with toothed lateral structures concave anteriorly, membranous oval structures absent (Figure 12A,D).
Distribution. This species has been reported from Central, North, West, and South European territory (including Russia), known from the Urals territory, West and East Siberia. Orthops basalis has had records from the Caucasus and Iran. For more details, see Kerzhner and Josifov [3], Lis and Lis [46], Linnavuori [47], Linnavuori [48], Kment and Banar [49], Hosseini [16], and Rabitsch et al. [50].
Material examined (see details in the Supporting Information 4: Data SI2): Armenia, Azerbaijan, Belarus, Bulgaria, Estonia, France, Georgia, Germany, Italy, Poland, Russian Federation (Central European Territory, North European Territory, South European Territory, West Siberia), Switzerland, Turkey, Ukraine, Serbia.
Orthops campestris (Linnaeus, 1758)
Figures 1B, 3, 5E–H, 6B,F, 7B,G, 10M,N, 11B, and 12B,E.
Cimex campestris Linnaeus, 1758: 448 (original description).
Orthops campestris [51]: 23 (distribution); [43]: 176 (catalogue); [52]: 279 (distribution, hosts, life history information); [53]: 364 (distribution, hosts, life history information); [13]: 944 (diagnosis, distribution, hosts); [15]: 416 (description, hosts, distribution); [9]: 855 (catalogue); [10]: 92–93 (figures of male genitalia); [3]: 132–133 (catalogue); [8]: 223–225 (description, figures of male genitalia); [16]: 58, 60 (diagnosis, figures of male genitalia). For more references, see Carvalho [43], Schuh [9], and Kerzhner and Josifov [3].
Diagnosis. Body size in males 3.7–4.6, in females 3.5–4.6. This species can be discriminated from its congeners using the following set of characters: main body color green, color pattern on the dorsum pale brown to brown, weekly contrasting, clypeus pale, usually with brown to dark brown apex or stripe, dark marking on the scutellum basally small, the femora usually uniformly yellow (Figure 3); the left paramere with prominent subquadrangular basal lobe (Figure 6B), the apex of the right paramere T-shaped, with the apex at least 0.3× as wide as the left paramere medially (Figure 7B,G); the apex of the left paramere tapering, without apical outgrowth in the posterior view (Figure 6F); the dorsal labiate plate with sickle-shaped membranous structure between sclerotized rings; the posterior wall with toothed lateral structures convex anteriorly, membranous oval structures present (Figure 12B,E).
Distribution. This species has been reported from Central, North, West, and South European territory (including Russia), known from the Urals territory, West and East Siberia. Orthops campestris has had records from the Caucasus and Iran. For more details, see Kerzhner and Josifov [3], Linnavuori [47], Kondorosy [54], Kment and Banar [49], and Hosseini [16].
Material examined (see details in the Supporting Information 4: Data SI2): Belarus, Georgia, Germany, Kazakhstan, Lithuania, Moldova, Russian Federation (Central European Territory, North European Territory, South European Territory, West Siberia), Turkey, Ukraine, Serbia.
Orthops ferrugineus (Reuter, 1906)
Figures 6C,G, 7C,H, 9, 11C, and 12C,F.
Lygus kalmii ferruginea (Reuter, 1906): 46 (original description).
Orthops ferrugineus [55]: 253 (new status, discussion); [3]: 133 (catalogue); [41]: 499 (diagnosis, records, figures of male genitalia); Carvalho [43]: 175; [9]: 855. For more references, see Carvalho [43], Schuh [9], and Kerzhner and Josifov [3].
Diagnosis. Body size in males 5.2–5.4, in females 4.5–5.3. This species can be distinguished from other species in the following set of characters: body large; frons color reddish; femora with red markings, hemelytron uniformly yellowish brown to rusty red (Figure 9); apex of the right paramere r-shaped (Figure 7C); the left dorsal lobe of the vesica without spined area and with additional membranous lobe; spicule in the vesica reaching secondary gonopore in repose (Figure 11C); the dorsal labiate plate with membranous swelling covering sclerotized rings; the posterior wall of the bursa copulatrix with membranous fold covering the basal half of the wall, and with paired triangular sclerotized areas basally (Figure 12C,F).
Distribution. This species has been reported from China. For more details, see Kerzhner and Josifov [3], Schwartz and Kerzhner [55], and Zheng and Lu [41].
Material examined (see details in the Supporting Information 4: Data SI2): China.
Orthops frenatus Horváth, 1894
Figures 3, 5I–L, 6D,H, 7D,I, 11E, and 12G,J.
Lygus kalmii frenatus Horvath, 1894: 182 (original description).
Orthops frenatus [9]: 856 (catalogue), [3]: 133 (catalogue); [16]: 58, 59 (diagnosis, figures of male genitalia). For more references, see Carvalho [43], Schuh [9], and Kerzhner and Josifov [3].
Diagnosis. Body size in males 4.2–4.5, in females 4.2–4.6. This species can be discriminated from its congeners using the following set of characters: main body color yellow or green, markings pale brown to brown, weekly contrasting, the femora yellow with two brown rings apically; the right dorsal lobe with elongate sclerotized area narrow bearing small spines, not differentiated into rows (Figure 11E); the posterior wall of the bursa copulatrix with acute sclerotized area between the interramal lobes (Figure 12J); the lateral sclerite near the sclerotized ring absent (Figure 12G).
Distribution. This species has been reported from Central Asia, South Caucasus, and West Asia (Iran). For more details, see Kerzhner and Josifov [3], Linnavuori and Modarres [56], Linnavuori [47], Linnavuori [48], Hosseini [16], and Shamsi [57].
Material examined (see details in the Supporting Information 4: Data SI2): Azerbaijan, Georgia, Iran, Kazakhstan, Kyrgyzstan, Moldova, Russian Federation (West and East Siberia), Tajikistan, Turkmenistan, Uzbekistan.
Orthops kalmii (Linnaeus, 1758)
Figures 1C, 4, 5M–P, 6I,L, 7E,J, 8A,B, 10E–H, 11D, and 12A,D.
Cimex kalmii Linnaeus, 1758: 448 (original description).
O. kalmii [51]: 33 (distribution, habitat); [43]: 178 (catalogue); [52]: 280 (distribution, hosts, life history information); [53]: 365 (distribution, hosts, life history information); [44]: 43 (diagnosis, hosts); [58]: 945 (diagnosis, distribution); [14]: 215 (synonymy, description, life history information, distribution); [59]: 180 (habitat, hosts, distribution); [15]: 417 (description, distribution, hosts); [9]: 856, 857 (catalogue); [10]: 92–93 (figures of male genitalia); [3]: 133–134 (catalogue); [8]: 223–225 (description, figures of male genitalia); [16]: 60, 62, 63 (diagnosis, figures of male genitalia). For more references, see Carvalho [43], Kerzhner and Josifov [3], and Schuh [9].
Diagnosis. Body size in males 3.9–4.5, in females 4.2–4.6. This species can be distinguished from its congeners in the following set of characters: coloration yellow or green with contrasting dark brown to black markings (Figure 4); the frons with single, rounded, trapezoidal, or rectangular marking (Figure 5M–P), the femora yellow with two brown rings apically (Figure 4); the left side of the vesica without spined area and without row of spines (Figure 11D); the left paramere at least three times as long as wide, with rounded basal lobe (Figure 6I); the right paramere r-shaped apically (Figure 6L); the dorsal labiate plate with three separate thin sclerites, toothed lateral structures concave anteriorly, membranous oval structures absent (Figure 12A,D).
Distribution. This species has been reported from Central, North, West, and South European territory (including Russia), known from the Urals territory, West and East Siberia. O. kalmii is also recorded from the Caucasus and Iran, Kazakhstan, and Kyrgyzstan. For more details, see Kerzhner and Josifov [3], Linnavuori and Modarres [56], Linnavuori [48], Kondorosy [54], Kment and Banar [49], and Hosseini [16].
Material examined (see details in the Supporting Information 4: Data SI2): Armenia, Azerbaijan, Belarus, Croatia, Finland, Georgia, Germany, Hungary, Iran, Kazakhstan, Kyrgyzstan, Lithuania, Moldova, Mongolia, Russian Federation (Central European Territory, North European Territory, South European Territory, West Siberia), Turkey, Ukraine, Serbia.
Orthops scutellatus Uhler, 1877
Figures 1D, 4, 5Q–T, 6J,K,M,N, 7K–M,Q, 10B–D,I,J,L,P, 11F,G, and 12H,L.
O. scutellatus Uhler, 1877: 420 (original description); [43]: 182 (catalogue); [60]: 17 (distribution); [61]: 40 (new combination, synonymy, discussion); [42]: 100 (diagnosis, hosts, distribution); [8]: 224 (new synonymy, diagnosis, discussion, figures of male genitalia). Schuh (1995) [9]: 858 (catalogue); [10]: 92–93 (figures of male genitalia); [8]: 223–225 (description, figures of male genitalia). For more references, see Carvalho [43], Schuh [9], and Kerzhner and Josifov [3].
Diagnosis. Body size in males 4.6–5.2, in females 4.1–4.8. The species can be distinguished from its congeners in the following set of characters: general color pale brown to brown with dark brown markings; the scutellum partly yellow with dark marking (Figure 4); black spot оn head variable, usually Y-shaped; the clypeus brown, usually with dark apex (Figure 5Q–T); the femora yellow with two brown rings apically (Figure 4); the dorsal labiate plate with swollen membranous structure between sclerotized rings (Figure 12H,L).
Palearctic form:
Diagnosis. The left paramere with rectangular basal lobe (Figure 6J); the right paramere with T-shaped apical process having the right outgrowth only slightly protruding (Figure 7K,M).
Distribution. This form has been reported from East Siberia to East Asia, including the Russian Far East. Has been recorded from India. For more details, see Kelton [42], Kerzhner and Josifov [3], Zheng and Lu [41], Kim et al. [62], Oh et al. [63], Vinokurov and Rudoi [64], and Harshini [65].
Material examined (see details in the Supporting Information 4: Data SI2): China, Japan, Mongolia, Russian Federation (West Siberia, East Siberia, Far East).
Nearctic form:
Diagnosis. The left paramere with rounded basal lobe (Figure 6K); the right paramere with T-shaped apical process having right outgrowth distinctly protruding (Figure 7L,N).
Distribution. This form has been reported from Canada, Mexico, and the United States. For more details, see Uhler [66], Wheeler, Henry and Mason [67], and DeWaard et al. [68].
Material examined (see details in the Supporting Information 4: Data SI2): Canada, Mexico, and the United States.
Orthops vitticeps (Reuter, 1906)
Lygus kalmi vitticeps (Reuter, 1906) (original description); Orthops vitticeps [55]: 253 (new status, discussion); [3]: 134 (catalogue); [41]: 500 (Figure 4) (diagnosis, records, figures of male genitalia); [9]: 859 (catalogue). For more references, see Carvalho [43], Schuh [9], and Kerzhner and Josifov [3].
Diagnosis. Body size in males 5.2–5.5, in females 4.4–4.7. This species can be distinguished from its congeners in the following set of external characters: coloration yellow with contrasting dark markings, median black marking on the head narrowly triangular, backwardly extending to the hind margin of the vertex, the femora yellow with two brown rings apically (Figure 9); hypophysis of the right paramere straight (Figure 3b in [41]); the basal lobe of the left paramere small and quadrangular (Figure 3a in [41]); the spicules of the vesica not reaching secondary gonopore in repose (Figure 3c in [41]); the dorsal labiate plate without sclerotized areas proximally (Figure 12I,N).
Distribution. This species has been reported from China. For more details, see Schwartz and Kerzhner [55], Kerzhner and Josifov [3], and Zheng and Lu [41].
Material examined (see details in the Supporting Information 4: Data SI2): China.
3.4. Sequence Distances
The p-distance and Kimura 2-parameter distance ranges were almost identical in all the cases. All distances are provided in Supporting Information 5: Table SI4. Intraspecies average distances are less than 0.01 (1%) in most cases. For COI, they are around 0.002 for O. kalmii, 0.005 in O. basalis and O. campestris, and 0.017 in O. scutellatus. For 12S rRNA, sequences are identical in O. kalmii, O. basalis, and O. campestris, and the intraspecies average distance is 0.004 for O. scutellatus. For nuclear ITS1, the sequences are identical within O. basalis and O. campestris, and the intraspecies average distance equal 0.001 in O. kalmii and 0.002 for O. scutellatus. For Ca-ATPase, intraspecies average distances are equal to 0.001 in O. basalis, 0.016 in O. campestris, and 0.013 in O. kalmii. The greatest intraspecific genetic diversity for COI, 12S rRNA, and ITS1 is detected for O. scutellatus. The average genetic distances between Asian and American representatives of this species are 0.038 for CO1 and 0.008 for 12S rRNA. However, the COI distances within each of those groups are also less than 0.01. We do not have any results for the intraspecific differences for Ca-ATPas of O. scutellatus, because this marker for only a single specimen of this species was obtained.
The genetic distances between four Orthops species are the largest for COI and ITS1. These values vary from 0.036 to 0.085 for the COI marker. The minimum distance is detected between O. campestris and O. scutellatus. The COI distance between O. basalis and O. kalmii is around 0.05. Other pairs have interspecific distances subequal to 0.08. Orthops campestris and O. scutellatus have the smallest difference in ITS1, which is 0.007. The distance between O. basalis and O. kalmii is around 0.02. Other pairs have differences equal 0.06. For 12S rRNA and Ca-ATPase, the interspecies genetic distances are less than 0.01 for the pairs O. campestris–O. scutellatus and O. basalis–O. kalmii, and they are between 0.02 and 0.033 for other pairs. According to the data obtained for two mitochondrial and two nuclear markers, O. campestris and O. scutellatus are the most genetically similar species.
3.5. Phylogenetic Relationships Between Orthops Species
The topologies are shown in Figures 2, 13, and Supporting Information 3: Figures SI1–SI12.
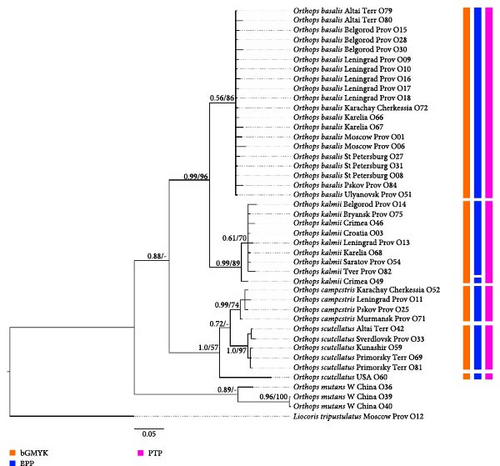
Phylogenetic analyses show different results depending on the marker. However, in all analyses except for Ca-ATPase, O. kalmii form a clade with high support, and this species form a clade with O. basalis also with high supports. However, Orthops mutans is absent in Dataset 4 (Ca-ATPase), and Nearctic O. scutellatus is absent in the nuclear marker datasets (Datasets 4 and 5).
Phylogeny based on all markers (Dataset 7, Figures 13, Supporting Information 3: Figure SI9) shows that O. basalis, O. campestris, and O. kalmii are monophyletic groups (PP = 0.56 and BS = 86, PP = 0.99 and BS = 74, and PP = 0.99, BS = 89, respectively). Orthops basalis and O. kalmii form a clade with high supports (posterior probability [PP] = 0.99, bootstrap support [BS] = 96); O. campestris and O. scutellatus also form a clade (PP = 1.0, BS = 57); Palearctic representatives of O. scutellatus form a clade with high supports (PP = 1, BS = 97). This groups are sister toO. campestris with low supports in the Bayesian analysis (PP = 0.72), and the relationships between Palearctic O. scutellatus and Nearctic O. scutellatus and O. campestris are unresolved in the RAxML analysis. Subgenus Orthops is either monophyletic in Bayesian analysis (PP = 0.88), but in the RAxML analysis, the position of O. mutans in respect to this group is unclear.
The phylogenetic trees based on the COI + 12S rRNA dataset (Dataset 6, Supporting Information 3: Figures SI6 and SI8) is similar to that based on all markers, but it is less resolved, especially in the RAxML tree. O. scutellatus and O. campestris form a clade only in the Bayesian tree (PP = 1.0), but relationships between Palearctic O. scutellatus and Nearctic O. scutellatus and O. campestris are unresolved in both analyses. Orthops basalis is monophyletic only in the Bayesian analysis tree (BS = 68), and O. campestris is monophyletic only in the RAxML tree (BS = 68). O. kalmii and Palearctic O. scutellatus are monophyletic in both cases (PP = 0.99 and BS = 85 and PP = 1.0 and BS = 87, respectively). Subgenus Orthops is either monophyletic in Bayesian analysis (PP = 1), but in the RAxML analysis, the position of O. mutans in respect to this group is unclear.
The topologies of the phylogenies based on COI only (Datasets 1 and 2, Figures 2, Supporting Information 3: Figures SI1, SI2, and SI11) are similar to those based on all markers (Dataset 7). Orthops basalis, O. campestris, and O. kalmii are monophyletic groups (PP > 0.99 and BS > 91, PP > 0.75 and BS > 72, and PP = 1.0 and BS > 89, respectively). Orthops basalis and O. kalmii form a clade with high supports (PP = 1.0, BS = 93). Orthops campestris and O. scutellatus form a clade in most cases (PP > 0.94, BS = 0.72), except for the RAxML based on Pachylygus root dataset (Dataset 2). Palearctic representatives of O. scutellatus and Nearctic representatives of O. scutellatus form a clade each (PP > 0.98 and BS > 94 and PP = 1.0 and BS > 92, respectively). However, relationships between those two groups and O. campestris are unresolved. Subgenus Orthops is either monophyletic in the Bayesian analyses although with low supports (PP = 0.64–0.69), but in the RAxML analysis, the position of O. mutans in respect to this group is unclear (Dataset 1), or O. mutans form a clade with O. basalis + O. kalmii clade (PP = 84) (Dataset 2).
In the dataset based on 12S rRNA (Dataset 3, Supporting Information 3: Figures SI3 and SI7), O. basalis and O. kalmii form a clade with high supports (PP = 1.0, BS = 93), and O. campestris and O. scutellatus also form a clade (PP = 0.94, BS = 82). Among all species, only O. kalmii is a monophyletic group (PP = 1.0, BS = 95). Nearctic O. scutellatus is a sister group (PP = 0.94, BS = 82) to a clade formed by Palearctic O. scutellatus and O. campestris (PP = 0.94, BS = 86). Subgenus Orthops form a clade (PP = 1.0, BS = 72).
In the dataset based on ITS1 (Dataset 5, Supporting Information 3: Figures SI5 and SI12), O. basalis, O. campestris, O. kalmii, and Palearctic O. scutellatus are monophyletic groups (PP = 1.0 and BS = 100, PP = 0.99 and BS = 99, PP = 1.0 and BS = 98, and PP = 0.89 and BS = 90, respectively). Nearctic O. scutellatus is absent in this dataset. Orthops basalis and O. kalmii form a clade with high supports (PP = 1.0, BS = 99); O. campestris and O. scutellatus also form a clade (PP = 1.0, BS = 87). Orthops mutans forms a sister group relationship with the Orthops basalis + O. kalmii clade (PP = 1.0, BS = 99).
Phylogenies based on Ca-ATPase (Dataset 4, Supporting Information 3: Figures SI4 and SI10) are less resolved. Orthops basalis is monophyletic (PP = 0.98, BS = 85). O. kalmii is monophyletic only in the Bayesian analysis (PP = 0.99). Orthops campestris and O. scutellatus form a clade only in the Bayesian analysis results (PP = 0.95). The relationships between O. basalis, O. kalmii, and O. campestris + O. scutellatus clade are unresolved. Orthops mutans is absent in this dataset.
In the phylogeny based on COI (Datasets 1, Figures 2, and Supporting Information 3: Figure SI11), more Mirinae genera were included. The subgenus Orthops, O. mutans, Pachylygus nigrescens, Peltidolygus scutellatus, and L. annexa form a clade (PP = 1.0, BS = 65). Specimens of Orthops palus from Indian Ocean islands do not form a clade with other Orthops species, but it is a sister group to Lygidolon hecate.
3.6. Species Delimitation
The species delimitation analyses bGMYC, PTP (based on RaxML), and bPTP (based on Bayesian inference) were conducted on the separate markers, COI + 16S dataset, and a full dataset. The ABGD analysis was performed for the separate markers only, and we applied BPP for the COI + 12S rRNA and full datasets.
COI dataset (Figure 2). Orthops basalis has been delimited as a single species in all analyses. O. kalmii was delimited as a single species by ABGD and bGMYC, but PTP and bPTP assigned specimen from Crimea (O49) to a separate species. Only bGMYC delimited O. campestris as a single species, and all other analyses delimited two species within this group, treating either specimen from Murmansk province (O71) or specimen from Karachay-Cherkessia (O52) as a separate species. O. scutellatus from Palearctic was delimited as a separate single species by all the analyses. Only ABGD analysis assigned O. scutellatus from North America to a single species; other analyses found two or three species within this group.
12S rRNA dataset (Supporting Information 3: Figure SI3). In this case, ABGD method failed to delimit Orthops species. The PTP and bGMYK analyses resulted in three groups: (1) O. basalis + O. kalmii, (2) O. campestris + Palearctic O. scutellatus, and (3) Nearctic O. scutellatus. The bPTP method resulted in the same groups as PTP but also separated O. basalis and O. kalmii from each other.
ITS1 dataset (Supporting Information 3: Figure SI5). All delimitation analyses conducted on the ITS1 dataset have resulted in three groups: (1) O. campestris and O. scutellatus, (2) O. basalis, and (3) O. kalmii.
Ca-ATPase dataset (Supporting Information 3: Figure SI4). For the Ca-ATPase dataset, all four methods fail to delimit species within the subgenus Orthops.
12S rRNA + COI dataset (Supporting Information 3: Figure SI6). All analyses delimited five groups: (1) O. basalis, (2) O. kalmii, (3) O. campestris, (4) Palearctic O. scutellatus, and (5) Nearctic O. scutellatus.
12 Sr RNA + COI + Ca-ATPase + ITS1 dataset (Figure 13). Most specimens were assigned to the separate species (41 out of 45) with bPTP. Those results are apparently wrong; therefore, we do not discuss them further. The analyses bGMYK and PTP delimited five groups: (1) O. basalis, (2) O. kalmii, (3) O. campestris, (4) Palearctic O. scutellatus, and (5) Nearctic O. scutellatus. In addition to the mentioned five groups, the BPP analysis assigned O. kalmii from Crimea (O49) to a separate species.
4. Discussion
4.1. Delimitation and Identification of the Subgenus Orthops Species
Kerzhner and Josifov [3] placed seven species in the subgenus Orthops: O. basalis, O. campestris, O. frenatus, O. ferrugineus, O. kalmii, O. udonis, and O. vitticeps. Asian O. udonis was synonymized with North American O. scutellatus [8].
Morphological characters in the color and paramere structures were used to separate the species (see Results). To date, there have been no studies on the species delimitation within the subgenus Orthops. In the present work, we have applied an integrative approach for the species delimitation and identification within this group, combining analysis of mitochondrial and nuclear markers and detailed study of the morphological characters, including genital structures.
We found that all species of the subgenus Orthops have unique paramere shape, and it is the most reliable identification character complex. The structure of female genitalia also bears species-specific characters, except for Orthops basalis and O. kalmii. Aedeagus structure is less variable; however, it can be used for the species identification in some cases. The color pattern, which is most often used in the various keys to species, is not a reliable diagnostic character (see Results section). The markings on the frons usually correspond to the genitalia structures of O. basalis and O. kalmii, but sometimes, they can be misleading. The present analysis shows that the sequences of the different genetic markers (COI, 12S rRNA, ITS1, and Ca-ATPase) mostly correspond to the morphological characters for O. basalis, O. campestris, O. kalmii, and O. scutellatus. The markers COI and ITS1 can be used to separate all those species, and they have considerable interspecific variation in comparison to the intraspecific variation. The COI is most variable with interspecific variation ranging from 4% to 9% (Supporting Information 5: Table SI4). The sequences of ITS1 are less variable with interspecific difference ranging from 1% to 6%. The 12S rRNA and Ca-ATPase are the least variable and do not show stable differences between O. campestris and O. scutellatus.
We applied different species delimitation analyses for six datasets, and in each case, the different approaches showed similar results. Among all of them, ABGD shows less species than the others (Figures 2, 13, and Supporting Information 3: Figures SI3–SI6). The species delimitation analyses based on the COI, both mitochondrial markers, and the combined datasets assign O. basalis, O. campestris, O. kalmii, and O. scutellatus to different species, although most of the trees show more than one species within all species except for O. basalis. Most or all analyses based on 12S rRNA and ITS1 failed to delimit some of those species, and the species delimitation analyses did not delimit any species when only Ca-ATPase was used. Our study suggests that 12S rRNA, ITS1, and Ca-ATPase should not be used alone in the species delimitation analysis of Orthops species.
The phylogenetic analysis shows that O. scutellatus is subdivided into Palearctic and Nearctic clades, which is supported by the species delimitation analyses. We also found that those two groups slightly differ in the structure of parameres. The structure of the parameres of the Nearctic O. scutellatus depicted in this work (Figures 6K and 7L) is very similar to that published in Kelton [42]. However, more material has to be examined to test whether those differences are stable within the clades. Initially, O. scutellatus was described from the Nearctic [66], and O. udonis was described from Japan [69]. Subsequently, O. udonis was synonymized with O. scutellatus [8]. The results of our work suggest that O. udonis can be a separate species. However, to test for this, the mitochondrial and nuclear sequences of more specimens from Nearctic should be compared with those from Palearctic.
The sequences of O. ferrugineus, O. frenatus, and O. vitticeps were not obtained for this work. Those three species have significant differences in the genitalia structures, and it is very likely that they are distinct species.
4.2. Phylogeny of the Orthops Species
We included four species of the subgenus Orthops into the dataset, as well as O. mutans and O. palus. We have downloaded COI sequences for Orthops palus from GenBank. The phylogenetic analysis shows that this species is not close to other Orthops representatives. Externally, O. palus is more similar to Taylorilygus Leston, 1952 (see habitus figures in [70]), where it was placed by Taylor [21]. Linnavuori [71] placed this genus to Orthops. However, our phylogenetic tree suggests that generic assignment of this species should be reconsidered in the future.
The results show that the monophyly of the subgenus Orthops is questionable. This groups is monophyletic with high supports in the phylogenies obtained using 12S rRNA and in the Bayesian analyses based on COI + 12S rRNA and all markers datasets forming sister group to O. mutans. The subgenus Orthops is not monophyletic, and Orthops mutans forms a sister group relationship with the O. basalis + O. kalmii clade with high supports in the phylogenies based on ITS1 and COI RAxML phylogeny based on the dataset with Pachylygus nigrescens used as a root. Other analyses do not clarify the monophyly of the subgenus Orthops.
Orthops mutans does not fit our diagnosis of the subgenus Orthops. Based on the previously diagnoses and keys [16, 72] and our observations, it has salient features similar to Montanorthops, that is, deep punctation on the dorsum, pale brown to yellow spines on the tibia, and the collar with the setae shorter than the collar length. We also examined male genitalia of this species, and they distinctly differ from those of the subgenus Orthops representatives (cf., Figures 8 and 11 and [72]). Therefore, it is unlikely that O. mutans is within this group, and the hypothesis that it should be related to the members of Montanorthops is more plausible. The clades Orthops campestris + scutellatus and O. basalis + O. kalmii appear in most phylogenies, although sometimes with low supports. The trees based on Ca-ATPase and COI + 12S rRNA (RAxML) show unresolved relationships for those species pairs.
There are few explanations of the discrepancies between the different markers, and they are not mutually exclusive. First, unresolved position of O. mutans can be due to the bias toward the subgenus Orthops representatives, and adding more species from Montanorthops can clarify the position of Orthops mutans. Second, missing data can affect the results based on the COI + 12S rRNA and all markers datasets [73, 74]. Third, the differences in the markers variability also can affect the topology. In particular, COI is more variable than 12S rRNA in the subgenus Orthops. Fourth, several microevolutionary processes can lead to the discrepancies between different markers [1, 75, 76]. As soon as all species are well delimited and the markers used for this study mutate with relatively high rate, the incomplete lineage sorting is unlikely. Hybridization and the genetic hitchhiking due to the presence of Wolbachia can be at least partly responsible for those inconsistences; however, those processes would lead to the discrepancies between nuclear and mitochondrial markers, and in this case, the differences between two mitochondrial markers are also present. The differences between the mitochondrial markers can be explained by the pseudogenes (NUMTs). Within the protein coding genes, NUMTs usually can be recognized with the presence of stop codons and gaps, and they are absent in COI in our dataset. However, the NUMTs still can be present for 12S rRNA. Therefore, other mitochondrial and nuclear markers should be analyzed to test the topology.
4.3. Other Species of the Genus Orthops
According to the previously published molecular-based phylogenies [5, 28], Orthops is more closely related to Agnocoris Reuter, 1875; Cyphodemidea Reuter, 1903; Eolygus Poppius, 1915; Henrylygus Schwartz and Foottit; Knightomiris Kelton, 1973; Nonlygus Schwartz and Foottit; Liocoris Linnaeus, 1858; Lygidea Reuter, 1875; Lygus Hahn, 1833; Proba Distant, 1884; and Tolongia Poppius, 1915. However, among Orthops species, only representatives of the subgenus Orthops were included in those analyses.
The subgenus Orthops differ from other species of this genus in the characters provided in the diagnosis. All species within this group have similar parameres and vesica, and interspecific differences are small. However, the female genitalia are more diverse; for example, the structures of the dorsal labiate plate and posterior wall of bursa copulatrix in O. ferrugineus are very different from other species.
Our study also shows that the genus Orthops is not monophyletic, because O. palus forms a clade with Lygidolon hecate. It also has very different parameres [71]. According to the drawings of those structures, provided by the previous authors, the systematic position of some African Orthops species also should be reconsidered. For example, the right paramere in O. nigriscutum (Poppius 1912) and O. sjostedti (Poppius 1910) is four times as long as wide with concave right margin and long hypophysis [71], and it is very different from that of the other representatives of the genus Orthops, including Montanorthops (e.g., [16, 72, 77, 78]).
4.4. Mitochondrial and Nuclear Markers for the Miridae Species Delimitation
There are only three studies on Miridae, where a combination of morphological and molecular data are used for the species delimitation. In the previous works, only mitochondrial markers were utilized for this purpose [1, 79, 80]. In the study on Dicyphus Fieber 1858 based on three mitochondrial markers (COI, 12S rRNA, and 16S rRNA), new synonyms were proposed, and more robust diagnoses were provided. The combined dataset was congruent with the morphological data; however, the authors did not provide the inter- and intraspecific distances and phylogenies for the separate markers. In the study of Stenodema Laporte, 1832, and Lygus species [1, 80], the 16S rRNA was less variable than COI but showed better congruence with the morphological data at least for the Lygus species identifications. The analyses of Lygus also showed that the molecular and morphological data do not always correspond to each other, which might be connected with the recent speciation, genetic sweep, or introgression.
The mitochondrial markers are often used in the species delimitation studies, and barcoding region of COI is widely used for the species identification. However, those regions do not always fit for such studies [81–83]. In this work, we also used two nuclear markers, ITS1 and Ca-ATPase, for the species delimitation for the subgenus Orthops. In the previous studies on different insects, including Hemiptera, it was shown that those markers are highly variable and can be used for the species level studies [27, 83–85]. Those sequences are harder to obtain in comparison to the mitochondrial markers. However, the study shows that ITS1 is even more variable than 12S rRNA and provides the results which mostly correspond to the morphological data. This marker can be used in the further study of Orthops and other Miridae genera on the species level at least in combination with the mitochondrial markers. The sequences of Ca-ATPase are more conservative, and less fit for the species-level studies. The phylogeny based on ITS1 also shows incongruence with those based on the mitochondrial DNA sequences regarding species interrelationships. The causes of those results should be investigated in the future.
5. Conclusion
Subgenus Orthops is widely distributed in the Palearctic and includes seven species. Among them, Orthops basalis, O. campestris, O. frenatus, and O. kalmii are known from Europe and Asia. O. scutellatus is also widespread but inhabits Siberia and East Asia. Previously, those species were mostly separated using the color pattern and small differences in parameres. Using an integrative approach, we tested whether the widely distributed Palearctic species represent monophyletic lineages and found relationships between them. The results showed that three widespread species, O. basalis, O. kalmii, and O. campestris, are monophyletic each, whereas O. scutellatus might represent a complex of two species: Nearctic and Palearctic lineages. Although the O. basalis + O. kalmii and O. campestris + O. scutellatus clades are present in most phylogenies, sometimes they are absent or have low supports. The results also showed that the subgenus Orthops can be nonmonophyletic. The discrepancies between the mitochondrial and nuclear markers affect the phylogenies, and this can be a result of the different processes connected with the speciation or presence of Wolbachia, which should be studied in the future. This work demonstrates the importance of the integrative species delimitation studies even for the taxa which can be separated morphologically. Our results reveal the underlying problems possibly connected with microevolution, which is important for answering the fundamental questions on speciation and biodiversity assessment.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (grant 075-15-2021-1069).
Acknowledgments
The molecular and SEM works were performed at the Core Facility Centre “Taxon” at ZIN. We thank our colleagues who helped us with obtaining the specimens; they are Alexandra Tokareva (Museum and Institute of Zoology, Polish Academy of Sciences, Poland), Alexey Neelov (ZIN), Armand Matocq (Muséum National d’Histoire Naturelle, France), Kirill Fadeev (ZIN), Larisa Sundukova (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch of the Russian Academy of Science, Russia), Michael Schwartz (Agriculture and Agri-Food Canada, Canadian National Collection of Insects), Nikolai Vinokurov (Institute for Biological Problems of Cryolithozone, Siberian Branch Russian Academy of Sciences, Russia), Sergey Belokobylskij (ZIN), Shamil Davletshin (Saint Petersburg State University, Russia), Veronica Tyts (Saint Petersburg State University, Russia), Viktor Golub (Voronezh State University, Russia), and Yurii Sundukov (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch of the Russian Academy of Sciences, Russia). Some specimens for our research were collected in the Belogorye Nature Reserve (Belgorod Province, Russia), Kandalakshskiy State Nature Reserve (Murmansk Province, Russia), Lazovskiy Nature Reserve (Primorskiy Territory, Russia), and Sebezhskiy National Park (Pskov Province, Russia). We also thank Verionica Tyts for the help with adapting scripts for R and Python for our study. This study was supported by the Ministry of Science and Higher Education of the Russian Federation (grant 075-15-2021-1069).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The datasets generated during and analyzed during the current study are available in the GenBank NCBI (https://www.ncbi.nlm.nih.gov/genbank/). All specimens are preserved at the Zoological Institute of the Russian Academy of Sciences, St Petersburg, Russia (ZISP). A label with the unique specimen identifier (USI) was attached to each examined specimen. The data on the specimens, that is, i.e. collecting event and host plant records, were entered in the Arthropod Easy Capture Locality Database (https://research.amnh.org/pbi/locality/). All images taken are stored in Zoological Institute, Russian Academy of Sciences. These images are available from the authors upon reasonable request.




