Taxonomic Studies of the Ground Beetle Subgenus Rhagadus Motschulsky (Coleoptera: Carabidae: Pterostichus): Unraveling the “Female Holotype Problem” Using External Geometric Morphometrics and Novel Male Genital Morphology
Abstract
Clarifying the identity of type specimens without information and developing novel procedures for this task are important issues in taxonomy. Because female insects frequently lack taxonomically useful morphological characters, their taxonomic assignment is challenging without sufficient locality information. Using the cryptic species complex previously treated as “Pterostichus (Rhagadus) polygenus Bates” as a model, this study presents a novel procedure based solely on morphological data for the taxonomic assignment of female type specimens that lack sufficient locality information. Without a priori species hypotheses, males were divided into morphotypes based on the morphology of the endophallus, a genital structure that has recently been found to be useful in the taxonomy of insects, particularly Coleoptera. Then, geometric morphometrics of the pronotum followed by discriminant analysis were conducted using females obtained from the same localities as the males (not including female type specimens). The obtained discriminant function unambiguously assigned all female type specimens to the morphotypes, including those without sufficient locality information, resulting in the following Japanese Rhagadus Motschulsky species: Pterostichus microcephalus (Motschulsky) ( = Pterostichus kimurai Morita syn. nov.), Pterostichus negylopus sp. nov. (type locality: Tokyo, Hachiôji-shi, Todorimachi), Pterostichus nimbatidius (Chaudoir), Pterostichus polygenus ( = Pterostichus brittoni Habu, Pterostichus harponifer Tanaka, both syn. nov.), Pterostichus takaosanus Habu ( = Pterostichus freyellus Jedlička, Pterostichus komiyai Morita, both syn. nov.), Pterostichus thorectes Bates ( = Pterostichus latemarginatus (Straneo), Pterostichus mundatus Jedlička, Pterostichus straneoi Habu, Pterostichus satsumanus Habu, all syn. nov.), and Pterostichus thorectoides Jedlička ( = Pterostichus ishiii Morita, Kurosa, and Mori syn. nov.). A morphological phylogeny of Japanese and Korean Rhagadus species was constructed, and their differentiation process is discussed. In addition, based on the observed genital morphology, the possible functions of male and female genitalia are discussed in terms of sexual conflict. Pterostichus glabripennis Jedlička from China was removed from Rhagadus based on type examinations.
1. Introduction
The Linnaean taxonomy is based on a nomenclatural system in which the name of a species is assigned based on a specific type specimen. This type-based nomenclatural system guarantees the stability and objectivity of species classification and prevents confusion about species identity, which is a prerequisite for scientific studies using organisms. However, in groups composed of many closely related species that are difficult to distinguish by superficial morphology, also referred to as cryptic species complexes, the type-based nomenclatural system can hamper subsequent research. The most conspicuous case is the loss of type specimens of uncertain identity. In such cases, relationships to the unidentified specimens are unresolved, the names of other members of the complex are forced to remain tentative or undetermined, and descriptions of new species within the complex are often withheld (e.g., [1, 2]). This problem can be resolved by designating a neotype under the nomenclatural code, although various requirements must be met for the designation [3]. Surprisingly, the presence of type specimens can hinder taxonomic studies when there is insufficient information to determine the species. For example, when type specimens lack important taxonomic characters or sufficient collection data [4–6]. Such type specimens can render cryptic species complexes taxonomically unstable, impeding subsequent taxonomic studies. Given the lack of fundamental solutions, such as neotype designation, problems caused by type specimens lacking sufficient information may be more serious than those caused by loss of type specimens. To understand biodiversity correctly, methodological studies addressing these issues are necessary.
Among insects, type specimens lacking sufficient information can generally be divided into two categories, both of which cause taxonomic problems in cryptic species complexes. The first category comprises specimens without sufficient information on the collection locality; such specimens may have been collected historically, when detailed geographic information was difficult to obtain. For example, marked regional differentiation within the Japanese Archipelago is often observed for taxa with low dispersal ability [7]. Some of these species, described by researchers outside Japan between 1800 and the early 1900s, were described with “Japan” as their type locality (e.g., [8–10]). Such insufficient locality information can make species identification difficult among taxa for which species identification relies on detailed information about the collection site. Similar cases have been reported for various insects worldwide (e.g., [6, 11, 12]). The second category comprises female type specimens. In insect taxonomy, the male genitalia are usually examined and used to define species, due to their marked morphological diversification, which provides useful diagnostic characters. Consequently, female type specimens may pose a problem in terms of sufficient morphological discrimination among related species (e.g., [4, 5, 13]).
When only one of these two problems occurs, that is, insufficient locality data or female holotype, it may be relatively easily addressed. For example, when the holotype is a male without sufficient locality information, the solution is to identify and describe characters that are useful in distinguishing the species from related species (e.g., [14, 15], this study); in this case, accumulation of information may lead to the discovery of candidate type localities. When the holotype is a female with sufficient locality information, the solution is to examine conspecific males obtained from the type locality and redefine the species (e.g., [4, 13, 16]). Thus, a small number of additional analyses or the analysis of a small number of additional specimens can resolve either one of these taxonomic problems. However, when the two problems occur simultaneously (i.e., female type specimens with insufficient locality information), many additional specimens and additional analyses are required to find a solution.
This problem of determining the identity of type specimens lacking sufficient morphological and locality information can be solved logically, albeit with much effort, through a three-step procedure. First, without a priori species hypotheses, specimens from the entire distribution range of all candidate species for the target type specimen are divided into groups based on reliable data and methods. Second, the taxonomic group to which the type specimen belongs is determined based on reliable data and methods. Third, the name of the taxonomic group is assigned on the basis of nomenclatural priority. For cryptic species complexes, molecular data and techniques are predominantly used in the first and second steps of this procedure (e.g., [17–19]). However, in rare or historical specimens, which include a substantial proportion of type specimens, molecular data are not always available for various reasons [6]. Furthermore, groupings based on molecular data are occasionally incongruent with actual species groupings due to introgressive hybridization among species or random sorting of ancestral polymorphisms, among other reasons (e.g., [20]). To address these problems, it is necessary to establish a procedure based on information other than molecular data and to evaluate the utility of that procedure.
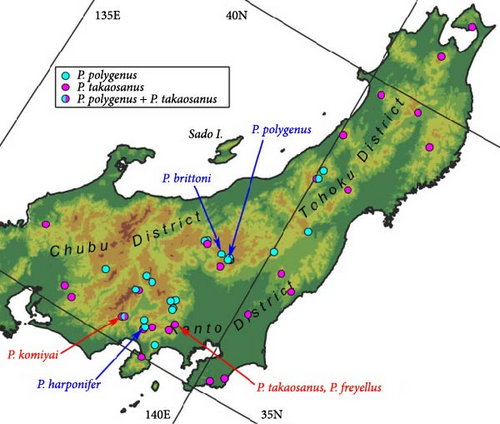
A simple solution would be to perform the procedure using only morphological data. For this purpose, this study focused on the flightless carabid beetle subgenus Rhagadus Motschulsky (genus Pterostichus Bonelli), given its unresolved taxonomic issues, the status of its type specimens, and available methods for its morphological analysis. Rhagadus is distributed in East Asia, and 19 species are recognized in the current taxonomic system: 12 endemic to Japan, 2 distributed in Japan and the Asian continent, and 5 found only on the Asian continent [21]. These species are very similar in external appearance and are mainly distinguished only by partial structures of the male genitalia [22, 23]. Thus, most of the interspecies differences are ambiguous, and validation of their species status is required. Preliminary research for this study demonstrated that a species of this subgenus, treated to date as “Pterostichus polygenus Bates,” may be an undescribed species, because two names thought to refer to this species (P. polygenus and Pterostichus freyellus Jedlička) were found to refer to two other species (Pterostichus brittoni Habu and Pterostichus takaosanus Habu; Figure 1A,B,D–G,J–L). This unexpected finding necessitates a taxonomic revision of the species previously treated as P. polygenus, as well as these related species. Moreover, among the seven names that may refer to the focal species, four were found to have been based on type specimens that lack sufficient information. Specifically, the oldest name (Pterostichus thorectes Bates) was based on a male type specimen from “Japan” (Figure 1C,H,I,M,N); the second oldest name [Pterostichus latemarginatus (Straneo)] was based on a female type specimen, also from “Japan” (Figure 2A,D); and the remaining two names (Pterostichus mundatus Jedlička and Pterostichus thorectoides Jedlička) (Figure 2B,C,E,F) were based on female type specimens from a locality that may be inhabited by multiple species. For morphological analysis, two relatively new methods are available: comparative morphology of the male genital endophallus and geometric morphometrics of external morphology. In the former method, the endophallus, a membranous inner sac within the aedeagus of the male genitalia, is everted and its structure is examined. This genital structure provides novel morphological data that are taxonomically useful, but cannot be obtained based on previous superficial examinations of genital morphology. The taxonomic utility of the endophallus structure has been demonstrated in various groups of the Coleoptera, including Carabidae (e.g., [24, 25]). The latter method (geometric morphometrics of external morphology) is based on landmark coordinates and can quantitatively evaluate subtle morphological differences that are difficult to detect via traditional morphometrics based on linear measurements [26]. This technique has been applied in studies on various insects. Subtle morphological differences between females of related species within the Carabidae have been reported to be detectable using this method of analysis [27, 28]. These two morphology-based methods provide classification and identification accuracy comparable to those of molecular-based methods (e.g., [19, 25, 29]). In summary, its taxonomic issues, type specimens, and available morphological methods all indicate that Rhagadus is a suitable model for performing the focal procedure based only on morphological data. Therefore, this study attempted to solve taxonomic problems in this subgenus, most of which are attributed to type specimens lacking sufficient morphological and/or locality information, by combining comparative male genital morphological and external geometric morphometric analyses. Additionally, a morphological phylogenetic tree of members of Rhagadus was constructed, including species redefined in this study, and the process of subgenus differentiation is discussed.


2. Materials and Methods
The following seven species are potentially identical to or related to the species treated as “Pterostichus polygenus”: P. thorectes; P. latemarginatus; P. mundatus; P. thorectoides Jedlička; Pterostichus straneoi Habu; Pterostichus satsumanus Habu; and Pterostichus ishiii Morita, Kurosa & Mori. Among these, type localities were clearly mentioned for the three species most recently described by Japanese researchers (P. straneoi, P. satsumanus, and P. ishiii), and the taxonomically important morphological characters (including genitalia) were described. Therefore, the key to solving taxonomic problems related to the species previously treated as “P. polygenus” is to clarify the identity of the following four type specimens: the P. thorectes lectotype male from “Japan,” the P. latemarginatus holotype female from “Japan,” the P. mundatus holotype female from “Osaka,” and the P. thorectoides holotype female from “Kobe.” Three of these four type specimens are females, which lack distinct diagnostic characters. Among their type localities, “Japan” offers virtually no information, and “Osaka” and “Kobe” are similarly insufficient because they are proximate to the distribution boundary between species; as discovered in this study, two different species were found in “Osaka.” To identify these type specimens, this study (i) classified male specimens potentially identical or related to “P. polygenus” into morphotypes based on the endophallus morphology, (ii) generated a discriminant function of the morphotypes based on external morphometric values of female specimens from the same localities as males examined in (i), (iii) assigned the three female holotypes to morphotypes based on the discriminant function obtained in (ii) and determined their resulting species-level taxonomy, and (iv) performed morphological phylogenetic analysis for all available members of Rhagadus to infer the differentiation process of this subgenus.
2.1. Specimens Examined
For the taxonomic study of “P. polygenus,” about 360 specimens from species that were potentially of—or related to—this species were examined. In each collection, these were treated as P. polygenus, P. thorectes, P. latemarginatus, P. mundatus, P. thorectoides, or P. ishiii. The specimens directly examined included type specimens of four species, P. thorectes, P. latemarginatus, P. mundatus, and P. thorectoides, and topotype males of the remaining three species (P. straneoi, P. satsumanus, and P. ishiii), covering sufficient specimens to discuss the nomenclatural priorities for the seven names concerned. For morphological phylogenetic analysis, several ingroup species, including species redefined here, and several outgroup candidates were investigated, as explained in detail in the morphological phylogenetic analysis section and Table S1.
Specimens examined are housed in the following collections: the private collection of Toshiaki Dejima, Takamatsu, Kagawa, Japan (CTD); Ehime University Museum, Ehime, Japan (EUM); Hokkaido University Museum, Hokkaido, Japan (HUM); Kashihara City Museum of Insects, Nara, Japan (KCMI); Kitakyushu Museum of Natural History & Human History, Kitakyushu, Fukuoka, Japan (KMNH); K. Sasakawa collection deposited in the Laboratory of Zoology, Department of Science Education, Faculty of Education, Chiba University, Chiba, Japan (KS); Minoh Park Insect Museum, Osaka, Japan (MPIM); Natural History Museum, London, U.K. (NHMUK); Museum of Nature and Human Activities, Hyogo, Japan (MNHAH); National Agriculture and Food Research Organization, Tsukuba-shi, Ibaraki, Japan (NARO); Natural History Museum Basel, Basel, Switzerland (NMB); National Museum, Prague, Czech Republic (NMP). For label data, a backslash (\) was used to separate lines on the same label, a double backslash (\\) to separate different labels, and an asterisk indicates an unreadable character.
2.2. Comparative Morphology of Genitalia
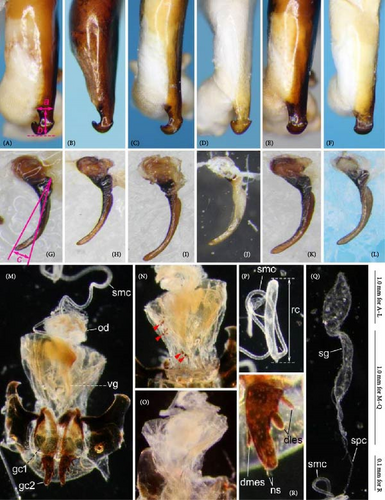
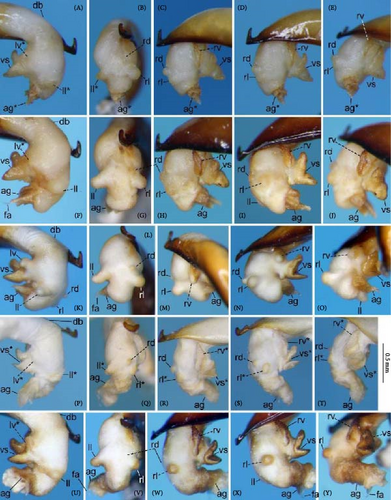
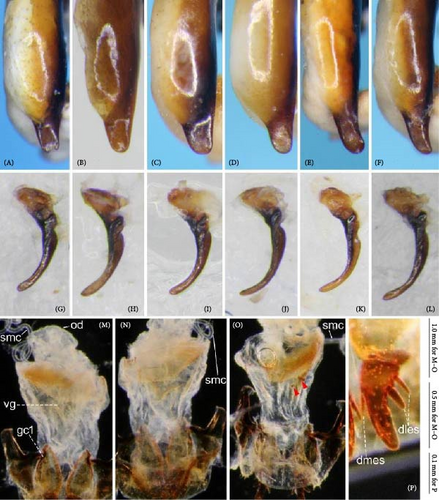
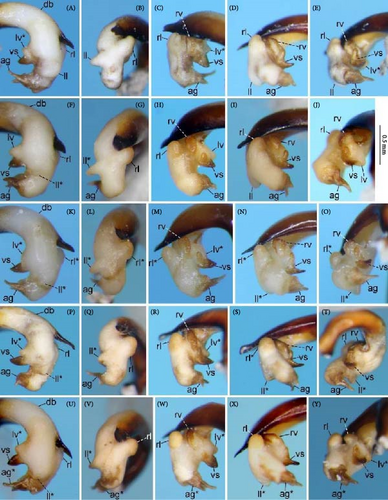
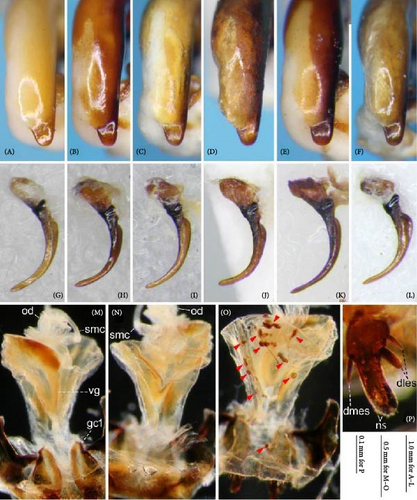
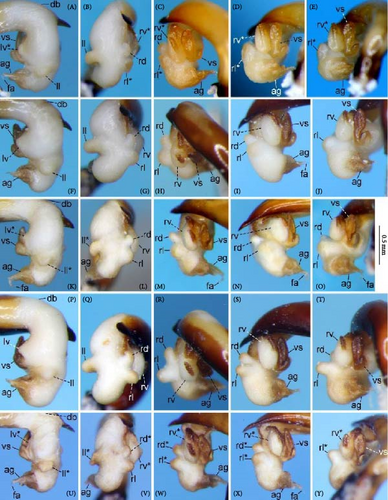
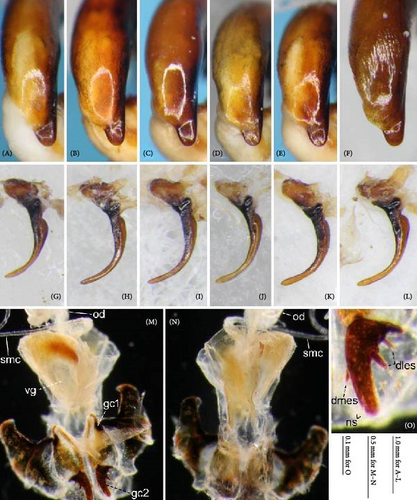
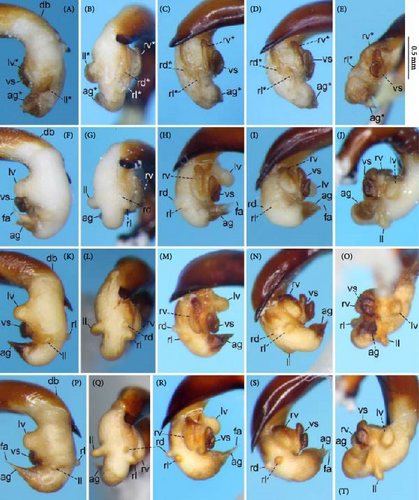
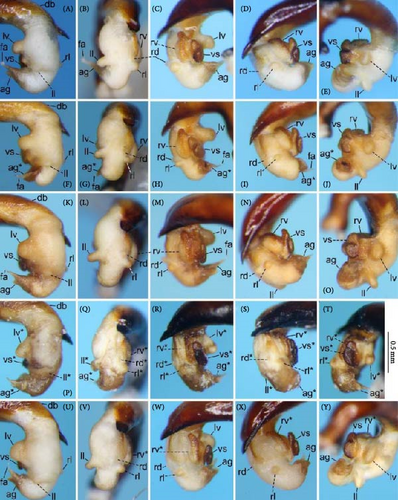
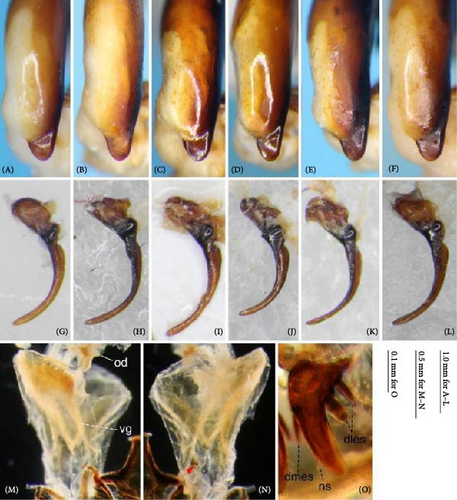
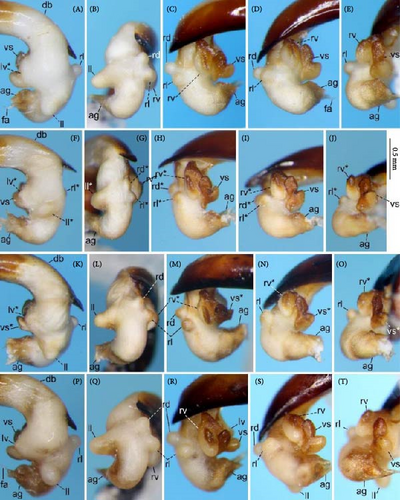
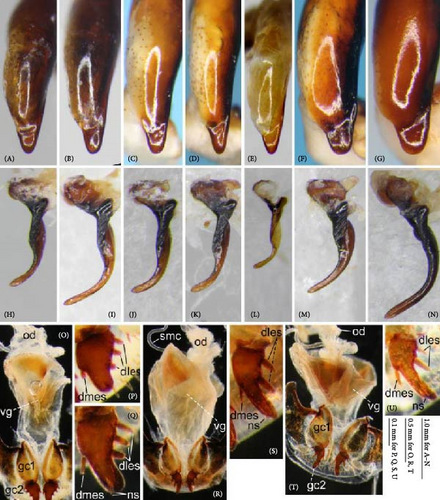
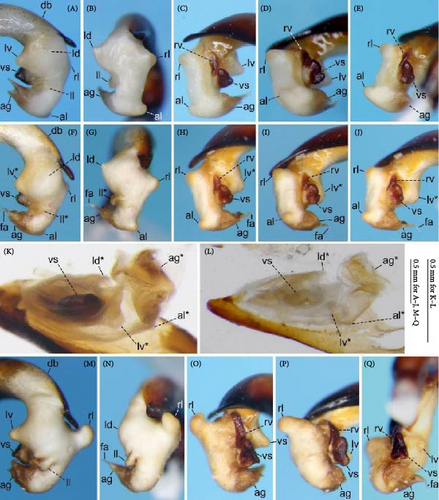
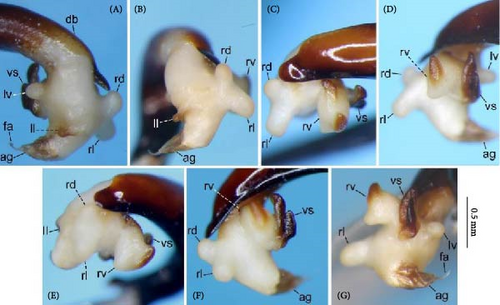
Because closely related species of Rhagadus are virtually indistinguishable based on superficial external morphology, examination of the genitalia is necessary for identification. In addition to the male genital aedeagus and right paramere, which have been examined in previous studies, the male genital endophallus and female genital membranous parts were newly examined. The endophallus was observed after eversion and full inflation by injecting toothpaste from the base of the aedeagus. The figures show visible interspecies differences viewed from parallel directions; unless otherwise stated, each set of photographs is taken from the following five angles, from left to right: left lateral view, dorsal view, right ventrolateral view (to show the upper surface of the right ventral lobe), right lateral view (to show the right lateral view of the ventral sclerite), and ventral view (to show the ventral view of the ventral sclerite). For the female genitalia, muscles around the genitalia were dissolved using 5% potassium hydroxide, and the organs were cleaned and observed in pure water.
Abbreviations of genital components used in figures are as follows: ag, aggonoporius; al, apical lobe; do, dorsal surface near ostium; dles, dorsolateral ensiform setae of gonocoxite 2; dmes, dorsomedial ensiform seta of gonocoxite 2; fa, filamentous apex of aggonoporius; gc1, gonocoxite 1; gc2, gonocoxite 2; ld, left dorsolateral lobe; ll, left lateral lobe; lv, left ventral lobe; ns, nematiform setae of gonocoxite 2; od, oviduct; rc, receptaculum; rd, right dorsolateral lobe; rl, right lateral lobe; rv, right ventral lobe; sg, spermathecal gland; smc, seminal canal; spc, spermathecal canal; vs, ventral sclerite; vg, vagina; α, sclerotized area on left ventrolateral surface; β, lobe on right anterolateral surface near gonopore; γ, bifid lobe; ε, plate-shaped sclerite.
2.3. Morphotype Recognition
An initial attempt to divide the specimens into morphotypes based on male and female genital morphology revealed that male nonendophallic and female genital membranous parts showed little variation and provided no features that could be unambiguously defined. Therefore, morphotypes were grouped based solely on the male endophallus. Thus, while the morphotypes of male specimens were determined based on direct evidence (i.e., endophallus structure), those of female specimens were indirectly determined based on the morphotypes of males from the same collection site. Consequently, although the morphotypes of the P. thorectes lectotype male and topotype males of P. straneoi, P. satsumanus, and P. ishiii could be determined, those of the three female holotypes lacking male specimens from the same collection site remained undetermined. The morphotypes of these female holotypes were determined by subsequent discriminant analysis.
2.4. Geometric Morphometric Analysis
A discriminant analysis based on morphometric values was conducted to determine female holotype morphotypes; therefore, geometric morphometrics was performed only on female specimens of determinate morphotype (i.e., morphotypes of males from the same collection site were determined by the endophallus) and on the three female holotypes. The dorsal view of the pronotum was used in this analysis (Figure 3). The tilt of the anteroposterior axis of the pronotum was adjusted by maintaining the line connecting the middle of the posterior margin (landmark 1) and the middle of the anterior margin (landmark 50) perpendicular to the camera angle, and scaled digital images were obtained using a digital camera attached to a microscope. All coordinates were plotted on the left side; when the left side was unavailable (e.g., damaged), the image was inverted laterally and the inverted right side was used.
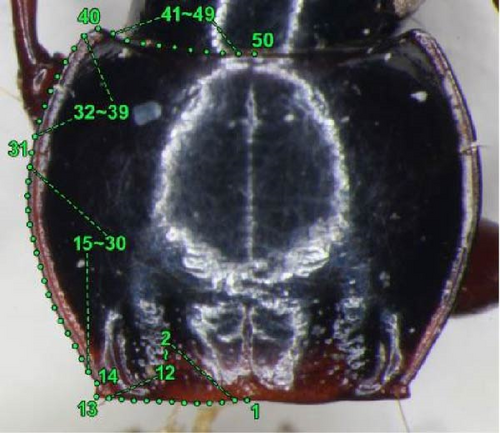
Using the software tpsDig version 2.31 [30], six landmarks and 44 semi-landmarks distributed along the contour at regular intervals between landmarks were identified (Figure 3). The landmarks on the contour are as follows: 1, middle of posterior margin; 13, apex of hind angle; 14, bending point anterior to the hind angle; 31, point at the level of anterior marginal seta; 40, apex of anterior angle; 50, middle of anterior margin. The semi-landmarks are as follows: 2–12, between landmarks 1 and 13; 15–30, between landmarks 14 and 31; 32–39, between landmarks 31 and 40; and 41–49, between landmarks 40 and 50. Using the software tpsRelw version 1.53 [30], the raw coordinates were converted into Procrustes coordinates, in which variations due to rotation, position, and size were removed with semi-landmarks being “slid” along the contours. Relative warp analysis and visualization of shape differences were also performed using this software. The obtained relative warp scores and centroid size were used for subsequent discriminant analysis. The raw data used in the analysis are provided in Tables S2 and S3.
2.5. Discriminant Analysis
Using the “lda” function of the MASS package in R (R Core Team, Vienna, Austria), linear discriminant analyses were performed with morphotype as the response variable and centroid size and all noncollinear relative warp scores as explanatory variables. First, classification accuracy was confirmed in a dataset that excluded female holotypes through cross-validation using the “lda” function. Then, a discriminant function was obtained for this dataset and used to determine the morphotypes of the three female holotypes. To capture the results visually, scatterplots based on the first two canonical variates were also created.
2.6. Morphological Phylogenetic Analysis
All Japanese species of Rhagadus, most of which were redefined here, were used in the analysis. Two continental consubgeners, Pterostichus solskyi (Chaudoir) and Pterostichus glabripennis Jedlicka, were also examined. The former is distributed in Korea and inland China and was examined as a candidate ingroup species; specimens from the Korean Peninsula and the adjacent Jejudo Island were examined. The latter (P. glabripennis) was examined as a candidate outgroup species for Japanese species + P. solskyi within Rhagadus, because its distribution is limited to Jiangxi, China, and it is assumed to be distantly related to Japanese and Korean species; the holotype male, which is the only known specimen, was examined. Based on previous studies, the following three species were examined as outgroup candidates: Pterostichus ventralis (Say), Pterostichus sayanus Csiki, and Pterostichus eximius rishiridakensis Sasakawa, Berlov and Okuzaki. The first two species are members of the North American endemic subgenus Gastrosticta Casey, which Bousquet [31] suggested might be related to Rhagadus. The third species occurs in Japan and is a member of subgenus Petrophilus Chaudoir (s. lat., i.e., Petrophilus sensu [32]), which was determined to be sister to Rhagadus in the molecular phylogenies, although statistical support was not sufficient [33, 34]. Judging from their genital structures, some of these ingroup and outgroup candidates were later found to be unrelated to Rhagadus and were excluded from the final phylogenetic analysis. Detailed information on specimens examined is provided in the later taxonomic section for five Japanese endemic species redefined here and in Table S1 for the other species.
Morphological phylogenetic trees were constructed through maximum parsimony analysis using TNT v1.6 [35]. Adult morphological characters that showed variation among ingroup species were evaluated and coded. Some of the characters of outgroup species for which the character state could not be determined were coded with “?.” The analysis was performed with the following parameter settings: Analyze > “Traditional search”; Starting trees > “Wagner trees” with “random seed” = 1 and 100 replicates; Swapping algorithm > “tree bisection reconnection (TBR)” with 10 trees saved per replication; default parameter settings for all other factors. All characters were, thus, treated as “non-additive” (unordered as per PAUP ∗) and given equal weight. Bootstrap values were calculated using ‘standard’ and ‘absolute frequencies’ options with 1000 replicates. Synapomorphies and autapomorphies were mapped on the strict consensus tree derived from the obtained trees, based on the list of these character state changes (found under Optimize > Synapomorphies > List common synapomorphies in the TNT output).
3. Results
3.1. Comparative Morphology of Genitalia and Morphotype Recognition
Male specimens were divided into three morphotypes, distinguished by endophallus structure (Figure 4A–R). Endophallus morphology was stable within each type, but showed distinct gaps between types, with no individuals having an intermediate character state. Variation in the contour of the aedeagal apex and right paramere, which have been treated as characters for species diagnosis in previous studies, was vague and continuous, showing no morphological gaps that might be used for morphotype grouping. Similarly, female genitalia did not show any morphological variation that could be used to classify morphotypes. Consequently, it was virtually impossible to divide specimens into groups based on the male aedeagal apex, right paramere, or female genital membranous parts. Therefore, the three types distinguished by the endophallus (morphotypes A, B, and C) were used in subsequent analyses. For female specimens, when males that were obtained from the same collection site were identified by the endophallus, the females from that collection site were assigned the same morphotype. When male specimens from the same collection site were unavailable, the female morphotype was determined by a combination of pronotum geometric morphometrics and geographical evidence (i.e., morphotypes of nearby populations).

Morphotype A is widely distributed in the eastern part of the distribution range of this group (Honshu, east of Nagano) (Figure 4S). Morphotype B is widely distributed west of the distribution range of morphotype A (Honshu, west of Aichi and Fukui Prefectures; Shikoku; and Kyushu) and includes the P. thorectes lectotype male and the topotype males of P. straneoi and P. satsumanus. The type localities of P. mundatus, P. thorectoides, P. straneoi, P. satsumanus, and P. ishiii were located within the distribution range of morphotype B. The distribution range of morphotype C is relatively narrow, generally coinciding with the northeastern part of the distribution range of morphotype B, but sympatric occurrence of the two morphotypes has not been confirmed. The topotype males of P. ishiii were included in morphotype C. The type localities of P. mundatus, P. thorectoides, and P. ishiii were located within the distribution range of morphotype C.
3.2. Geometric Morphometric Analysis
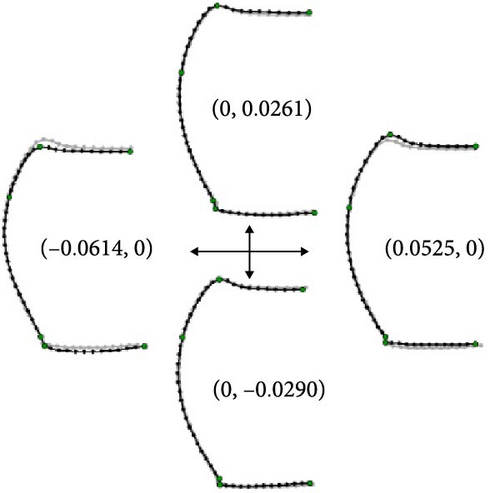
This analysis was based on 63 females from 12 localities of morphotype A, 57 females from 19 localities of morphotype B, 8 females from 3 localities of morphotype C, and the three female holotypes. The first relative warp (RW1) scores accounted for 50.5% of the total variance and were mainly associated with protrusions of the anterior and posterior angles and the relative length of the posterior half of the pronotum; positive values indicated strongly protruding anterior and posterior angles and a shorter posterior half of the pronotum, whereas negative values indicated less protrusion of the anterior and posterior angles and a longer posterior half of the pronotum (Figure 5B). The second relative warp (RW2) scores accounted for 15.5% of the total variance and were mainly associated with pronotum length–width ratio; positive and negative values indicated a longer and transverse pronotum, respectively (Figure 5B). The third relative warp (RW3) scores accounted for 12.5% of the total variance and were mainly associated with protrusions of anterior and posterior angles and width of pronotum; positive values indicated a weakly protruding anterior angle, a strongly protruding posterior angle, and a narrower pronotum, whereas negative values indicated a strongly protruding anterior angle, a weakly protruding posterior angle, and a broader pronotum (Figure 6B). The fourth relative warp (RW4) scores accounted for 8.7% of the total variance and were mainly associated with the curvature of the contour of the pronotum lateral margins; positive values indicated a less cordate pronotum with the lateral margins uniformly arched, whereas negative values indicated a more cordate pronotum with the anterior half of the lateral margins more strongly arched than the posterior half (Figure 6B).
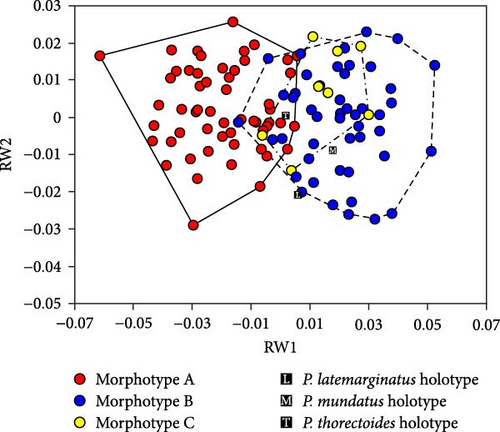
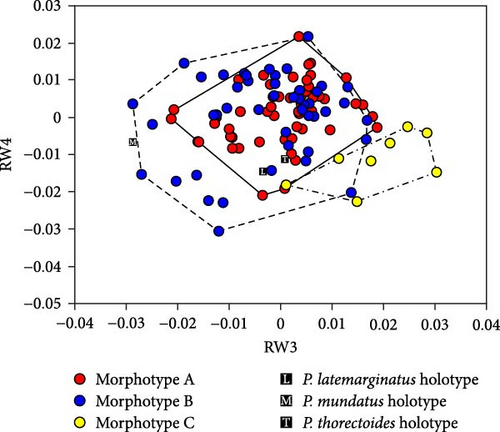
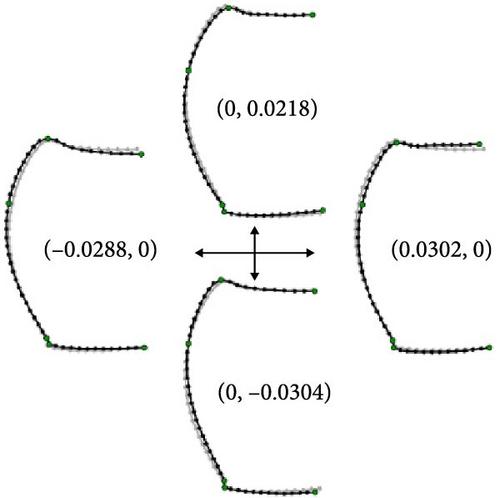
Scatterplots of RW1 and RW2 scores (Figure 5A) showed that the three morphotypes were continuous and did not form separate areas. However, morphotype A had smaller RW1 scores and overlapped only partially with morphotypes B and C. This pattern indicates that morphotype A is generally distinguished from the other two morphotypes based on the traits represented by RW1 scores. The P. latemarginatus holotype was circumscribed to the areas of morphotype B. The P. mundatus holotype was located within the area of morphotype B but very close to that of morphotype C. The P. thorectoides holotype was located within the overlapping area of the three morphotypes. Scatterplots of RW3 and RW4 scores (Figure 6A) showed that the three morphotypes did not form separate areas. However, morphotype C had larger RW3 scores and overlapped only partially with morphotypes A and B. This pattern indicates that morphotype C is generally distinguished from the other two morphotypes based on the traits represented by RW3 scores. The P. latemarginatus holotype was circumscribed to the area of morphotype B. The P. mundatus and P. thorectoides holotypes were located within the overlapping area of morphotype A and morphotype B.
3.3. Discriminant Analysis
Cross-validation of a dataset of 128 non-type specimens using 1–51 relative warp scores (all non-collinear values) and centroid size showed correct classification rates of 96.8% (61/63) for morphotype A, 96.5% (52/54) for morphotype B, and 87.5% (7/8) for morphotype C, justifying the use of the obtained function for the classification of female holotypes. Analysis with this discriminant function classified the P. latemarginatus and P. mundatus holotypes to morphotype B and the P. thorectoides holotype to morphotype C. This result is consistent with the scatterplot of the first and second canonical variates (Figure 7). A scatterplot showed that the three morphotypes were clearly segregated and did not overlap. Although none of the three female holotypes was within the morphotype areas, the P. latemarginatus and P. mundatus holotypes were located very close to the area of morphotype B, and the P. thorectoides holotype was located very close to that of morphotype C.
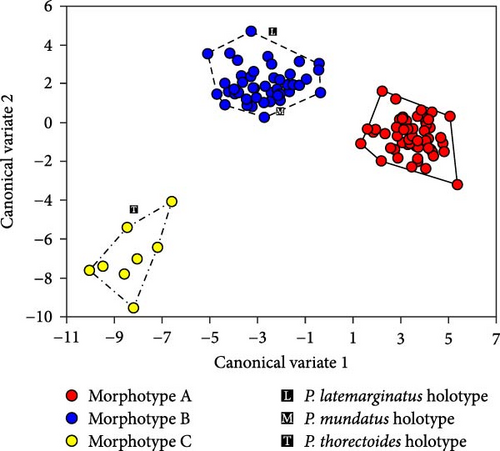
3.4. Taxonomy
Based on the comparative morphology of the male genital endophallus and geometric morphometric analysis of the female pronotum followed by discriminant analysis, the type specimens of P. thorectes, P. latemarginatus, and P. mundatus and the species P. straneoi and P. satsumanus were assigned to morphotype B, and the P. thorectoides type specimen and the species P. ishiii were assigned to morphotype C. Based on this result, those of previous studies, and geographical information about the type localities, the resulting taxonomic treatments of morphotypes A, B, and C are provided below, following the morphological redefinition of subgenus Rhagadus and taxonomic treatments of both P. polygenus and P. freyellus. The latter two names have been used incorrectly for some individuals among the morphotypes treated in this study (particularly morphotype A). The Japanese names of Japanese species of Rhagadus are provided in Table S4. Body length was measured from the mandible apices to the elytral end.
3.5. Subgenus Rhagadus Motschulsky, 1866


-
Rhagadus Motschulsky 1866 [36]: 261 (original description; type species Argutor microcephalus Motschulsky, 1860, by monotypy); Habu 1958 [22]: 1 (description); Jedlička 1962 [37]: 204 (synonym of Badistrinus Motschulsky 1865); Tanaka 1985 [38]: 113 (description); Bousquet 2003 [39]: 513 (catalogue); Bousquet 2017 [21]: 744 (catalogue).
Notes: Two genus-group taxa, Pseudadelosia Tschitschérine 1889 [type species: Pterostichus punctatipennis (Tschitschérine, 1889) = P. solskyi (Chaudoir 1878)] and Rhagadulus Tschitschérine 1897 [type species: Pterostichus modicellus (Tschitschérine, 1897)], have traditionally been treated as synonyms of Rhagadus. However, as shown in this study, the identity of a given Pterostichus species is indeterminable without detailed examinations of the male genitalia. Therefore, until the conspecificity of P. punctatipennis with P. solskyi is confirmed with reliable evidence, the treatment of Pseudadelosia as a synonym of Rhagadus is refrained. Similarly, the synonymic treatment of Rhagadulus is also refrained until P. modicellus is adequately examined.
Diagnosis: Subgenus Rhagadus is distinguished from other Pterostichus subgenera by the following combination of characters: small to medium body size; hind wings atrophied, except in rare macropterous individuals of P. microcephalus; pronotum laterobasal impressions double, with the surface densely punctate; elytral scutellar stria absent; elytral interval 3 with two setigerous punctures; ventrolateral sides of the thorax and abdomen with punctures; male sternum 7 without sexual modification; ostium of the male aedeagus directed to the left, with everted endophallus strongly bent ventrally; endophallus surface with two or three sclerites; aggonoporius elongated in the apical direction, with one end forming a filamentous structure; gonoporal piece absent; right paramere arcuately curved, elongated apically.
Description: Dorsal habitus: Small to medium in size. Dorsal body surface glossy, not opaque; mouthpart appendages, legs, margins of the pronotum and elytra reddish brown to brownish black; other body parts almost black. Hind wings atrophied but rarely developed in P. microcephalus.
Head: Normal in size, widest at mid-eye level. Mandibles stout; length of the portion protruding from the labrum almost the same as that of the labrum; inner margin weakly and uniformly arcuate except for the apices, which are strongly bent inward; surface more or less wrinkled. Labrum 6-setose, transverse square, with anterior margin straight or slightly emarginate. Clypeus bisetose, with anterior margin slightly emarginate. Frontal grooves distinct, with the posterior end reaching the apical 1/3–1/2 of the eye. Tempora not developed, with its anteroposterior length less than 1/3 the anteroposterior length of the eye (P. microcephalus and P. nimbatidius) or weakly developed, with the anteroposterior length more than half the anteroposterior length of the eye (other species examined). Anterior and posterior supraorbital setae both present, the anterior at the level of the posterior end of the frontal grooves and the posterior at the level of or behind the posterior end of the eye. Labrum, clypeus, frons, and tempora (dorsum) surfaces smooth except for frontal grooves in some specimens, in which the surface is sparsely punctate. Antennal segments 1 and 2 each with one seta; segment 3 with usually five, rarely six setae; pubescence absent on segments 1–3 but present on the other segments. Eyes convex, normally developed, with the ratio of distance between the apices of the eyes to the inter-eye distance at 1.3–1.6. Mentum with a pair of setae at the middle part; mentum tooth shallowly bifid; labial pits distinct. Submentum with two setae on each side. Genae with surface smooth. Ventral side of tempora smooth in most specimens; in some specimens, more or less wrinkled and/or punctate.
Pronotum: Transverse, widest near the middle; disc convex. Anterior margin weakly emarginate. Anterior angles more or less produced. Lateral margin arcuate, with curvature greater at the anterior half than at the posterior half. Hind angles distinct in the species studied. Posterior margin almost straight. Anterior impression absent. Median line distinct but disappearing near the anterior and posterior margins. Laterobasal impressions double, with the lateral impression shorter. Marginal setae in two pairs, the anterior at apical 1/4–1/3 and the posterior near hind angles. Pronotum surface smooth except for laterobasal impressions, which are densely punctate.
Elytra: Lateral sides weakly arcuate. Basal margin with a distinct transverse line along the anterior end of the elytral intervals, uniformly impressed along the elytral anterior ends, more strongly than that of the stria. Shoulders distinct. Plica distinct. Apices rounded, not denticulate. Scutellar stria absent. Intervals less convex. One setigerous puncture (parascutellar seta) present on interval 2 near or adjoining stria 2. Two setigerous punctures on interval 3, the anterior near the middle and the posterior near the apical 1/4. 13–15 marginal setigerous punctures on interval 9.
Thorax and abdomen ventral sides: Proepisternum, sides of prosternum, mesepisternum, metepisternum, sides of metasternum, and sides of abdominal sterna with punctures; in some specimens, median area of abdominal sterna also punctate. Metepisternum elongate. Sterna 4–5 with a pair of setae in both sexes; sternum 7 with a pair of setae in male, two pairs in female; male sternum 7 simple in shape, without secondary sexual modification.
Legs: Protibia with 2 clip setae. Seta of mesotrochanter present; mesofemur with two posterior setae; mesotibial ctenidium well differentiated. Metacoxa with two setae, lacking medial seta; seta of metatrochanter present. Meso- and metatarsomeres with distinct carina on outer sides of first two and three segments, respectively; meso- and metatarsomere 5 both without setae on ventral side.
Male genitalia: Aedeagus stout, bent to ca. 90° at the basal third; right dorsal surface near ostium sclerotized, orienting the ostium to the left; left dorsal surface near ostium more or less sclerotized, except in P. polygenus, in which surface not sclerotized; apical lamella with hooked projection on both lateral sides in P. polygenus; in other species examined, apical lamella tongue-shaped, without projections on the lateral sides. Left paramere subquadrate. Right paramere arcuately curved, apically elongated.
Endophallus strongly bent at the base, directed ventrally; apex bent further, with the gonopore opening in the anterior direction (directions are defined in the aedeagal apical two-thirds). Endophallus surface with some lobes and sclerites; one sclerite present on the ventral surface (ventral sclerite) in all species examined; ventral sclerite composed of a basal part, attached to the endophallus, and a protruding structure (digitate part); four lobes present in all species examined, unsclerotized lobe on the ventral surface at the left side of the ventral sclerite (left ventral lobe), partially-sclerotized lobe on the ventral surface at the right side of the ventral sclerite (right ventral lobe), sclerotized (in P. solskyi) or unsclerotized (in the other species examined) lobe on the left lateral surface at the middle (left lateral lobe), and unsclerotized lobe on the right lateral surface between the base and middle (right lateral lobe); additional lobes present in some species, unsclerotized lobe on the dorsolateral surface near the right lateral lobe in all examined species except P. takaosanus, P. microcephalus, and P. nimbatidius (right dorsolateral lobe), and unsclerotized lobe on the left dorsolateral surface (left dorsolateral lobe) in P. microcephalus and P. nimbatidius, and on the surface at the bending apical part (apical lobe) in P. microcephalus. Gonopore with weakly sclerotized rim (aggonoporius); aggonoporius elongated in the apical direction, with one end forming a filamentous structure (but often destroyed and lost during dissection); gonoporal piece absent.
Female genitalia: Seminal canal simple, tubular in shape, elongate. Seminal canal and receptaculum differentiated. Receptaculum long, slightly curved. Spermathecal canal shorter than the spermathecal gland, inserted at the junction of the seminal canal and receptaculum. Spermathecal gland elongate, longer than the receptaculum. Vagina surface more or less sclerotized, with the dorsal side more strongly sclerotized than the ventral side. Apophyses of the seminal canal and median oviduct not sclerotized. Gonocoxite 2 with one ensiform seta on the medial side (dorsomedial ensiform seta), with three (P. microcephalus) or two (other species examined) ensiform setae on the lateral side (dorsolateral ensiform setae); two nematiform setae in the subapical depression.
Distribution: East Asia around the Sea of Japan. Based on species appropriately identified (i.e., by male genital morphology), the distribution range includes Hokkaido, Honshu, Shikoku, and Kyushu in the Japanese Archipelago; Sakhalin Island [40]; the Korean Peninsula; and Jejudo Island. Given that P. glabripennis, which was assigned to Rhagadus and the only Chinese species examined in this study, was found not to be a member of Rhagadus, the inclusion of China in the distribution of this subgenus awaits the confirmation of further specimens from this area.
Species studied: P. microcephalus, P. thorectes, P. nimbatidius, P. solskyi, P. polygenus, P. takaosanus, P. thorectoides, P. negylopus sp. nov. Most of these species are redefined in this paper.
3.6. Pterostichus (Rhagadus) polygenus Bates, 1883
-
Pterostichus polygenus Bates 1883 [10]: 247 (original description; type locality: “Nikko”; Pt. (Omaseus?)); Jedlička 1962 [37]: 209 (description; Pt. (Badistrinus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)).
-
Pterostichus brittoni: Habu 1958 [22]: 10 (original description; type locality: “Katashina, Gumma Prefecture”; Pt. (Rhagadus)); Morita 2007 [23]: 405 (description; Pt. (Rhagadus)); Yoshitake et al. 2011 [41]: 130 (catalogue; Pt. (Rhagadus)); [21]: 744 (catalogue; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 100 (part; catalogue; Pt. (Rhagadus)). Syn. nov.
-
Pterostichus harponifer Tanaka 1987 [43]: 118 (original description; type locality “Mt. Fuji, Shizuoka and Yamanashi Prefectures”, Pt. (Rhagadus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 100 (part; catalogue; Pt. (Rhagadus)). Syn. nov.
Notes: Specimens with a hooked aedeagal apex have previously been treated as P. brittoni or P. harponifer, although the species status of the latter has been questioned (e.g., [23]). The present study revealed that type specimens of P. polygenus, which was thought to refer to another species, also have a hooked aedeagal apex, and despite geographical variation in the shape of the hooked projections, specimens with a hooked aedeagal apex have the same endophallus structure throughout their wide distributional range. Based on these results and the earliest publication date of P. polygenus, P. brittoni and P. harponifer were synonymized with P. polygenus. In most of the distribution range, the hooked projections are asymmetrical in dorsal view, with the left and right projections large and small, respectively (Figure 9A–C); the type specimens of P. polygenus (Figure 1D,E) and P. brittoni ([22], fig. 4) have the same type of aedeagal apex. However, near the western margin of the distribution, they are symmetrical, either large (Yatsugatake Mountains; Figure 9D) or small (southern Akaishi Mountains and Kiso Vally; Figure 9E,F) on both lateral sides. Specimens with small projections on both lateral sides have been treated as P. harponifer ([43], fig. 1).
Diagnosis: This species is distinguished from other consubgeners by the following combination of characters: pronotum anterior angles barely produced; posterior half of the pronotum lateral margin barely arcuate; pronotum hind angles obtuse; pronotum laterobasal impression deeply concave near its outer margin; elytra without spectral iridescence; apical lamella of the aedeagus dorsally hooked on both lateral sides.
Description: Body length: ♂, 7.88–9.42 mm (mean ± SD: 8.6 ± 0.49 mm, n = 19); ♀, 8.05–9.33 mm (mean ± SD: 8.69 ± 0.37 mm, n = 17).
Dorsal habitus: Color of dorsal surface varying among individuals, ranging from uniformly black (Figure 8A,B), to a black head and pronotum and reddish-brown elytra (Figure 1A). Elytra without obvious spectral iridescence.
Pronotum: Anterior angles weakly protruded (Figure 8A,B). Posterior half of lateral margin barely arcuate. Hind angles obtuse. Laterobasal impression most deeply concave near its outer margin. The area between the laterobasal impression between the lateral margins strongly convex.
Male genitalia: Aedeagus (Figures 1D,E and 9A–F) with dorsal surface near ostium sclerotized only on the right side from the midline; apical lamella with hooked projections on both lateral sides; hooked projections varying in size among populations, including small on both sides, large on both sides, and large on the left side and small on the right side; length of the apical lamella equal to or greater than its width, varying among populations. Right paramere (Figure 9G–L) moderately curved; in lateral view, ventral margin simply arcuate, not bisinuate. The basal part of the ventral sclerite a semi-trapezoidal plate, with about half its total length protruding (Figure 10A–Y); total length about the same as its width and the width of the endophallus at the base of the aggonoporius; apex not bent. Digitate part of the ventral sclerite an equilateral triangular plate, protruding perpendicularly to the basal part; lateral sides of the equilateral triangular plate structure weakly arcuate; length about equal to its width, about equal to the width of the endophallus at the base of the aggonoporius, and slightly shorter than the length of the ventral sclerite basal part. Left ventral lobe semi-elliptical, with its diameter about 1/3 of the width of the endophallus at the base of the aggonoporius; in left lateral view of the endophallus, apex not beyond the distal margin of the ventral sclerite. Right ventral lobe small; basal part only weakly protruding toward the base of the endophallus, with the surface sclerotized; lateral sides barely protruding and continuous with the surface near the base of the ventral sclerite; apex semi-ellipsoid, protruding toward the apex of the endophallus; upper surface sclerotized entirely or in a band along the midline of the right ventral lobe, varying individually; degree of sclerotization about equal at the basal part and along the midline, but weaker at other parts; all sclerotized parts connected. Left dorsolateral lobe absent. Right dorsolateral lobe semi-ellipsoid, with the diameter at its base smaller than half the width of the base of the apical lamella of the aedeagus. Left lateral lobe moderate in size; length from its base to the apex about half the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view; surface not sclerotized. Right lateral lobe semi-ellipsoid; length from its base to apex about 1/3 of the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view. Apical lobe absent.
Distribution: Honshu east of Chubu District, not including Sado Island (Figure 24). The northernmost collection site of the specimens examined in this study is Mt. Gassan (Yamagata, Nishikawa-machi, Shizu), and the westernmost is the Kiso Vally (Nagano, Agematsu-machi).
Specimens examined: Type specimens: P. polygenus lectotype ♂ (NHMUK), by present designation, “ ∗∗∗∗ 22/81 81 [back side of mount card] \\ Type \ H.T. \\ Japan. \ G. Lewis. \ 1910–320. \\ Chiuzenji. \ 19.VIII.-24.VIII.81. \\ Omaseus? \ polygenus \ Bates \\ [QR Code] \ NHMUK 015551571”; P. polygenus paralectotype ♂ (NHMUK), “ ∗i ∗∗h [back side of mount card] \\ Japan. \ G. Lewis. \ 1910–320. \\ Ex coll. \ Brit. Mus. \\ Co- \ type \\ Pseudomaseus \ polygenus \ Bates \\ H. E. Andrewes Coll. \ B.M.1945-97. \\ [QR Code] \ NHMUK 015551573”; P. brittoni holotype ♂ (NARO), “Holotype \ Pterostichus \ brittoni \ HABU \\ VII. 25, 1954 \ Katashina, \ near Oze,Gum- \ ma P. A.Habu” [through high-quality photos, which are available in the type-specimen database of NARO [44]].
Specimens from type localities (not types): 2♂1♀ (KS), Mt Fujisan [type locality of P. harponifer] (1♀, Shizuoka, Fujinomiya-shi, Awakura, the Fujisan-Skyline, alt. ca 1600–1900 m, 23–34-VI-2004, K. Sasakawa & H. Ikeda leg.; 2♂, Yamanashi, Narusawa-mura, alt. 1693 m, 1–2-VII-2013, K. Sasakawa leg.).
Other specimens: 2♂1♀ (KS), Yamagata, Nishikawa-machi, Shizu, alt. ca 900 m, 24-VI–8-VII-2006, K. Sasakawa; 1♂ (KS), Yamagata, on the border between Yanagawa, Ôe-machi and Shirakura, Asahi-machi, Buna-tôge Pass, 9–10-IX-2004, K. Sasakawa leg.; 2♂ (KS), Fukushima, Fukushima-shi, Tsuchiyuonsenmachi, Tsuchiyu-tôge-shitsugen wetland, 23–24-V-2003, K. Sasakawa leg; 1♂ (KS), Tochigi, Nasu-machi, Yumoto, Yawata-Onsen, alt. 1200 m, 18–19-VII-2010, K. Sasakawa leg.; 1♀ (KS), Tochigi, Nikkô-shi, Chȗgȗshi, Kotoku-Bokujô Ranch, alt. 1440 m, 15-IX-2007, W. Toki leg.; 2♂ (KCMI), Tochigi, Nikkô-shi, Yumoto, near Lake Yunoko, 20-VII-1975, T. Matsuda leg.; 1♀ (KS), Tochigi, Nikkô-shi, Yumoto, Konsei-zawa, alt. ca. 1550 m, 15–16-IX-2012, K. Sasakawa leg; 1♂2♀ (KS), Gunma, Minakami-machi, Yubiso, 15–17-VII-2001, K. Sasakawa leg. (1♂, near Tenjindaira Station on Tanigawadake Ropeway, alt. 1380 m; 2♀, near Doaiguchi Station on Tanigawadake Ropeway, alt. 750m); 1♂ (KS), Gunma, Minakami-machi, Nagai, Mikuni-tôge Pass, 25–27-VIII-2002, H. Ikeda leg.; 1♂3♀ (EUM), Tokyo, Okutama-machi, Nippara, Mt. Kintaisan, R. Shiiba leg. (1♂1♀, 17.I.2016; 2♀, 6.III.2016); 1♀ (KS), Tokyo, Okutama-machi, Nippara, Mt. Kumotoriyama, near the summit, 5–12-VIII-1999, K. Sasakawa leg.; 1♀ (KS), Kanagawa, Ashigara-shi, Hakonemachi, Motohakone, Mt. Komagatake, 24–29-VI-2002, K. Sasakawa leg.; 1♂ (KS), Yamanashi, Tabayama-mura, Mt. Nanatsuishiyama, near the summit, 10–18-X-1999, K. Sasakawa leg.; 1♂ (KS), Yamanashi, Tabayama-mura, Mts. Okutama, Mizunashione ridge, 19-VI-1999, K. Sasakawa leg.; 1♂1♀ (KS), Yamanashi, Hokuto-shi, Takanecho, Kiyosato, 24–25-IX-2022, T. Shimizu leg.; 1♂1♀ (KS), Yamanashi, Minobu-cho, Ôjiro, Abe-tôge Pass, alt. 1100 m, 1–2-X-2003, K. Sasakawa leg; 1♂2♀ (KS), Yamanashi, Kôshû-shi, Enzan, Ichinosetakahashi~Kamihagiwara, Yanagisawa-tôge Pass, 27-V-1971, R. Ishikawa leg.;1♀ (KS), Nagano, Chino-shi, Tamagawa, alt. ca 1600 m, 15–16-IX-2009, K. Sasakawa & H. Ikeda leg.; 1♂ (KS), Nagano, Agematsu-machi, Ogawa, Ashizawa, 11-X-1999, M. Hama leg.; 1♀ (KS), Nagano, Ina-shi, Hase, Kurogouchi, Kitazawa-tôge Pass, 15-X-1998, M. Hama leg.
3.7. Pterostichus (Rhagadus) takaosanus Habu, 1958
-
Pterostichus takaosanus Habu 1958 [22] : 247 (original description; type locality: “Vicinity of Mt. Takao, Tokyo Metropolis”; Pt. (Rhagadus)); Tanaka 1985 [38]: 113 (description; Pt. (Rhagadus)); Morita 2007 [23]: 403 (description; Pt. (Rhagadus)); Yoshitake et al. 2011 [41]: 133 (catalogue; Pt. (Rhagadus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 102 (part; catalogue; Pt. (Rhagadus)).
-
Pterostichus freyellus Jedlička 1958 [45]: 910 (original description; type locality: “Japan: Takao-san, Tokyo”; Pt. (incertae sedis)); Jedlička 1962 [37]: 235 (description; Pt. (Morphohaptoderus)); Bousquet 2017 [21]: 744 (synonym of polygenus; catalogue; Pt. (Rhagadus)). Syn. nov.
-
Pterostichus komiyai Morita 2007 [23]: 401 (original description; type locality: “Abe Pass, Shizuoka-shi Shizuoka Prefecture”; Pt. (Rhagadus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)). Syn. nov.
Notes: This study revealed that P. freyellus, which was previously considered a synonym of another species, actually refers to P. takaosanus. Although both P. takaosanus and P. freyellus have the same type locality (Mt. Takaosan) and year of description (1958), the publication date of the former is February 25, which precedes the publication date of the latter, October 31, making P. takaosanus the valid name for this species. Populations west of the Akaishi Mountains have been treated as P. komiyai due to a longer apical lamella that is weakly bent to the right [23]. However, P. komiyai and P. takaosanus were found to have the same endophallus structure; therefore, P. komiyai was synonymized with P. takaosanus.
Diagnosis: This species is distinguished from other consubgeners by the following combination of characters: small body size; shallow pronotum laterobasal impression, without conspicuous concavity near its outer margin; elytra without spectral iridescence; apical lamella long and tongue-shaped (i.e., width < length).
Description: Body length: ♂, 6.73–8.51 mm (mean ± SD: 7.69 ± 0.47 mm, n = 30); ♀, 6.94–8.11 mm (mean ± SD: 7.69 ± 0.37 mm, n = 15).
Dorsal habitus: Color of dorsal surface varying among individuals, ranging from uniformly black (Figure 8D), to a brownish-black head and pronotum and reddish-brown elytra (Figures 1B and 8C). Elytra without spectral iridescence.
Pronotum: Anterior angles varying among individuals, ranging from weakly to distinctly protruded (Figure 8C,D). Posterior half of lateral margin barely arcuate. Hind angles obtuse or right angled, varying individually. Laterobasal impressions shallow, with the degree of concavity almost unform throughout. The area between the laterobasal impression between the lateral margin less convex.
Male genitalia: Aedeagus (Figures 1F,G and 11A–F) with dorsal surface near the ostium sclerotized on both the right and left sides; on the left side, sclerotized part narrow and sclerotization weak at the level not visible from left lateral view; apical lamella roughly tongue-shaped, with shape and size varying among populations; laterally symmetrical or bent right-laterally in dorsal view; length approximately equal to or greater than its width. Right paramere (Figure 11G–L) moderately curved; in lateral view, ventral margin simply arcuate, not bisinuate. The basal part of the ventral sclerite forms a weak bulge (Figure 12A–Y); length about the same as the width and less than half the width of the endophallus at the base of the aggonoporius. Digitate part of the ventral sclerite an equilateral triangular plate, protruding perpendicularly to the basal part; lateral sides of the equilateral triangular plate structure weakly arcuate; length about the same as the width, the width of the endophallus at the base of the aggonoporius, and the length of the ventral sclerite basal part; the width of the base twice as wide as width of the ventral sclerite basal part. Left ventral lobe conical; in left lateral view of the endophallus, apex not exceeding or just reaching the apex of the digitate part of the ventral sclerite. Right ventral lobe small; basal part only weakly protruding toward the base of the endophallus, with surface sclerotized; lateral sides barely protruding and continuous with the surface near the base of the ventral sclerite; apex not protruding; upper surface sclerotized entirely or around lateral sides, varying individually; degree of sclerotization about equal at the basal part and lateral sides, but weaker in other areas; all sclerotized parts connected. Left and right dorsolateral lobes absent. Left lateral lobe moderate in size; length from the base to the apex about half the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view; surface not sclerotized. Right lateral lobe semi-ellipsoid; in dorsal view, contour of the margin of the apical side of the endophallus more arcuate; length from the base to the apex about equal to or greater than half the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view. Apical lobe absent.
Distribution: Honshu east of Chubu District, not including Sado Island (Figure 24). The northernmost collection site of specimens examined in this study is the Shimokita Peninsula (Aomori, Mt. Kamafuseyama), and the westernmost is Mt. Hekosan (Fukui).
Specimens examined: Type specimens: P. takaosanus holotype ♂ (NARO), “Holotype \ Pterostichus \ takaosanus \ HABU \\ Col.AHABU \ Near Mt.Ta- \ kao,Tokyo P. \ V. 2. 2603.” [through high-quality photos, which are available in the type-specimen database of NARO [44]; Pterostichus freyellus holotype ♂ (NMB), “Takao-san \ Tokyo-Japan \. 3-5-1952 \\ Pterostichus \ freyellus \ sp. n. \ det. ING.JEDLIČKA \\ TYPUS \\ Museum Frey \ Tutzing \\ Coll. G Frey \ NMB”.
Specimens from type localities (not types): 1♂1♀ (EUM), Tokyo, Hachiôji-shi, Takaomachi, Mt. Takaosan [type locality of P. takaosanus and P. freyellus], R. Shiiba leg. (1♂, 20-IX-2015; 1♀, alt. 400–600 m, 21-V-2017); 1♂1♀ (KS), Yamanashi, Minobu-cho, Ôjiro, Abe-tôge Pass, alt. 1100 m [type locality of P. komiyai], 1–2-X-2003, K. Sasakawa leg.
Other specimens: 1♂ (KS), Aomori, Mutsu-shi, Mt. Kamafuseyama, alt. 620 m, 19–20-VII-2005, H. Ikeda leg.; 1♂1♀ (KS), Aomori, Aomori-shi, Mts. Hakkôdasan, Mt. Maedake, alt. 650–750 m, 18-VIII-2010, S. Hoshino leg; 2♂2♀ (KS), Iwate, Hanamaki-shi, The northern foot of Mt. Kojiramori, between Kasadumezawa and Kawaranobou, alt. 650–1000 m, 1–2-VII-2004, K. Sasakawa & H. Ikeda leg.; 1♀ (KS), Iwate, Takizawa-shi, Mt. Iwatesan, Umagaeshi Trailhead, alt. ca. 650 m, 30-VI–2-VII-2004, K. Sasakawa & H. Ikeda leg.; 1♂ (KS), Miyagi, Taiwa-cho, Mt. Funagatayama, 4–5-VI-2002, K. Sasakawa leg.; 2♂ (KS), Akita, Akita-shi, Mt. Taiheizan, 7-VI-2003, H. Sato leg.; 1♂ (KS), Yamagata, Sakata-shi, Mt. Chokaisan, alt. ca 900 m, near Lake Tsurumaike, 24-VI–8-VII-2006, K. Sasakawa; 1♂ (KS), Yamagata, Ôe-machi, Mt. Ôzunomoriyama, alt. 900 m, 9–12-IX-2004, K. Sasakawa leg.; 1♂ (KS), Yamagata, Nishikawa-machi, Ôisawa, Higuresawa, alt. 625 m, 9–12-IX-2004, K. Sasakawa leg.; 1♂ (KS), Ibaraki, Daigo-machi, Mt. Yamizosan, near the summit, 21–22-V-2002, K. Sasakawa leg.; 1♂ (KS), Ibaraki, Takahagi-shi, Kamikimita, Kamikimita-Kamitashiro Experimental Station (Forestry and Forest Products Research Institute), 13–21-XI-2002, S. Niwa leg.; 1♂1♀ (KS), Ibaraki, Tsukuba-shi, Tsukuba, Mt. Tsukubasan, 12–13-V-2001, K. Sasakawa leg.; 3♂ (KS), Tochigi, Nasu-machi, Yumoto, Yawata-Onsen, alt. 1200 m, K. Sasakawa leg. (1♂, 21–22-V-2002; 1♂, 2–3-VI-2004; 1♂, 18–19-VII-2010); 1♂ (EUM), Gunma, Midori-shi, Azumachokonaka, 19-VIII-2023, R. Shiiba leg.; 1♀ (KS), Gunma, Minakami-machi, Yubiso, 28–30-V-2002, K. Sasakawa leg.; 1♀ (KS), Chiba, Isumi-shi, Misakichôkamone, alt. ca 30 m, 28-IX–2-X-2011, K. Sasakawa leg.; 1♂ (KS), Chiba, Kamogawa-shi, Kiyosumi, Ippaimizu, 20–22-V-1981, K. Kubota leg.; 2♂ (KS), Tokyo, Hachiôji-shi, Todorimachi, 18–19-V-2004, H. Ikeda leg.; 1♂1♀ (KS), Kanagawa, Hadano-shi, Horiyamashita, Mts. Tanzawasanchi, Ôkuraone ridge (1♂, alt. 600 m, 10–12-VIII-2000, S. Niwa leg.; 1♀, alt. 835 m, 15–21-X-2000, K. Sasakawa leg.); 1♂1♀ (KS), Fukui, Ôno-shi, Hôkyôji, Mt. Hekosan, alt. 1100 m, S. Inoue leg. (1♂, 13-XI-2019; 1♀, 10-X-2019); 1♂ (MPIM), Yamanashi, Hokuto-shi, Mukawa-cho, 20-V-1994, T. Saito leg.; 1♂ (KS), Shizuoka, Gotenba-shi, Kagosaka-tôge Pass, 15–16-X-2003, H. Ikeda & M. Wakabayashi leg.; 2♂ (KS), Shizuoka, Fuji-shi, Ôbuchi, Mt. Fujisan, alt. 1440 m, 24–25-VII-2020, T. Shimizu leg.; 1♀ (KS), Shizuoka, Izu-shi, Shuzenji, 12–13-VI-2002, K. Sasakawa leg.; 1♀ (KS), Shizuoka, Shizuoka-shi, Umegashima, Igawa-tôge Pass, 10–11-VII-2022, T. Shimizu leg.; 2♂ (KS), Aichi, Seto-shi, Sonocho, Mt. Yamaboshiyama∼Ôbora-tôge Pass, alt. ca 200 m, 10–11-IV-2023, H. Nishida leg.; 1♀ (KS), Aichi, Toyota-shi, Rendanicho, near Isegami-tôge Pass, alt. ca 800 m, 20–23-V-2022, H. Nishida leg.
3.8. Pterostichus (Rhagadus) negylopus sp. nov.
-
Pterostichus polygenus: Habu 1958 [22]: 4 (description; Pt. (Rhagadus)); Habu 1972 [46]: 4 (taxonomy; Pt. (Rhagadus)); Tanaka 1985 [38]: 113 (description; Pt. (Rhagadus)); Yoshitake et al. 2011 [41]: 131 (part; catalogue; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 101 (part; catalogue; Pt. (Rhagadus)).
-
Pterostichus sp.: Morita 2007 [23]: 405 (description; Pt. (Rhagadus)).
-
Pterostichus freyellus: Habu 1972 [46]: 4 (synonym of polygenus; Pt. (Rhagadus)).
Zoobank LSID: http://zoobank.org:act:B61B02F6-AD23-43F0-A02A-7F7DF453F8D3
Notes: This new species has been treated incorrectly as P. polygenus, and P. freyellus has been considered a synonym of this misidentified P. polygenus. As mentioned above, examinations of type specimens revealed that P. polygenus and P. freyellus in fact refer to species that have been treated as P. brittoni and P. takaosanus, respectively. Additionally, the results of the current integrative morphological analyses showed that the names P. thorectes, P. latemarginatus, P. mundatus, P. thorectoides, P. straneoi, P. satsumanus, and P. ishiii do not refer to this species. Consequently, this species was found to have no scientific name, so it is described as a new species. This new species was treated above as belonging to morphotype A.
Diagnosis: This species is distinguished from other consubgeners by the following combination of characters: posterior half of the pronotum lateral margin only slightly arcuate; pronotum hind angles obtuse; elytra with obvious spectral iridescence; left dorsal side of the aedeagal dorsum near the ostium weakly sclerotized; and basal part of the ventral sclerite of the endophallus about three times longer than the digitate part.
Description: Body length: ♂, 8.55–10.55 mm (mean ± SD: 9.49 ± 0.4 mm, n = 71); ♀, 8.92–10.92 mm (mean ± SD: 9.78 ± 0.5 mm, n = 90).
Dorsal habitus: Dorsal surface uniformly black (Figure 8E). Elytra with obvious spectral iridescence.
Pronotum: Anterior angles varying among individuals, ranging from slightly to distinctly protruded (Figure 8E). Posterior half of the lateral margin only slightly arcuate. Hind angles obtuse. Laterobasal impressions most deeply concave near the outer margin. The area between the laterobasal impression between the lateral margins more or less convex, with convexity varying among individuals.
Male genitalia: Aedeagus (Figures 4A and 13A–F) with dorsal surface near ostium sclerotized on both the right and left sides; on the left side, sclerotized part narrow and sclerotization weak at the level not visible in left lateral view; apical lamella laterally symmetrical, tongue-shaped, with the width greater than the length. Right paramere (Figure 13G–L) strongly curved; in lateral view, ventral margin simply arcuate, not bisinuate. The basal part of the ventral sclerite tongue-shaped, protruding toward the base of the endophallus (Figure 14A–Y); length of the protruded part about half the total length; total length more than twice the width and greater than the width of the endophallus at the base of the aggonoporius (but less than twice the width); apex not bent. Digitate part of the ventral sclerite tongue-shaped, directed toward the base of the endophallus along the ventral sclerite basal part; total length about equal to the width, less than the width of the endophallus at the base of the aggonoporius, and about 1/3 of the length of the ventral sclerite basal part. Left ventral lobe conical; in left lateral view of the endophallus, apex not extending beyond the distal margin of the ventral sclerite. Right ventral lobe large; basal part narrowly protruding toward the base of the endophallus, with surface strongly sclerotized; lateral sides distinctly protruding and discontinuous, with the surface near the base of the ventral sclerite; apex semi-ellipsoid, protruding toward the apex of the endophallus; upper surface of the middle strongly sclerotized in a band ca. 30° off the midline right ventral lobe; sclerotized parts of the basal part and upper surface not connected. Left dorsolateral lobe absent. Right dorsolateral lobe semi-ellipsoid, slightly curved toward the base of the endophallus; diameter at its base larger than half the width of the base of the apical lamella of the aedeagus. Left lateral lobe moderate in size; length from the base to the apex about half the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view; surface not sclerotized. Right lateral lobe semi-ellipsoid; length from the base to the apex about 1/3 of the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view. Apical lobe absent.
Distribution: Honshu east of Chubu District, including Sado Island (Figure 4S as morphotype A). The northernmost collection site of the specimens examined in this study is Mt. Iwatesan (Iwate), and the westernmost is Mt. Kisoontakesan (Nagano).
Type specimens: Holotype: ♂, Tokyo, Hachiôji-shi, Todorimachi, Tama Forest Science Garden, 19-V-2003, K. Matsumoto leg., in the K. Sasakawa collection deposited in the Laboratory of Zoology, Department of Science Education, Faculty of Education, Chiba University, Chiba, Japan.
Paratypes 70♂90♀: 1♀ (KS), Iwate, Takizawa-shi, Mt. Iwatesan, Umagaeshi Trailhead, alt. ca. 650 m, 30-VI–2-VII-2004, K. Sasakawa & H. Ikeda leg.; 1♂1♀ (KS), Iwate, Morioka-shi, Ueda, Kitayama-sansakuro, N. Nakaya leg. (1♂, 23-VIII-2019; 1♀, 5-V-2019); 1♀ (KS), Miyagi, Ôsaki-shi, Furukawakawakuma, Lake Kejonuma, the western shore, 20-XII-1999, K. Sasakawa leg.; 1♂ (KS), Miyagi, Ôhira-mura, Komaba, 20-XII-1999, K. Sasakawa leg.; 1♂1♀ (KS), Miyagi, Zaô-machi, Mt. Zaôsan, the eastern foot, alt. ca 880 m, along the Zaô Echô Line, 4–5-VI-2002, K. Sasakawa leg.; 1♂ (KS), Akita, Yuzawa-shi, Minase, 24–25-IX-2021, T. Shimizu leg.; 1♀ (KS), Yamagata, Ôe-machi, Mt. Ôzunomoriyama, alt. 900 m, 9–12-IX-2004, K. Sasakawa leg.; 1♂ (KS), Yamagata, Nishikawa-machi, Ôisawa, Higuresawa, alt. 625 m, 9–12-IX-2004, K. Sasakawa leg.; 2♂ (KS), Fukushima, Iwaki-shi, Mt. Shibayama, 24–25-V-2003, K. Sasakawa leg.; 1♀ (KS), Fukushima, Shirakawa-shi, Shirasaka, Miwadai, 26–27-V-2000, K. Sasakawa leg.; 1♀ (KS), Fukushima, Sukagawa-shi, Shioda, Mt. Utsumine, 2–3-VI-2004, K. Sasakawa & H. Ikeda leg; 3♂2♀ (KS), Ibaraki, Kitaibaraki-shi, Nakagôchô, Onoyasashi, 21–22-V-2002, K. Sasakawa leg.; 2♀ (KS), Ibaraki, Kitaibaraki-shi, Isoharacho, 21–22-V-2002, K. Sasakawa leg.; 2♀ (KS), Tochigi, Nikkô-shi, Sannai, near Shiraito-no-taki, alt. ca. 690 m, 1–2-IX-2012, K. Sasakawa leg.; 2♂10♀ (KS), Gunma, Minakami-machi, Yubiso, near Doaiguchi Station on Tanigawadake Ropeway, alt. 750 m, 15–17-VII-2001, K. Sasakawa leg.; 1♂1♀ (KS), Gunma, Minakami-machi, Yubiso, Yubisokôen Park, 28–30-V-2002, K. Sasakawa leg.; 4♂5♀ (KS), Gunma, Minakami-machi, Yubiso, 28–30. v.2002, K. Sasakawa leg.; 4♂2♀ (KS), Gunma, Takasaki-shi, Yoshiimachi, Nanyôdai, 28–30-V-2002, K. Sasakawa leg.; 7♂6♀, Tokyo, Hachiôji-shi, Todorimachi, Tama Forest Science Garden, K. Matsumoto leg. [1♀ (KS),13-VI-2001; 1♂1♀ (KS), 16-IV-2002; 2♂ (KS), 29-IV-2002; 2♂ (KS), 28-V-2002; 2♀ (KS), 29-VI-2002; 1♀ (KS), 26-VII-2002; 1♀ (KS), 4-X-2002; 1♂ (KS), 22-IV-2003; 1♂ (NHMUK), 28-VI-2003]; 4♂9♀ (KS), Tokyo, Hachiôji-shi, Todorimachi, 18–19-V-2004, H. Ikeda leg.; 2♀, Tokyo, Hachiôji-shi, Mt. Takaosan (1♀ (KS), 7–8-VI-2003, K. Sasakawa leg.; 1♀ (MNHAH), 5-V-1955, Karasawa leg.); 3♂1♀, Tokyo, Hachiôji-shi, Takaomachi, Mt. Takaosan, R. Nakamura leg. [2♂ (KS) and 1♂ (NMP), alt. 400–600 m, 22-VI-2019; 1♀ (KS), alt. 400–500 m, 31-X-2020].; 1♀ (MNHAH), Kanagawa, Sagamihara-shi, Wakayanagi, Okuhata, 7-IV-1957, Y. Karasawa leg.; 1♂ (KS), Niigata, Murakami-shi, Mt. Shinbodake, alt. 550 m, 4–19-IX-2016, H. Itô leg.; 1♀ (KS), Niigata, Murakami-shi, Warabi-tôge Pass, alt. 415 m, 8-IX-2018, H. Itô leg.; 2♀ (KS), Niigata, Nagaoka-shi, Teradomarinozumi, Mt. Yahikosan, alt. 510 m, 22–23-V-2021, H. Itô & M. Anazawa leg.; 1♂ (MPIM), Niigata, Nagaoka-shi, Happodai, 7-VIII-1996, T. Saito leg.; 15♂11♀ (HUM), Niigata, Sado Island, Sado-shi, Toyooka, Y. Kaneko leg. (15♂10♀, 19-VI-2005; 1♀, 23-V-2005); 1♂ (MNHAH), Nagano, Nagano-shi, Nagano, Zenkôji Temple, 28-IV-1940, N. Matsuzawa leg.; 1♀ (KS), Nagano, Hakuba-mura, Kamishiro, Teirinji Temple, 18-VIII-2005, W. Taki leg.; 6♂6♀ (KS), Nagano, Fujimi-machi, 29–30-IV-2001, K. Shindo leg.; 2♂2♀ (KS), Nagano, Minamiminowa-mura, Ôshibakôen Park, 1-XI-1999, M. Hama leg.; 1♀ (KS), Nagano, Ina-shi, Nozoko, 3-XI-1998, M. Hama leg.; 1♀ (KS), Nagano, Tatsuno-machi, Ono, Shidareguri, 4-IV-2000, M. Hama leg.; 1♀ (KS), Nagano, Agematsu-machi, Nezame, 4–6-VI-2001, K. Sasakawa leg.; 8♂8♀ (KS), Nagano, Kiso-machi, Mt. Kisoontakesan, alt. 1300 m, Kaidakôgen, 25–26-VIII-1998, K. Kubota leg.; 1♀ (KS), Yamanashi, Minobu-cho, Ôjiro, Abe-tôge Pass, alt. 1100 m, 1–2-X-2003, K. Sasakawa leg.; 4♀ (KS), Shizuoka, Fujinomiya-shi, Mts. Tenshisanchi, 16–18-V-2001, M. Wakabayashi leg.; 1♀ (KS), Shizuoka, Hamamatsu-shi, Misakubochô, Yamazumi, Yachônomori, Yamagaranomon, 13–14-VI-2017, M. Ujiie leg.; 1♂ (KS), Shizuoka, Shizuoka-shi, Umegashima, Igawa-tôge Pass, 10–11-VII-2022, T. Shimizu leg.
Etymology: The name negylopus is an anagram of the specific name of P. polygenus, which has been thought to be the valid name of this species.
3.9. Pterostichus (Rhagadus) thorectes Bates, 1873
-
Pterostichus thorectes Bates 1873 [9]: 287 (original description; type locality: Japan; Pt. (Omaseus)); Habu 1958 [22]: 3 (description; Pt. (Badistrinus)); Jedlička 1962 [37]: 209 (description; Pt. (Badistrinus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 102 (catalogue; Pt. (Rhagadus)).
-
Platysma polygenum v. latemarginatum Straneo 1936 [47]: 148 (original description; type locality: “Giappone”; Habu 1958 [22]: 5 (synonym of straneoi; Pt. (Rhagadus)); Jedlička 1962 [37]: 209 (description; Pt. (Badistrinus (s.l.)); Bousquet 2017 [21]: 744 (synonym of straneoi; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 100 (catalogue; Pt. (Rhagadus)). Syn. nov.
-
Pterostichus mundatus Jedlička 1940 [48]: 13 (original description; type locality: “Japan: Osaka”; Pt. (incertae sedis)); Jedlička 1962 [37]: 301 (description; Pt. (incertae sedis); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)). Syn. nov.
-
Pterostichus straneoi Habu 1958 [22]: 5 (original description; type locality: “Mt. Hiko, Fukuoka Prefecture”; Pt. (Rhagadus)); Yoshitake et al. 2011 [41]: 131 (catalogue; Pt. (Rhagadus)); Yoshimatsu et al. 2018 [42]: 101 (catalogue; Pt. (Rhagadus)). Syn. nov.
-
Pterostichus satsumanus Habu 1958 [22]: 6 (original description; type locality: “Mts. Kirishima, Kagoshima Prefecture”; Pt. (Rhagadus)); Yoshitake et al. 2011 [41]: 130 (catalogue; Pt. (Rhagadus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)). Syn. nov.
Notes: P. thorectes was described based on specimens from “Japan,” and detailed locality information was not provided in the original description [9] or lectotype labels (Figure 1M,N). This study revealed that specimens with the same male genital structure (including endophallus structure) as found in the P. thorectes lectotype occur in Honshu, in the west of Fukui and Aichi Prefectures; Shikoku; and Kyushu. Thus, the lectotype is assumed to have been collected somewhere within these areas. The synonymized species P. latemarginatus was described based on a single female from “Japan” ([47]; fig. 2D). Although there is no detailed locality information, the holotype female was unambiguously determined to be P. thorectes in the present discriminant analysis (Figure 7). Another synonymized species, P. mundatus, was described based on a single female specimen from “Osaka” (Figure 2E), but with no further locality information [37]. Two species occur in Osaka; P. thorectoides, which will be redefined later, is found in the northeast and P. thorectes in the southwest (Figure 4S); however, the P. mundatus holotype female was unambiguously determined through discriminant analysis to be P. thorectes. Therefore, the P. mundatus holotype is assumed to have been collected somewhere in southwestern Osaka. The remaining two synonymized species, P. straneoi and P. satsumanus, have the same endophallus structure as the P. thorectes lectotype male (Figures 16A–E and 17K–T). These two species were described from northern and southern Kyushu, respectively, and their type localities are within the distribution range of P. thorectes defined in this study, and distant from that of the other species (Figure 4S). Therefore, the conspecificity of these two species with P. thorectes is unambiguously determined based on both morphological and geographical evidence. This redefined P. thorectes was treated above as belonging to morphotype B.
Diagnosis: This species is distinguished from other consubgeners by the following combination of characters: pronotum anterior angles distinctly produced; posterior half of the pronotum lateral margin arcuate; elytra with obvious spectral iridescence; left dorsal side of the aedeagal dorsum near the ostium strongly sclerotized; and digitate part of the ventral sclerite of the endophallus shaped as a semicircular plate.
Description: Body length: ♂, 8.13–10.36 mm (mean ± SD: 9.25 ± 0.49 mm, n = 80); ♀, 8.09–10.91 mm (mean ± SD: 9.5 ± 0.52 mm, n = 86).
Dorsal habitus: Dorsal surface uniformly black (Figures 1C, 2A,B, and 8F). Elytra with obvious spectral iridescence.
Pronotum: Anterior angles distinctly protruded (Figures 1C, 2A,B, and 8F). Posterior half of the lateral margin arcuate. Hind angles varying among individuals, ranging from obtuse to projecting acute angles. Laterobasal impressions most deeply concave near the outer margin. The area between the laterobasal impressions between lateral margins more or less convex, with convexity varying among individuals.
Male genitalia: Aedeagus (Figures 1H,I, 4G, 15A–F) with dorsal surface near the ostium sclerotized on both the right and left sides; on the left side, sclerotized part wide and sclerotization strong at the level visible in left lateral view; apical lamella laterally symmetrical, tongue-shaped, with width greater than the length. Right paramere (Figure 15G–L) strongly curved; in lateral view, ventral margin simply arcuate, not bisinuate. The basal part of the ventral sclerite tongue-shaped, protruding toward the base of the endophallus (Figures 16A–T and 17A–Y); length of the protruded part about 1/4 of the total length; total length more than twice the width and about equal to the width of the endophallus at the base of the aggonoporius; apex not bent. Digitate part of the ventral sclerite shaped as a semicircular plate, along the base of the ventral sclerite basal part; apex oriented left laterally; length about the same as the width, about half the width of the endophallus at the base of the aggonoporius, and about half the length of the protruded part of the ventral sclerite basal part. Left ventral lobe semi-elliptical, with diameter more than 2/3 of the width of the endophallus at the base of the aggonoporius; in left lateral view of the endophallus, apex far beyond the distal margin of the ventral sclerite. Right ventral lobe moderate in size; basal part narrowly protruding toward the base of the endophallus, with surface strongly sclerotized; lateral sides weakly protruding and continuous with the surface near the base of the ventral sclerite; apex semi-ellipsoid, protruding toward the apex of the endophallus; upper surface of the middle sclerotized in a band along the midline of the right ventral lobe, with the degree of chitinization more or less weaker than that of the basal part; sclerotized parts of the basal part and upper surface connected. Left dorsolateral lobe absent. Right dorsolateral lobe semi-ellipsoid, with diameter at its base smaller than half the width of the base of the apical lamella of the aedeagus. Left lateral lobe moderate in size; length from the base to apex about half the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view; surface not sclerotized. Right lateral lobe semi-ellipsoid; length from the base to the apex about 1/5 of the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view. Apical lobe absent.
Distribution: Honshu, in the west of Fukui and Aichi Prefectures; Shikoku; and Kyushu (Figure 4S as morphotype B).
Specimens examined: Type specimens: P. thorectes lectotype ♂ (NHMUK), by present designation, “1872 [back side of mount card] \\ Japan. \ G. Lewis. \ 1910–320. \\ Ex coll. \ Brit. Mus. \\ Co- \ type \\ Pseudomaseus \ thorectes \ Bates \\ H. E. Andrewes Coll. \ B.M.1945-97. \\ [QR Code] \ NHMUK 015212459”; Platysma polygenum v. latemarginatum holotype ♀ (NHMUK), “TYPUS \\ Japan. \ G. Lewis. \ 1910–320. \\ Rhagadus \ polygenus \ v. latemarginatus \ mihi. \\ det. Ing. Straneo \\ [QR Code] \ NHMUK 015543662”; P. mundatus holotype ♀ (NMP), “OSAKA NIPON \ Japan-1 ∗36 \ H.Jamamoto \\ TYPUS \\ Mus. Nat. Pragae \ Inv. 26,004 \\ mun ∗datus \ sp.n. \\ DET.ING.JEDLIČKA”; Pterostichus straneoi holotype ♂ (NARO), “Holotype \ Pterostichus \ straneoi \ HABU \\ 69 \\ 800~ \ 900 m \\ leg.A.HABU \ Mt. Hiko \ Fukuoka P. \ X. 29, 1951. \\ not Pterostichus \ thorectes Bates \ E.B.Britton det. \ Comp. with Type.” [through high-quality photos, which are available in the type-specimen database of NARO [44].
Specimens from type localities (not types): 5♂2♀, Fukuoka, Soeda-machi, Mt. Hikosan [type locality of P. straneoi] (2♂ (CTD), 7-IX-2012, T. Dejima leg.; 2♀, 24-IV-1974, Y. Takakura leg.; 1♂ (KMNH), Mt Takanosuyama [A small peak that is only 1.7 km from the summit of Mt. Hikosan and virtually can be regarded as the same as the type locality], 13-IX-1970, Y. Takakura leg.; 1♂ (KMNH), Mt. Takanosuyama, 12-IX-1973, Y. Takakura leg.; 1♂ (KMNH), Mt. Takanosuyama, 23-V-1979, Y. Takakura leg.; 1♂ (KMNH), Miyazaki, Mts. Kirishimayama, Mt. Takachihonomine [type locality of P. satsumanus], 23-IX-1957, M. Amano leg.
Other specimens: 1♂ (KS), Fukui, Awara-shi, Kiyotaki, Mt. Kariyasuyama, alt. 500 m, 26-IV-2002, S. Inoue leg.; 1♀ (KS), Fukui, Awara-shi, Une, alt. 160 m, 5-IV-2017, S. Inoue leg.; 2♂1♀ (KS), Fukui, Awara-shi, Kumasaka, alt. 300 m, 8-VII-2014, S. Inoue leg.; 1♂ (KS), Fukui, Sakai-shi, alt. 580 m, 7-V-2008, S. Inoue leg.; 1♂ (KS), Fukui, Katsuyama-shi, Nomukicho, Yokokura, alt. 800 m, near Shinmata-tôge Pass, 9-VI-2021, S. Inoue leg.; 1♂6♀ (KS), Fukui, Fukui-shi, Mt. Kunimidake, alt. 660 m, S. Inoue leg. (6♀, 9-VII-2012; 1♂, 13-VII-2012); 2♂2♀ (KS), Fukui, Echizen-cho, Myôga, Mt. Rokushoyama, alt. 540 m, S. Inoue leg. (1♂1♀, 25-VI-2013; 1♂1♀, 3-VIII-2013); 2♂5♀ (KS), Fukui, Ôno-shi, Hôkyôji, Mt. Hekosan, alt. 1100 m, S. Inoue leg. (1♂, 13-IX-2019; 1♀, 20-IX-2019; 1♀, 26-X-2021; 1♂, 13-XI-2019; 2♀, 1-IX-2020; 1♀, 12-IX-2020); 1♂ (KS), Aichi, Nagoya-shi, Kawahigashiyama, 20-XII-2021, T. Tenma leg.; 4♂7♀ (KS), Aichi, Nagoya-shi, Makinogaike-ryokuchi (west side), H. Nishida leg. (4♂6♀, 17–19-IV-2020; 1♀, 26–28-IV-2020); 1♀ (MNHAH), Mie, Iga-shi, Aoyama, 13-I-1946, M. Ohkura leg.; 1♀ (MNHAH), Mie, Tsu-shi, Okuichigi, Hirakura, 23-XI-1957, M. Ohkura leg.; 1♂1♀ (MNHAH), Mie, Tsu-shi, Hirakura Forest, Mie University, M. Goto leg. (1♂, 23-XI-1957; 1♀, 14-V-1957); 1♂2♀ (KS), Mie, Mihama-cho, Kamiichigi, Mt. Sengensan, 7–8-VII-2004, K. & N. Kubota leg.; 1♀ (MNHAH), Mt. Hieizan, on the border between Shiga and Kyoto, 4-IX-1938, M. Ohkura leg.; 1♂ (MPIM), Osaka, Nose-cho, 5-XI-1972, A. Jusan leg.; 1♂2♀ (MPIM), Osaka, Minoh (1♀, 23-X-1981, M. Ishii leg.; 1♂, 23-IV-1982, T. Saito leg.; 1♀, 14-V-1988, T. Saito leg.); 1♂ (MPIM), Osaka, Osaka-shi, Himejima, 8.xi.1970, S. Jusan leg.; 1♂ (MNHAH), Osaka, Kawachinagano-shi, Kihata, Mt. Iwawakisan, 8-XII-1940, M. Ohkura leg.; 1♀ (KS), Mts. Kongôsanchi, on the border between Gose-shi, Nara and Chihayaakasaka-mura, Osaka, 27-V-2000, M. Wakabayashi leg.; 1♂ (MPIM), Hyogo, Inagawa-cho, Kamiakotani, 13-XI-1988, T. Saito leg.; 1♂ (MPIM), Hyogo, Kato-shi, Mt. Mikusayama, 21-XII-1975, T. Saito leg.; 1♂ (MNHAH), Hyogo, Mt. Hokkesan, 15-X-1967, M. Ohkura leg.; 2♀ (KCMI), Nara, Mt. Kasugayama, 1-VII-1973, T. Matsuda leg.; 1♂ (MNHAH), Nara, Uda-shi, Murôji, 7-V-1961, M. Ohkura leg.; 1♂1♀ (MNHAH), Nara, Mt. Kongôsan, 4-X-1964, M. Ohkura leg.; 1♂3♀, Mt. Ôdaigahara (2♀ (MPIM), on the border between Nara and Mie, 6-VIII-1956, T. Uemura leg.; 1♂ (MNHAH), Nara, 21-VII-1961, M. Ohkura leg.; 1♀ (MNHAH), Nara, 22-VII-1961, M. Ohkura leg.); 1♂ (MNHAH), Wakayama, Kitayama-mura, Shimazu, Kitayama-kyô, Y. Okuda leg. [no data on collection date]; 1♀ (MPIM), Wakayama, Kushimoto-cho, Shionomisaki, 28-III-1987, T. Saito leg.; 1♂ (KS), Tottori, Wakasa-cho, Wakasa Hyounosen Ski Area, alt. ca 700 m, 25-VII-1991, K. Kosaka leg.; 2♀ (KS), Hiroshima, Mihara-shi, Kuichôyoshida, alt. ca 500 m, 31-III-2016, T. Kosaka leg.; 2♂ (KS), Hiroshima, Higashihiroshima-shi, Saijôcho, Fukumoto, alt. ca 300 m, 9-VII-2006, K. Terada leg.; 6♂2♀ (KS), Hiroshima, Hiroshima-shi, Motoujina, alt. ca 50 m, 24-VII-1999, K. Terada leg.; 6♂2♀ (KS), Hiroshima, Akiôta-cho, Mt. Osorakanzan, alt. ca 1200 m, 22-VI-1996, K. Terada leg.; 7♂ (KS), Hiroshima, Hatsukaichi-shi, Yoshiwa, Ômukai-Chôjabara-rindô, alt. ca 800 m, 12-V-2003, K. Kakushima leg.; 1♀ (KMNH), Yamaguchi, Hikari-shi, Asae, 8-II-1992, K. Miyoshi leg.; 1♂ (KS), Tokushima, Kamikatsu-cho, Mt. Shibakoyayama, alt. 1220 m, 29-IX-2006, K. & N. Kubota leg.; 1♀ (EUM), Tokushima, Kamiyama-cho, Mt. Kumosayama, alt. 1150 m, 2–3-VIII-2021, R. Shiiba leg.; 1♂ (EUM), Tokushima, Miyoshi-shi, Mt. Tsurugisan, Minokoshi, alt. 1280 m, 30-V-2020, R. Shiiba leg.; 1♂ (EUM), Tokushima, Miyoshi-shi, Mt. Tsurugisan, Minokoshi, 16-X-2021, R. Shiiba leg.; 1♂ (CTD), Kagawa, Shôdoshima-chô, Shôdoshima Island, Mt. Hoshigajôyama, 15-X-1995, T. Dejima leg.; 1♂1♀, Kagawa, Takamatsu-shi, Shionoecho, Mt. Ôtakisan, (1♀ (KS), alt. 930 m, 26-IX-2007, K. Kubota leg.; 1♂ (EUM), 5-V-2020, R. Shiiba leg.); 6♂9♀ (EUM), Kagawa, Marugame-shi, Ayautacho, Tomikuma, 23-III-2024, K. Kuroda leg.; 3♂2♀ (EUM), Ehime, Saijô-shi, Mt. Kanpûzan, M. Motonaga leg. (1♂, 25–28-X-2013; 2♂2♀, alt. 1120 m, Kanpûzan-tsuidô, Fujinoishi, 16–29-X-2015); 1♂2♀ (EUM), Ehime, Tôon-shi, Mt. Saragamine (1♂, 29-V-2022, R. Shiiba & N. Azuma leg.; 2♀, 28-VI-2020, R. Shiiba leg.); 1♂3♀ (EUM), Ehime, Matsuyama-shi, Mt. Takanawasan, 9-VIII-2017, R. Shiiba leg.; 2♀ (EUM), Ehime, Uwajima-shi, Tsushimachosanzai, Mt. Hachimenzan, alt. 960 m, R. Shiiba leg. (1♀, 24-V-2021; 1♀, ~Kurosongawa River, alt. 780 m, Shimanto-cho, Kochi, 24–25-V-2021); 3♀ (EUM), Kochi, Mihara-mura, Mt. Imanoyama, 20–21-V-2022, R. Shiiba leg; 1♂1♀ (KMNH), Natsuyoshi, Tagawa-shi, Fukuoka, Y. Takakura leg. (1♂, 31-III-1975; 1♀, 28-III-1972); 1♀ (KMNH), Fukuoka, Mt. Fukuchiyama, 9-VII-1978, Y. Takakura leg.; 1♂ (EUM), Nagasaki, Isahaya-shi, Mt. Taradake, Kinsenji, alt. 828 m, 14–16-VIII-2022, R. Shiiba leg.; 3♂4♀ (KCMI), Kumamoto, Kumamoto-shi, Mt. Kinpôzan, alt. 500 m, 22-III-1977, T. Matsuda leg. (1♂1♀, 22-III-1977; 2♂1♀, 19-XI-1977; 2♀, 14-I-1979); 2♀ (EUM), Kumamoto, Yatsushiro-shi, Izumimachi, Hagi, Nigakoube Valley, alt. 1200 m, 21–23-VII-2021, R. Shiiba leg.; 1♂ (KS), Oita, Beppu-shi, Mt. Tsurumidake, 12-X-1999, T. Sota leg.; 1♀ (KMNH), Oita, Mts. Kujȗsan, 10-X-1978, M. Fukamachi leg.; 1♂ (KS), Oita, Taketa-shi, Mt. Sobosan, near Kôbaru Trailhead, 30-VI-2001 [no collector data]; 1♀ (KMNH), Oita, Hita-shi, Nakatsuemura, 15-III-1977, Y. Takakura leg.; 2♀ (EUM), Miyazaki, Takachiho-cho, Kamiiwato, Obiragoe, alt. 980 m, near Obira Tunnel, 9-V-2023, R. Shiiba leg.; 2♂3♀, Miyazaki, Tsuno-cho, Mt. Osuzuyama (1♂1♀ (KS), 4-V-2006, W. Toki leg.; 1♂2♀ (EUM), 3-V-2018, R. Shiiba leg.); 1♀ (EUM), Kagoshima, Minamiôsumi-cho, Satamagome, 25–27-VII-2021, R. Shiiba leg.
3.10. Pterostichus (Rhagadus) thorectoides Jedlička, 1958
-
Pterostichus thorectoides Jedlička 1958 [45]: 240 (original description; type locality: “Japan: Kobe”; Pt. (incertae sedis)); Jedlička 1962 [37]: 208 (description; Pt. (Badistrinus)); Morita et al. 2009 [49]: 208 (description; Pt. (Rhagadus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)).
-
Pterostichus ishiii Morita et al. 2009 [49]: 211 (original description; type locality: Mt. Yuzuruha-san, the Awaji-shima Island, Hyogo, Japan; Pt. (Rhagadus)); Bousquet 2017 [21]: 744 (catalogue; Pt. (Rhagadus)). Syn. nov.
Notes: P. thorectoides was described based on a single female specimen from “Kobe”. The holotype label contains more detailed locality information, including “Mont Roko,” which denotes the Rokko Mountains (Figure 2F). The comparative endophallus morphology (Figure 4A–R) and discriminant analysis (Figure 7) indicated that P. thorectoides and P. ishiii are conspecific. This result is consistent with other evidence. Specimens from the western foot of the Rokko Mountains and those from the type locality of P. ishiii have the same endophallus structure, and specimens with this type of endophallus are distributed across a range that includes the type localities of both P. thorectoides and P. ishiii. The synonymized P. ishiii was described from Awajishima Island off the coast of the Rokko Mountains. Morita et al. [49] justified distinct species status for P. ishiii based on its differences from P. thorectoides specimens including the female holotype. However, regarding male P. thorectoides, they examined only a single specimen from Okayama and did not examine specimens from the Rokko Mountains. Given that the collection site of the Okayama male specimen is located within the distribution range of P. thorectes (Figure 4S), this male is likely P. thorectes. This conclusion is consistent with the genital structure of the Okayama male specimen shown in Morita et al. [49]. In the line drawing of the aedeagus of this specimen, the boundary between sclerotized and membranous parts on the dorsal side was not depicted, implying that the focal male had an entirely sclerotized dorsal side of aedeagus, which is a diagnostic character of P. thorectes. However, the male genitalia of P. ishiii shown in line drawings by Morita et al. [49] are completely consistent with those of the true P. thorectoides in the nonsclerotized dorsal side of the aedeagus and the endophallus structure.
Diagnosis: This species is distinguished from other consubgeners by the following combination of characters: pronotum anterior angles barely produced; posterior half of the pronotum lateral margin arcuate; pronotum hind angles projecting acute angles; elytra with obvious spectral iridescence; left dorsal side of the aedeagal dorsum near the ostium weakly sclerotized; and ventral sclerite of the male endophallus boldly L-shaped in lateral view.
Description: Body length: ♂, 9.56–10.63 mm (mean ± SD: 10.2 ± 0.3 mm, n = 21); ♀, 8.91–10.68 mm (mean ± SD: 10.11 ± 0.63 mm, n = 8).
Dorsal habitus: Dorsal surface uniformly black (Figures 2C and 8G). Elytra with obvious spectral iridescence.
Pronotum: Anterior angles barely protruded (Figures 2C, 8G). Posterior half of the lateral margin arcuate. Hind angles projecting acute angles. Laterobasal impressions most deeply concave near the outer margin. The area between the laterobasal impressions between the lateral margins strongly convex.
Male genitalia: Aedeagus (Figures 4M, and 18A–F) with dorsal surface near the ostium sclerotized on both the right and left sides; on the left side, sclerotized part narrow and sclerotization weak at the level not visible in left lateral view; apical lamella laterally symmetrical, tongue-shaped, with the width greater than the length. Right paramere (Figure 18G–L) strongly curved; in lateral view, ventral margin arcuate, not bisinuate. Ventral sclerite boldly L-shaped in right lateral view (Figure 19A–T). Basal part of the ventral sclerite tongue-shaped, protruding toward the base of the endophallus; length of the protruded part about half the total length; total length about twice the width, and about equal to the width of the endophallus at the base of the aggonoporius; apex not bent. Digitate part of the ventral sclerite semi-ellipsoid, protruding perpendicularly to the basal part; total length about equal to the width, less than the width of the endophallus at the base of the aggonoporius, and about equal to the length of the protruded part of the ventral sclerite basal part. Left ventral lobe conical; in left lateral view of the endophallus, apex not extending beyond the distal margin of the ventral sclerite. Right ventral lobe large; basal part narrowly protruding toward the base of the endophallus, with surface strongly sclerotized; lateral sides distinctly protruding and discontinuous with surface near the base of the ventral sclerite; apex semi-ellipsoid, protruding toward the apex of the endophallus; upper surface of the middle strongly sclerotized in a band along the midline of the right ventral lobe; sclerotized parts of the basal part and upper surface not connected. Left dorsolateral lobe absent. Right dorsolateral lobe semi-ellipsoid, with diameter at the base larger than half the width of the base of apical lamella of the aedeagus. Left lateral lobe markedly large; length from the base to the apex more than 3/4 of the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view; surface not sclerotized. Right lateral lobe semi-ellipsoid; length from the base to the apex about 1/3 of the width of the endophallus, which is measured at the position of the base of the left lateral lobe in endophallus dorsal view. Apical lobe absent.
Distribution: Northwestern Chubu, northeastern Kinki, and eastern Chugoku Districts (Figure 4S as morphotype C). The easternmost confirmed collection site is Mt. Tsurugisan in the Hida Mountains (Toyama, Banbajima), the westernmost is Mt. Daisen (Tottori), and the southernmost is Awajishima Island (Hyogo).
Specimens examined: Type specimen: P. thorectoides holotype ♀ (NMP), “Mont Roko \ Kobe, |Japan \ Dr. Baum lgt \\ TYPUS \\ Pterostichus \ thorectoides \ sp.n. \ det.ING.JEDLIČKA \\ Pt. (Rhagadus) \ thorectoides \ Jedlička \ Det.S.Morita, 2004”.
Specimens from type localities (not types): 2♂ (EUM), Hyogo, Minamiawaji-shi, Awajishima Island, Mt. Yuzuruhasan [type locality of P. ishiii], 29–30-VI-2019, R. Shiiba leg.
Other specimens: 2♂2♀ (KS), Toyama, Kamiichi-machi, Iori, Banbajima, alt. 800 m, 27–29-IX-2002, K. Sasakawa leg.; 1♂ (MNHAH), Osaka, Daitô-shi, Mt. Iimoriyama, 30-XI-1952, M. Ohkura leg.; 1♂ (MPIM), Mt. Myôkensan, on the border between Osaka and Hyogo, 29-IV-1963, [no collector data]; 12♂4♀ (MNHAH), Hyogo, Kôbe-shi, Suma-ku, Shirakawa, M. Nagai leg. (2♂, 7-XII-1978; 1♂1♀, 26-I-1979; 6♂3♀, 22-II-1979; 3♂, 28.iii. [no data on collection year]); 3♂2♀ (KS), Tottori, Hôki-cho, Mt. Daisen, alt. 780 m, 11–12-VI-2005, H. Ikeda leg.
3.11. Morphological Phylogenetic Analysis
The following eight species were treated as ingroups based on the comparative morphological analysis results: the five Japanese endemic species described above; P. microcephalus and P. nimbatidius (Figures 20A–D,F–H, 21A–F,H–M,O–S and 22A–Q), which are distributed both on the Asian continent and Japan; and the continental species P. solskyi (Figures 20E, 21G,N,T,U, and 23A–G). This study includes “P. (R.) kimurai Morita, 1995 [50]” which was described from Nakanoshima Island, a small island off the coast of Kyushu, as syn. nov. of the widely distributed species P. microcephalus (type locality: “Japan”; Motschulsky 1960). Although “P. (R) kimurai” was apparently conspecific to P. microcephalus, the species has been neglected and has never been formally synonymized. This synonymization is based on the confirmation that both the external and genital structures of a P. kimurai topotype male (Figures 20C,H, 21E,L, and 22L) are identical to those of P. microcephalus specimens from various localities in Japan, from Hokkaido in the north to Kyushu in the south (Figures 20A,B,F,G, 21A–D,H–K, and 22A–K). These eight ingroup species share the same endophallus structure, whose homology is easily traceable. In contrast, P. glabripennis (Figure 25A–O), which has been treated as a member of Rhagadus, was found to have an endophallus structure quite different from those of other members of Rhagadus. Some external and non-endophallic genital structures that are common to true members of Rhagadus (e.g., two pairs of pronotum basal depressions with distinctly punctate surfaces, distinct elytral surface, and apically elongate right paramere) are also absent in P. glabripennis. Therefore, P. glabripennis was concluded not to belong to Rhagadus and was not used in phylogenetic analysis. The two Gastrosticta species examined as possible outgroups (Figure 26A–R) also had an endophallus structure quite different from those of Rhagadus, even in its overall shape, and the homology of endophallus structures between the two subgenera could not be identified (Figure 26F–H,P–R). Therefore, Gastrosticta was found not to be related to Rhagadus and the two species were not used as outgroups. As a result, P. eximius rishiridakensis, a member of subgenus Petrophilus (s. lat.) that was sister to Rhagadus in molecular phylogenies [33, 34], was used as an outgroup in the final analysis (for its characters, see [51]). Information on specimens examined for these species is provided in Table S1.

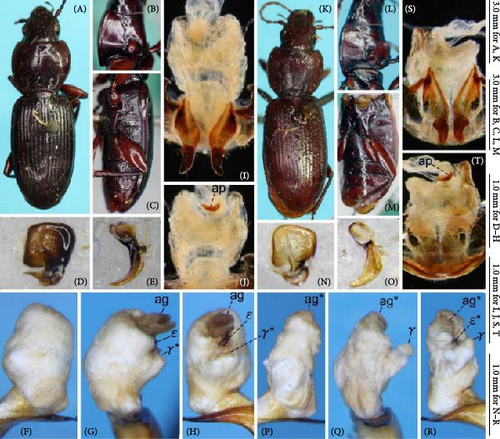
Nineteen morphological (15 male genital, 1 female genital, and 3 external) characters were found to show variation within the subgenus and were used in the analysis (Tables 1 and 2). Only one most parsimonious tree was obtained, with 39 scores (Figure 27). Two species, P. microcephalus and P. nimbatidius, formed a clade sister to the remaining six species. Within the clade of the remaining six species, P. takaosanus, P. polygenus, P. thorectes, and P. thorectoides diverged in this order and were paraphyletic to P. negylopus + P. solskyi. The monophyly of each clade was supported by 1–3 synapomorphies and moderate to high bootstrap values.
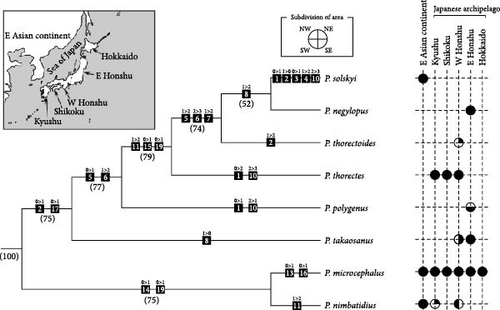
| Character number: Character: evaluation and coding |
|---|
| 1: Left ventral lobe: protruding in a conical shape (0); protruding in an elongated hemispherical shape, with diameter less than 2/3 of the endophallus width at the base of the aggonoporius, and apex not extending beyond the distal margin of the ventral sclerite in endophallus left lateral view (1); protruding in an elongated hemispherical shape, with diameter more than 2/3 of the endophallus width at the base of the aggonoporius, and apex extending far beyond the distal margin of the ventral sclerite in endophallus left lateral view (2); protruding widely, with diameter more than 2/3 of the endophallus width at the base of the aggonoporius, and apex bifurcate (3). |
| 2: Size of left lateral lobe: small, with length less than 1/3 of the endophallus diameter (0); medium in size, with length ca. half of the endophallus diameter (1); large, with length slightly less than the endophallus diameter (2). |
| 3: Sclerotization of left lateral lobe: absent (0); present (1). |
| 4: Right lateral lobe: absent (0); small, with length less than half of the endophallus diameter (1); large, with length more than half of the endophallus diameter (2). |
| 5: Right dorsolateral lobe: absent (0); present but rudimentary; markedly smaller than the right lateral lobe (1); present, similar in size to the right lateral lobe (2). |
| 6: Shape of right ventral lobe: absent (0); weakly swollen, with both lateral margins and gonopore side margin not protruding (1); swollen, with gonopore side margin but not lateral margins protruding (2); greatly swollen, with both lateral margins and gonopore side margin distinctly protruding (3). |
| 7: Number of sclerotized parts on the right ventral lobe: zero (0); one (1); two (2). |
| 8: Basal part of the ventral sclerite: rudimentary, smaller than half of the digitate part (0); about the same size as the digitate part, with length and width nearly equal (1); apparently larger than the digitate part, with length more than twice the width (2). |
| 9: Digitate part of the ventral sclerite: undifferentiated, raised only slightly from the basal part (0); differentiated a protruding structure clearly distinct from the basal part (1). |
| 10: Aedeagal dorsum near the ostium: sclerotized only near the right lateral side (0); sclerotized only on the right side from the midline (1); entirely sclerotized, with the left side weakly sclerotized (2); entirely sclerotized, with the left side strongly sclerotized (3). |
| 11: Length of apical lamella, evaluated by length/width ratio where length a and width b are measured, as shown in Figure 9A: long, with length/width >1.3 (0); moderate, with length/width 1.0–1.1 (1); short, with length/width <0.8 (2). |
| 12: Left dorsolateral lobe: absent (0); weakly swollen (1); distinctly swollen, with narrow apex (1). |
| 13: Apical lobe: absent (0); present (1). |
| 14: Ventral margin of the right paramere in lateral view: simply arcuate, not bisinuate (0); bisinuate (1). |
| 15: Degree of curvature of the right paramere, evaluated by the angle between the line segment between the apex of dorsal process and that of the paramere and the tangent line from the apex of the dorsal process to the inner margin of the paramere (Figure 9G): weak, with angle <20° (0); strong, with angle >20° (1). |
| 16: Dorsolateral ensiform setae of gonocoxite 2 of the female genitalia: two (0); three (1). |
| 17: Tempora: not developed, with anteroposterior length less than 1/3 of the anteroposterior length of the eye (0); weakly developed, with anteroposterior length more than half of the anteroposterior length of the eye (1). |
| 18: Pronotum laterobasal impressions: uniformly shallow throughout, continuous with the lateral margin, not forming distinct convexity between the impression and margin (0); uniformly shallow throughout, discontinuous with the lateral margin, forming distinct convexity between the impression and margin (1); distinct, most deeply concave near the outer margin, discontinuous with the lateral margin, forming distinct convexity between the impression and margin (2). |
| 19: Elytral surface: without spectral iridescence (0); with obvious spectral iridescence (1). |
| Taxon | Characters |
|---|---|
| P. (Rhagadus) microcephalus | 00010 11102 12110 1001 |
| P. (R.) negylopus | 01012 32212 20001 0121 |
| P. (R.) nimbatidius | 00020 11102 21010 0001 |
| P. (R.) polygenus | 11011 21111 a0000 0120 |
| P. (R.) solskyi | 10122 32213 20001 0121 |
| P. (R.) takaosanus | 01020 11012 a0000 0110 |
| P. (R.) thorectes | 21011 21113 20001 0121 |
| P. (R.) thorectoides | 02012 32112 20001 0121 |
| P. (Petrophilus) eximius rishiridakensis | 30000 00??0 10000 0000 |
- Note: “a” in character indicates polymorphic character state, 0 and 1, which is coded as “(01)” in data for TNT.
3.12. Key to Species of the Subgenus Rhagadus Treated in This Study
- 1.
Pronotum hind angle rounded (Figure 20D; [52], fig. 10)…………………….P. nimbatidius (Chaudoir)
- –
Pronotum hind angle angled (Figures 8 and 20A–C,E)……………………………………………………….2
- 2.
Pronotum less cordate; pronotum laterobasal impressions rather shallow and continuous with lateral margins, not forming distinct convexity between the impression and margin (Figure 20F–H); ventral margin of the right paramere bisinuate in lateral view (Figure 21H–L); gonocoxite 2 of the female genitalia with three dorsolateral ensiform setae (Figure 21P,Q)………………………..P. microcephalus (Motschulsky)
- –
Pronotum cordate; pronotum laterobasal impressions discontinuous with the lateral margin, forming distinct convexity between the impression and margin (Figures 8, and 20E); ventral margin of the right paramere simply arcuate, not bisinuate in lateral view (e.g., Figure 9G); gonocoxite 2 of the female genitalia with two dorsolateral ensiform setae (e.g., Figure 9R)……………………………………………………………………………..3
- 3.
Apical lamella of the aedeagus dorsally hooked on both lateral sides (Figure 9A–F)……………………………………………………………..P. polygenus Bates
- –
Apical lamella of the aedeagus without hooked projection on lateral sides (e.g., Figure 11A–F)………..……4
- 4.
Pronotum laterobasal impressions uniformly shallow throughout (Figure 8C,D; [23], figs. 1, 2); elytral surface without spectral iridescence (Figure 8C, D); apical lamella long, with length/width >1.0 (Figure 11A–F)………………………………….. P. takaosanus Habu
- –
Pronotum laterobasal impressions more deeply concave near the outer margin than the inner margin (e.g., Figure 8A); elytral surface with obvious spectral iridescence (e.g., Figure 20B); apical lamella short, with length/width <0.8 (e.g., Figure 13A)……………………….………………………………………5
- 5.
Left side of aedeagus dorsum near the ostium strongly sclerotized, with the sclerotized area wide at the level distinctly visible in left lateral view (e.g., Figure 16P)……………………………………………………….6
- –
Left side of aedeagus dorsum near the ostium weakly sclerotized, with the sclerotized area narrow at the level barely visible in left lateral view (e.g., Figure 14P)………………………………………………….…….7
- 6.
Body length >11 mm; degree of sclerotization of aedeagus dorsum near the ostium the same on both lateral sides (Figure 21G)…………………………………….…………………………………..P. solskyi (Chaudoir)
- –
Body length <11 mm; degree of sclerotization of aedeagus dorsum near the ostium weaker on the left side than on the right side (Figure 15A–F)………………………………………………………..…..P. thorectes Bates
- 7.
Pronotum hind angles projecting acute angles; posterior half of the pronotum lateral margin arcuate (Figure 8G; [49], fig. 3)…………………….P. thorectoides Jedlička
- –
Pronotum hind angles obtuse; posterior half of the pronotum lateral margin almost straight, only slightly arcuate (Figure 8E)…………………………………………………………………………..P. negylopus sp. nov.
4. Discussion
4.1. Methodology
Clarifying the identity of type specimens with insufficient morphological and locality information and developing novel procedures for this task are among the most important issues in taxonomy. This study tested the utility of a novel procedure to identify such problematic type specimens clearly based only on morphological data, using the carabid subgenus Rhagadus as a model. Its type specimens include females with insufficient locality information, which are particularly difficult to identify accurately. A combination of the comparative morphology of the endophallus and geometric morphometrics of external structures was applied to identify female Rhagadus type specimens with insufficient locality information unambiguously, demonstrating the utility of the proposed procedure. Although both morphological and morphometric methods have been used in previous insect taxonomic studies, most of these studies addressed general taxonomic issues using only one of the two methods. Exceptionally, Sasakawa [27] and Sasakawa [28] used both methods to discriminate and subsequently describe a new species; however, these studies used external geometric morphometrics only to obtain data that supported the results obtained by comparative endophallus morphological examination; thus, the taxonomic conclusions would not change even if the external morphometrics results were inconclusive. In contrast, the present study conducted geometric morphometrics of the female pronotum and subsequent discriminant analysis based on morphotype classification results obtained through comparative morphological examination of the endophallus. The successful application of this innovative procedure provides a method for the effective combined use of comparative morphology of the endophallus and external geometric morphometrics.
Several challenges remain to be addressed in future studies. Regarding the target species, it is necessary to evaluate the utility of the proposed procedure for taxa with distribution patterns different from those examined in this study. The three species treated here, P. negylopus, P. thorectes, and P. thorectoides, are parapatrically distributed. Therefore, studies involving sympatric species, for example, would be useful. In sympatric species pairs, interspecific differences in external morphology may differ from those observed in parapatric species, which could affect the accuracy of species discrimination.
Of the two methods, comparative morphology of the endophallus could alternatively be replaced by another morphological analysis method. For example, in insects, sclerotized structures of the male genitalia such as the aedeagus (median lobe) and parameres are generally easily observed (versus the endophallus); in some taxa, their external shape is a useful species diagnostic character (e.g., [53]). Furthermore, unlike the membranous endophallus, these sclerotized structures can be examined without specimen dissection, using new technology such as micro-computed tomography, providing an advantage when non-destructive investigation of type specimens is desired [54]. Other potential alternative characters include male secondary sexual traits other than weapon traits. In some insects, males have secondary sexual traits in body parts that may be associated with mating behavior (e.g., the last abdominal segment [55]). Although the morphology of these sexual traits may already have been described, their variation is rarely examined using methods such as morphometrics. Morphometrics may detect distinct interspecies differences in secondary sexual traits, in a manner similar to primary sexual traits such as the genitalia. Future research should test the utility of these potential alternative traits, in addition to searching for other useful morphological traits that can be examined non-destructively.
In contrast, no method appears to offer an alternative to geometric morphometrics of external structures. Geometric morphometrics is superior to other methods, including traditional linear morphometrics, not only in its high detectability but also in its visualization of shape differences [26]. Indeed, the visualization of morphological variation conducted in this study found several variations that have not previously been recognized as diagnostic characters in Rhagadus [22, 23, 49]. At this stage, geometric morphometrics is probably the most recommended method for the analysis of type specimens with subtle differences from related species. One advantage of morphometrics is that images, measurements, and coordinates obtained in one study can be used in subsequent studies, although this practice requires that they are obtained under the same (i.e., standardized) conditions (reviewed in [56]). The main condition that requires standardization in geometric morphometrics is the photographic angle of images used to acquire data. Despite this importance, many studies do not specify the photographic angle. Thus, the following two types of photographic angle are proposed as standardized conditions for dorsal-view images of the pronotum, which is widely used in geometric morphometrics of the Coleoptera, which includes Rhagadus. The first is the angle perpendicular to the line connecting the middle of the posterior margin and the middle of the anterior margin; this angle was used in this study; many previous studies are thought also to have used this angle, judging from their use of the term “dorsal” (e.g., [57, 58]) and the images provided. An advantage of images taken at this angle is that the pronotum length, which is a traditional measurement for this body part, can be calculated from scaled images and coordinates. The second is the angle perpendicular to the plane including the anterior and posterior angle apices of both lateral sides, which has been applied in some previous studies (e.g., [6, 28, 56, 59]). Compared with the first angle, images taken at this angle can capture the contour of the lateral margin more easily in a pronotum with lateral margins sloping strongly downward anteriorly; this shape of pronotum is often found in Coleoptera. Accumulation of images or coordinate data using these two standardized conditions would contribute to resolving many taxonomic problems, including the female holotype problem.
4.2. Phylogeny and Evolution of Rhagadus
The phylogenetic position of subgenus Rhagadus within the genus has remained unresolved despite previous molecular phylogenetic studies [28, 33, 60]. In this study, subgenus Gastrosticta, which was suggested as a possible sister group to Rhagadus by Bousquet [31], was initially examined as an outgroup candidate, but was found to be unrelated to Rhagadus. Judging from its straight endophallus and the results of a previous study that mapped endophallus morphology to a phylogenetic tree [33], Gastrosticta is assumed to hold a more basal position than Rhagadus within the genus. The apically bifid lobe (γ in Figure 26G,H,Q,R) and the plate-shaped sclerite between the lobe and the gonopore on its endophallus (ε in Figure 26G,H,R) likely represent autapomorphies of Gastrosticta and will therefore be useful for future phylogenetic studies of this subgenus. The Chinese species P. glabripennis, which has been treated as a member of Rhagadus, was also found to be unrelated to Rhagadus. Judging from the external and genital structures of this species and available information of other Pterostichus subgenera, P. glabripennis appears to belong to the Chinese endemic subgenus Circinatus Sciaky or its related groups [55]. Thus, this study failed to specify a sister group to Rhagadus. Nevertheless, endophallus morphological examinations, together with information from previous reports on the endophalli of other Pterostichus subgenera, revealed two traits that will be useful in searching for a sister group. One is the filamentous apex of the aggonoporius. Within Pterostichus, and possibly within the Carabidae, this structure has been found only in Rhagadus. The other is the sclerotized structure of the endophallus (sclerotized right ventral lobe surface and ventral sclerite); however, care must be taken to determine their homology due to the presence of similar structures in other subgenera.
In the obtained phylogeny, the ingroup species were divided into two clades, one consisting of P. microcephalus and P. nimbatidius, and the other consisting of the remaining six species. Some results for the former clade are noteworthy. For example, P. microcephalus has a wider distribution (East Asia including the entire Japanese Archipelago, versus only western Japan and South Korea in P. nimbatidius; [21]) and more autapomorphies than the other examined species (Figure 27), implying that P. microcephalus is more derived than P. nimbatidius. Moreover, intraspecific variation was observed in both the male and female genitalia of P. microcephalus (Figures 21A–E,H–L,O–Q and 22A–L), implying that geographic differentiation has occurred in this species. These findings will provide clues to the differentiation process of Rhagadus; however, the numbers of species and specimens of this clade examined in this study are insufficient for further discussion at this stage.
Among the six species of the other clade, P. takaosanus and P. polygenus, which form the basal lineages, are mainly distributed in eastern Honshu, but the western margins of their distribution reach Chubu District. Additionally, both P. takaosanus and P. polygenus were confirmed to show geographical differentiation within Chubu District, although the variants were not distinguished taxonomically in this study. Regarding three Japanese species that diverged subsequently in the phylogenetic tree, the distribution of P. thorectes + P. thorectoides and that of P. negylopus were parapatric, and their distribution boundary was also in Chubu District (Figure 4S), near the western margins of the distribution of the two basal species (P. takaosanus and P. polygenus) (Figure 24). The fact that the distribution margins of various species, resulting distribution boundaries among species, and geographical variation in some species were all found in Chubu District implies that the main differentiation of this clade occurred in Chubu District. This hypothesis is consistent with current knowledge on another carabid group, subgenus Ohomopterus Reitter (genus Carabus Linnaeus). In some species groups of Ohomopterus (albrechti and insulicola species groups), both morphological and molecular evidence have demonstrated that the main differentiation occurred in Chubu District [61, 62]. Because both Ohomopterus and Rhagadus are flightless, their differentiation is thought to have been caused mainly by geographic factors. The same geographic factors may be associated with their differentiation.
Both P. negylopus, which is endemic to eastern Honshu, and continental P. solskyi, were shown to be sister taxa; this result is unsurprising, given the similarity of their endophallus character states. The reconstruction of character state changes on the tree showed that P. negylopus has no autapomorphies (Figure 27). Considering that a limited number of traits were used in the present phylogenetic analysis and that traits not used may be autapomorphies, this result is interpreted to indicate that P. negylopus is morphologically similar to the common ancestor of P. negylopus + P. solskyi rather than that P. negylopus is the ancestral species. The ancestral species of P. negylopus + P. solskyi is assumed to have invaded the continent from the Japanese Archipelago, followed by speciation to P. solskyi, but how and when that invasion occurred remains unresolved. Given that P. solskyi has many autapomorphies compared to other species, there may be undiscovered species with character states intermediate between P. negylopus and P. solskyi. The P. solskyi specimens examined in this study were collected from the Korean Peninsula and an adjacent island (Table S1), and Rhagadus specimens from the continental region directly across the Sea of Japan from eastern Honshu (distribution area of P. negylopus) were not examined in this study. From a biogeographical perspective, any intermediate species between P. negylopus and P. solskyi are likely to be distributed in this opposite continental region.
Dissections of the female genitalia showed irregular melanized patches on the inner surface of the vagina in some specimens (e.g., Figure 13O), although the size of the patches varied among individuals. Given experimental evidence obtained from other insects, including a species of Carabidae (e.g., [63–65]), these patches likely resulted from wounds caused by males during mating. Indeed, among male endophallus structures, the sclerites on the right ventral lobe and the ventral sclerite have chitinized scaly projections on their surface, implying that these projections may damage the female membranous vagina. In females, the inner dorsal surface of the vagina, to which the right ventral lobe and ventral sclerites of the male endophallus would correspond during mating, was more strongly sclerotized than other parts of the inner vaginal surface, and thus appears to function as a defensive trait against harm caused by the corresponding male genital structures. This hypothesis, in which parts of the male and female genital structures behave as harmful and defensive traits, respectively, is consistent with observations in other congeneric species. For example, in species of subgenera such as Pseudomaseus Chaudoir, Nialoe Tanaka the raptor species group, Orientostichus Sciaky & Allegro, and Circinatus, the male has harmful sclerotized structures on the endophallus, and the female vagina has sclerotized areas and/or irregular melanized patches on its inner surface [55, 66–68]. In contrast, in species of subgenera such as Chinapterus Berlov, Nialoe the hisamatsui species group, and Gastrosticta, where the male lacks harmful endophallus structures, the female vagina has neither sclerotized areas nor irregular melanized patches on its inner surface ([69, 70], this study). Harmful and defensive genitalia characters in the male and female, respectively, are typical of species that engage in sexual conflict, and sexual conflict is considered to be a factor promoting rapid evolution of reproductive traits and resulting speciation (e.g., [71–73]). Thus, in addition to geographic factors, sexual conflict may have contributed to the development of diversified genitalia and species-level diversity in subgenus Rhagadus.
Conflicts of Interest
The author declares no conflicts of interest.
Funding
There was no direct funding for this research.
Acknowledgments
The author would like to thank H. Ikeda (University of Tokyo, Tokyo), S. Inoue (Fukui), R. Ishikawa (Tokyo), H. Itô (Niigata), J.-K. Kim (Korea), K. Kubota (Heisei International University, Saitama), the late K. Matsumoto (Hachioji, Tokyo), R. Nakamura (University of Tokyo, Tokyo), N. Nakaya (Institute of Science Tokyo, Tokyo), S. Niwa (Japan Wildlife Research Center, Tokyo), H. Nishida (Aichi), Y. Okuzaki (Osaka Metropolitan University, Osaka), R. Shiiba (Hyogo), T. Shimizu (University of Tokyo, Tokyo), K. Shindo (Japan), T. Sota (Kyoto), Y. Takami (Kobe University, Kobe), K. Terada (Hiroshima), W. Toki (Nagoya University, Aichi), M. Ujiie (Chiba), M. Wakabayashi (Chiba), and O. Yamaji (Okayama) for offering specimens; T. Dejima (Kagawa, Japan), C. Germann (NMB), J. Hájek (NHM), H. Ikeda (KCMI), Y. Minoshima (KMNH), H. Nakamine (MPIM), M. Nakamura (Hokkaido University, Japan), A. Okuda (Hokkaido University, Japan), D. Telnov (NHMUK), and T. Yamasaki (MNHAH) for the loan of specimens under their care. Thanks are also due to M. Zilioli (Museo di Storia Naturale, Milano, Italy), who examined the museum collections for the current study, M. Ohara (HUM) and H. Yoshitomi (EUM), who accepted some specimens to be deposited in the museum collections, and L. Toledano (Italy) for his help in translating a literature. Specimens from Sado Island were obtained in the Monitoring Sites 1000 Project launched by the Ministry of the Environment, Japan.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Data are available within the article or its supporting information.




