Exploring Grip, Voice, and Electromyography Signals to Initiate Elbow Flexion With a Wearable Robot Arm
Abstract
Background: Although several signals have been proposed to control the activation timing of wearable robots, there is no consensus on the suitability of these signals in terms of their ability to promote effective cooperation and smooth device activation.
Objective: This study investigated the effects of biomechanical (grip forces and voice commands), bioelectrical (electromyography [EMG]), and external sound (EX) signals on elbow flexion to trigger a wearable robot arm.
Methods: Constant torque control was applied to a single-degree-of-freedom (DOF) wearable robot arm, which operated within a range of 30°–130° of elbow flexion. Activation of the wearable robot arm was initiated using a grip force applied to a handle from either hand, the verbal command “go,” EMG signals, and an EX cue, while lifting a load equivalent to 15% of maximum voluntary contraction (MVC).
Results: Prior to initiating the wearable robot arm, the grip force used to activate the movement using the hand on the side of the assisted arm significantly induced activities of the biceps brachii (BB) (%MVCBB) by approximately 6%–10%MVCBB compared to bioelectrical and biomechanical signals. Nonetheless, after assistive forces were generated, the %MVCBB elicited during the initial phase of elbow flexion was similar to other activation signals. In contrast, EMG-induced activation increased the triceps brachii (TB) activity (%MVCTB) by approximately 7.2%MVCTB during elbow flexion, resulting in the highest joint angular acceleration during the initial phase among all signals.
Conclusion: These findings suggest that the grip force applied from the assistive hand was the most effective at triggering the wearable robot arm and may enhance muscle preparation and cooperation with the device more effectively.
1. Introduction
Wearable assistive devices have been engineered in multiple control systems to satisfy the increasing demand for motion assistance. Wearable assistive devices enhance the ability to provide natural movement through single- and multi-degrees-of-freedom (DOF) actuators. These devices can be developed as exoskeleton products, designed with passive to active control mechanisms, and are primarily used for rehabilitation [1] and worker support [2]. Moreover, wearable robots and devices, ranging from limb-supporting systems to full-body suits equipped with advanced sensors and actuators, are primarily used in industrial, military, and healthcare applications, where human effort and self-initiated control are required [3].
In the early development, wearable robots were mechanically designed as simple controllers for basic functions. Traditional motor systems provide specific control, which requires precise user cooperation and limits the flexibility of operations, leading to lower user acceptability and adaptability of wearable robots [4]. Movements with wearable robots are mainly aimed at reducing the physical effort and users’ energy expenditure of muscle contraction from organic actions using several external control systems, such as switches and buttons, and are programed via actuators that reflect human joints [5].
Originally, wearable robot use involved artificially coordinating the user’s movements with a device to synchronize their motion and force activation timing. Mobilization of wearable robots mostly supports exact timing, and a trigger for <100 ms ensures smooth assistance [6], while natural movement responses to sound and visual stimuli occur at 140–160 ms and 180–200 ms, respectively [7]. Natural movement control is primarily mediated by sensory inputs from proprioceptors, tactile receptors, and muscle spindles. These inputs provide continuous feedback to the central nervous system, enabling the anticipation of cognitive movements and the transmission of signals back to the muscles to adjust and refine these movements [8]. Thus, the self-intentional response time in natural movements is slower than that in wearable robots.
Recently, the hybrid assistive limb (HAL) was developed to provide an intuitive user experience and enrich the natural movements and capabilities of the user through the integration of robotics and human sensory input, referred to as biocybernetic control [9]; this includes biosignal-triggered wearable robots [10]. In the 2000s, myoelectric controls gained popularity and significantly improved movement expertise, which occupies the role of electrical signals from muscle contraction, in which electromyography (EMG) electrodes need to be attached and located at the closest or related to the aiming movements of muscle action [11, 12].
Although EMG-triggered wearable robots deliver powerful outcomes to assist and enhance mobility, EMG signals distribute artifact susceptibility via noise interference and individual variability, highlighting the difficulties of implication between users owing to the individualized anatomy, physiology, and cognition [13]. Jiang et al. [14] reported extensive variability in EMG signals across different users. The challenge of handling complex EMG signals and the time-consuming calibration process are crucial for advancing technologies such as EMG signal-processing algorithms and other reliable signal systems [15].
Using the values of biomechanical feedback, such as joint angles and velocity, force sensors, eye gaze, voice commands, and accelerometers, to trigger and control wearable robots is a well-researched area that provides robustness and complementarity to EMG for wearable robot control [8]. Tripathy et al. [16] validated the feasibility and capability of a voice-activated upper-body exoskeleton to reduce stress on the arms and shoulders of prolonged lifting workers. Recently, Sanders and Reinkensmeyer [17] corroborated the improvement in grip-lift-hold performance using pinch grip forces that effectively facilitated the hand exoskeleton. These researchers agreed with the ease of using biomechanical indications on account of straightforward program algorithm installation, simple sensor implementation, low-cost and durable sensors, uncomplicated calibration, and broad user accessibility [18]. As suggested by Losey et al. [19], feedback from the user challenges their cognitive processing to initiate and interact with wearable robots, particularly kinematic variables, simultaneously with motion intent, resulting in the appropriate completion of the movement [20].
Accordingly, promoting user intentions to interact with devices during the initiation phase is essential for achieving more precise movement outcomes. Voluntary initiation of user biomechanical signals has the potential to enhance this intention and effectively activate these devices. This study focused on exploring the biomechanical and bioelectrical signals to initiate a wearable robot arm. This study aimed to identify muscle activities and user perceptions during elbow flexion using a wearable robot arm triggered by EMG signals, grip forces, voice commands, and start sound cues.
2. Methods
2.1. Participants
A total of 14 healthy participants (Table 1) were enrolled as one sample group in this study. Participants were instructed to refrain from engaging in strenuous upper limb exercises for at least 24 h before the start of the study. Demographic and anthropometric data were collected after obtaining signed consent from the participants. All individuals exhibited right-hand dominance and reported no upper-limb or trunk pain in the preceding year. Ethical approval was granted by the Ethics Committee of the Faculty of Design at Kyushu University (approval number: 428).
Participants (Total n = 14) |
Age (years) |
Weight (Kg) |
Height (m) |
BMI (Kg/m2) |
Lower arm lengths (m) |
|---|---|---|---|---|---|
| Male (n = 8) | 25.8 ± 4.3 | 68.3 ± 9.6 | 1.75 ± 0.01 | 22.3 ± 1.7 | 0.27 ± 0.03 |
| Female (n = 6) | 27.1 ± 4.1 | 54.4 ± 5.5 | 1.60 ± 0.05 | 21.3 ± 2.6 | 0.22 ± 0.01 |
- Note: Descriptive data are presented as mean ± standard deviation.
- Abbreviations: BMI, body mass index; Kg, kilogram; m, meter.
2.2. Wearable Robot Arm and Control
In this study, the wearable arm was designed to function as a one-DOF robot arm providing an assistive force for elbow flexion (Figure 1). The seat position and shoulder guard were adjusted such that the rotational axis of the elbow joint was aligned with that of the robot arm. The robot arm was attached to the wrist of the participant at the TODO (wrist bony landmark), accounting for the difference in arm length of the participants.
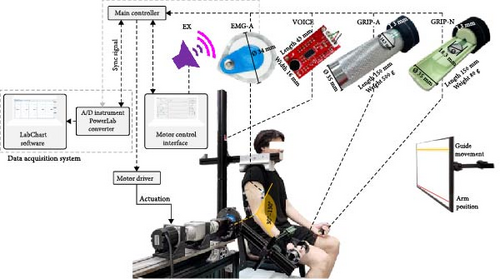
The microcontroller receives inputs from all sensors and initiates movement once the selected sensor exceeds a predefined threshold. Subsequently, the microcontroller sends a synchronizing signal to the data acquisition system to ensure that the recorded data is in synchronization with the motor’s movement and control signals. Moreover, the reference screen shows the red line as the arm position and the yellow line as a guided movement line. A guided movement line ends at the center of a yellow error band.
A constant assistive force was delivered using a brushless DC servomotor (M-3411P-LN-08D, Teknic, Inc.) controlled by a TMS320F28379D launchpad microcontroller connected to a BOOSTXL-3PHGANINV motor driver (Texas Instruments, USA). A switch-mode power supply (S8VK-G48048, Omron) and shunt regulator (DSR 70/30, Maxon) provided power to the device using the control software. Synchronization between the EMG signals and the wearable robot arm was achieved by synchronizing the signal of the microcontroller to an analog-to-digital (A/D) converter (PowerLab 16/30, ADInstruments, Dunedin, New Zealand). This was achieved by regulating the current provided to the motor using a motor driver. Additionally, a rotary encoder embedded in the servomotor was used to track the angle of the robot arm. This angle was used to indicate the current position of the arm on the reference screen.
2.3. Experiment Procedure
Prior to the tasks, EMG electrodes were attached to 1/3 of the origin of the biceps brachii (BB) and triceps brachii (TB). The maximum voluntary contraction (MVC) of the biceps and triceps was determined (MVCBB and MVCTB, respectively). The MVC was measured with the forearm supinated at 90° with 90° elbow flexion. To the force applied at MVC during elbow flexion, a soft strap was used to attach the right wrist of the participant to a tensile sensor (T.K.K. 1269f; Takei Scientific Co., Japan). The MVCBB was recorded as the participant pulled upward against an inelastic chain connected to the floor. MVCTB was measured as the participant pushed downwards against an inelastic chain connected to the above-right shoulder stand. A circuit of 5 s contraction holding and 120 s resting for three trials was compiled, and the center 3 s was analyzed.
A load equivalent to 15% of the MVCBB was used in this study [21]. The load was in the form of a dumbbell with an adjustable weight. The specified starting position at 30° and ending position at 130° of elbow flexion was based on the task guidance displayed on the monitor screen. An error band at the target angle was established at the concluding position, set at ±2 standard deviations (SDs) for a 95% confidence interval. The rise time was determined by considering the natural motion range of healthy adults at 30–130°elbow flexion and was deliberately set at 1.12 s. The assistive force was provided only for a fixed amount of time, and no assistive force was provided for the final phase of movement. This was done to account for the momentum of the robot arm and dumbbell and to minimize overshoots. The assistive forces were provided at 50% of the dumbbell load from the beginning to 75% of the rise time, corresponding to 0.84 s from the chosen start time (Figure 2).
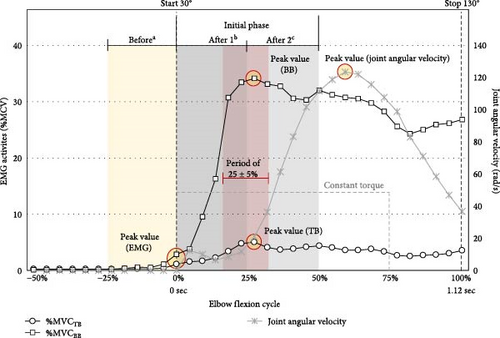
In the preparation phase, participants were instructed to maintain a dumbbell load equivalent to 15% MVCBB, allowing for the relaxation of the support. After receiving the readiness signal from the researcher, the participants were directed to intentionally lift their arms (represented by the red line) to match the yellow task guidance line on the feedback screen at their own pace and stop within the target yellow band (Figure 1). A trial was deemed complete when the participants could match their position reference line (depicted in red) with the task reference line (depicted in yellow) as much as possible and stop within the designated error band (illustrated by the light-yellow area). All participants were allowed to practice all the operations before the actual experiment. Confirmation that the participants gave feedback to reach the signal threshold was necessary during the practice session, especially the grip force, muscle contraction, and voice thresholds. The flow of the experiment was applied to all the condition sessions.
2.4. Assist Force-Triggered Conditions
In this study, the assistive forces functioned on the wearable robot arm exactly when the trigger signals reached a preset threshold. All the participants were introduced to the five trigger methods (Figure 1, Table 2) and performed in a randomized sequence. The bioelectrical and biomechanical signals activated the wearable robot arm in response to the user’s intent to move, allowing activation at their discretion. For the control condition, the switch-based control method using external sound (EX) signals was adopted. The sound signals were delivered via the motor control interface (laptop) to prompt the user to use the wearable robot arm.
| Task | Signal | Source | Sensor | Assistive force generation timing | |
|---|---|---|---|---|---|
| Specification | Location | ||||
| External signals | |||||
| EX | External sound | Device | A total of 3.5 s consists of four times programed “beep” sound events with three intervals of 1 s, 1 s, and 1.4 s. The fourth sound event lasts longer than the others, with a duration of 1 s. | Personal computer speaker | After the fourth sound event ends |
| Bioelectrical signals | |||||
| EMG-A | Biceps myoelectric | User |
|
1/3 from the BB origin on the assistive arm | Individual’s 10% MVCBB |
| Biomechanical signals | |||||
| GRIP-A | Grip force | User | 18.3 mm diameter × 56.3 mm length of flexible film force-sensitive resistor (FSR) | 38 mm diameter dumbbell handle on the assistive hand |
|
| GRIP-N | Grip force | User | 18.3 mm diameter × 56.3 mm length of flexible film force-sensitive resistor (FSR) | 38 mm diameter handle on the nonassistive hand |
|
| VOICE | Voice | User | SparkFun sound detector | 300 mm away from the user’s head | Adjustable for an individual’s normal speaking “Go” |
- Note: The sensors are integrated into the equipment.
- Abbreviations: EMG, electromyography; mm, millimeters; MVCBB, the maximum voluntary contraction of the biceps; s, seconds.
2.5. Measurements
The EMG activity and joint angular motion before and during the initial phase of natural elbow flexion (75% of 1.12 s) were analyzed (Figure 2). The primary movements of elbow flexion as BB and antagonist as TB activities were measured using an A/D converter with a Bio-amp ML 132 (AD Instrument, PowerLab 16/30, ML880, Australia). The 1000 Hz sampling EMG data were filtered using a bandpass finite impulse response with a 20 Hz low cutoff and a 500 Hz high cutoff via LabChart (version 7.3.8), in compliance with the licensing terms. A Python script was used to rectify the EMG data and interpret mean values at 100 Hz.
The EMG data were regularized as %MVCBB and %MVCTB. The EMG values (%MVC) at the start and peak times were observed, including the peak time (s) of the TB and BB. The mean EMG (%MVC) was 25% before elbow flexion (beforea), 0%–25% at the early initial phase after elbow flexion (after 1b), and 25%–50% at the late initial phase after elbow flexion (after 2c), the average of which were measured. Consequently, the co-contraction ratio was calculated as %MVCTB divided by %MVCBB to quantify the simultaneous activation of the TB relative to the BB across the three periods (beforea, after 1b, and after 2c). These periods were defined based on the ideal proactive and reactive actions during the acceleration phase of the elbow flexion.
Regarding the device parameter records, the movement dynamics related to EMG insights were analyzed to assess joint angular behavior during the torque-provided period (75% elbow flexion). The value of elbow joint angular velocity (rad/s) sourced from the wearable robot arm was reported as the peak value, and the peak time was considered when the mean values of 25% ± 5% elbow flexion (the center of the initial phase) were reached. Afterward, the joint angular acceleration (rad/s2) was computed, specifically at the midpoint of the acceleration phase, corresponding to 25% ± 5% elbow flexion.
Subjective examination was performed to present the user’s experiences with the wearable robot arm [22]. User-perceived exertion level by an 11 scoring represented 0%–100% via Borg Category-Ratio 10 [23] was used to examine the physical fatigue level. The effectiveness of the wearable robot arm was estimated by the user’s perceived assistive forces (%) and timeliness of assistive forces on a 7-point scale. Timeliness scored as −3 was “too slow,” as 0 “timely, and as +3 too fast. The usability and satisfaction of the wearable robot arm were evaluated by the questions of cooperation between the user and the wearable robot arm (%), level of learnability (%), and preference for the operation to use (%). In addition, users’ other experiences and opinions after using the wearable robot arm were collected through interviews and reported to supplement the subjective data and support standard indicators.
2.6. Statistical Analysis
Statistical analysis was performed using IBM SPSS (version 23.0, Chicago, IL, USA), with all usages adhering to the software licensing requirements. The Shapiro–Wilk test was used to assess data normality before formal analysis. The EMG activities (mean values of %MVCBB and %MVCTB, start and peak values, EMG value differences between elbow flexion periods, peak time, and co-contraction), joint angular values, and subjective examination were compared among the five triggered signals by one-way repeated measures analysis of variance (ANOVA). Post-hoc pairwise comparisons were conducted using Bonferroni’s test as a conservative method to control for type I errors. The Data were presented as mean ± SD. The significance level was set at p < 0.05.
3. Results
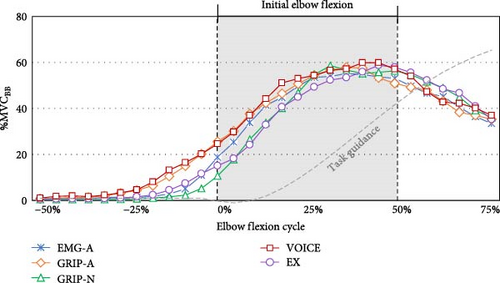
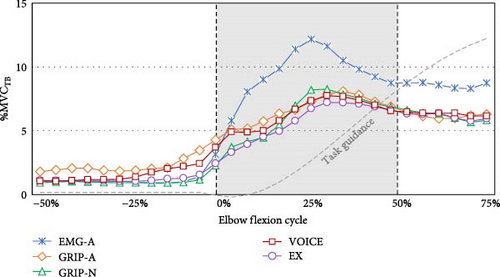
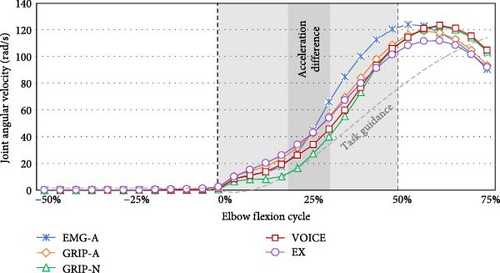
The differentiated EMG activities among five signal activations in the initial phase (50% of 1.12 s) of elbow flexion are presented in Table 3. In Figure 3, the temporal changes in the joint angular velocity triggered by all signals when using the assistive arm are also presented. According to the Bonferroni post-hoc test, GRIP-N exhibited lower TB activity at the start time than EX (p = 0.012) and GRIP-A (p = 0.052). At the peak time, EMG-A had a higher TB amplitude than VOICE (p = 0.007) and EX (p = 0.026), and EMG-A gained a range of TB amplitude from start to peak time that was greater than GRIP-A (p = 0.005), VOICE (p = 0.004), and EX (p = 0.018).
| EMG activities | Mean ± SD | p-Value | ||||
|---|---|---|---|---|---|---|
| EMG-A | GRIP-A | GRIP-N | VOICE | EX | ||
| Biceps activities (%MVCBB) | ||||||
| Start BB | 18.3 ± 12.0 | 25.6 ± 20.5 | 10.7 ± 10.7 | 15.1 ± 17.0 | 24.6 ± 23.3 | 0.027 |
| Peak BB | 71.0 ± 34.1 | 76.2 ± 31.4 | 76.4 ± 41.0 | 78.9 ± 40.3 | 84.9 ± 40.3 | 0.051 |
| Range BB | 52.1 ± 26.5 | 50.6 ± 25.0 | 65.8 ± 34.5 | 63.7 ± 42.4 | 60.3 ± 31.6 | 0.125 |
| Peak BB time (s) | 0.37 ± 0.09 | 0.33 ± 0.10 | 0.39 ± 0.11 | 0.35 ± 0.12 | 0.35 ± 0.12 | 0.581 |
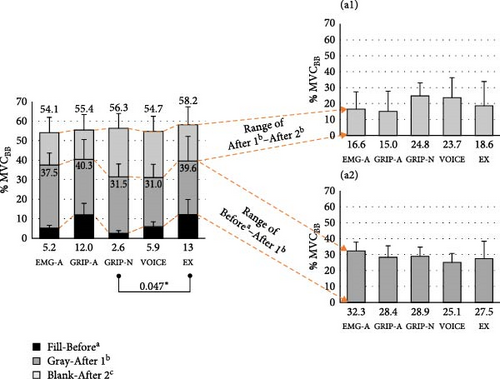
| Period beforea | 5.2 ± 4.2 | 12.0 ± 11.7 | 2.60 ± 2.5 | 5.9 ± 6.4 | 13.0 ± 12.6 | 0.006 |
| Period after 1b | 37.5 ± 16.5 | 40.3 ± 23.2 | 31.5 ± 24.3 | 31.1 ± 19.5 | 41.5 ± 31.3 | 0.234 |
| Period after 2c | 54.1 ± 28.3 | 55.4 ± 25.3 | 56.3 ± 28.8 | 54.7 ± 27.6 | 57.5 ± 26.7 | 0.885 |
| Differencesa,b | 32.4 ± 13.9 | 28.4 ± 16.1 | 28.9 ± 22.4 | 25.1 ± 15.7 | 28.5 ± 20.7 | 0.617 |
| Differencesb,c | 16.6 ± 15.1 | 15.0 ± 16.5 | 24.8 ± 20.5 | 23.7 ± 24.8 | 16.0 ± 25.6 | 0.353 |
| Triceps activities (%MVCTB) | ||||||
| Start TB | 3.1 ± 3.0 | 4.3 ± 2.4 | 2.3 ± 2.4 | 2.4 ± 2.1 | 3.7 ± 3.1 | 0.001 |
| Peak TB | 14.2 ± 9.6 | 11.3 ± 6.3 | 11.2 ± 7.8 | 10.5 ± 6.6 | 10.8 ± 6.3 | 0.002 |
| Range TB | 11.1 ± 6.9 | 7.0 ± 4.9 | 8.9 ± 5.7 | 8.0 ± 4.9 | 7.1 ± 3.9 | <0.001 |
| Peak TB time (s) | 0.29 ± 0.05 | 0.32 ± 0.11 | 0.38 ± 0.09 | 0.36 ± 0.10 | 0.33 ± 0.10 | 0.064 |
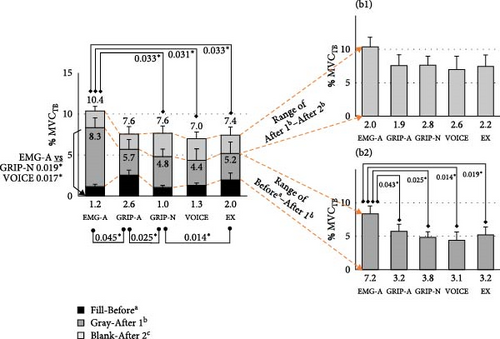
| Period beforea | 1.2 ± 0.7 | 2.6 ± 1.5 | 1.0 ± 0.7 | 1.3 ± 0.9 | 2.0 ± 1.3 | 0.002 |
| Period after 1b | 8.3 ± 6.4 | 5.7 ± 2.9 | 4.8 ± 4.0 | 4.4 ± 3.3 | 5.4 ± 3.9 | <0.001 |
| Period after 2c | 10.4 ± 7.1 | 7.6 ± 4.1 | 7.7 ± 5.2 | 7.0 ± 4.3 | 7.3 ± 4.2 | <0.001 |
| Differencesa,b | 7.2 ± 5.7 | 3.2 ± 2.7 | 3.8 ± 3.4 | 3.1 ± 2.7 | 3.3 ± 3.0 | 0.001 |
| Differencesb,c | 2.0 ± 2.4 | 1.9 ± 2.5 | 2.8 ± 2.1 | 2.6 ± 2.4 | 2.0 ± 2.6 | 0.454 |
| Co-activities (ratio) | ||||||
| Co-contraction beforea | 0.29 ± 0.18 | 0.82 ± 0.84 | 1.05 ± 0.94 | 1.06 ± 1.53 | 1.12 ± 1.76 | 0.168 |
| Co-contraction after 1b | 0.26 ± 0.24 | 0.21 ± 0.18 | 0.22 ± 0.19 | 0.20 ± 0.15 | 0.18 ± 0.12 | 0.475 |
| Co-contraction after 2c | 0.26 ± 0.29 | 0.16 ± 0.12 | 0.18 ± 0.17 | 0.16 ± 0.13 | 0.16 ± 0.13 | 0.038 |
- Note: One-way repeated analysis of variance among the triggered signals are presented p-values (2-tailed). Bold used to highlight significant values.
- Abbreviations: EMG, electromyography; EMG-A, assistive arm EMG-induced activation; EX, external sound; GRIP-A, grip on the assistive hand; GRIP-N, grip on the nonassistive hand; MVC, maximum voluntary contraction; VOICE, voice.
- aPeriod of 25% before the signals triggered.
- bPeriod of 25% after the signals triggered.
- cPeriod of 25%–50% after the signals triggered.
The period of EMG at 25% before, 25% after (early initial phase), and 25%–50% after (late initial phase) the signals initiated are reported in Table 3. Before the assistive forces were initiated, we observed lower BB activity in GRIP-N than in EX. GRIP-A featured higher TB activity than EMG-A and GRIP-N, whereas GRIP-N showed lower TB activity than EX. In the early initial phase, TB increased in EMG-A compared to other conditions during the late initial phase period. Considering the differences between these periods, EMG-A exhibited the highest TB among the five conditions. The Bonferroni post-hoc test indicated significant values, as shown in Figure 4. Additionally, the participants performed higher co-contractions in EMG-A during the late initial phase than in the other signal conditions.
As shown in Table 4, the joint angular acceleration during 25% ± 5% elbow flexion was significantly different among the five signals. Bonferroni post-hoc analysis revealed that EMG-A increased the joint angular acceleration more than GRIP-A (p = 0.070), GRIP-N (p = 0.129), VOICE (p = 0.014), or EX (p = 0.049). However, there was no significant difference in joint angular velocity among the five trigger conditions.
| Joint angular properties | Mean ± SD | p-Value | ||||
|---|---|---|---|---|---|---|
| EMG-A | GRIP-A | GRIP-N | VOICE | EX | ||
| Velocity (rad/s) | ||||||
| Peak value | 128.1 ± 17.2 | 129.9 ± 22.7 | 128.3 ± 21.3 | 131.6 ± 19.0 | 120.0 ± 25.1 | 0.434 |
| Peak time (s) | 0.63 ± 0.06 | 0.66 ± 0.11 | 0.67 ± 0.09 | 0.67 ± 0.10 | 0.63 ± 0.13 | 0.528 |
| 25 ± 5% period | 39.1 ± 13.7 | 38.6 ± 36.3 | 25.1 ± 16.1 | 30.6 ± 30.3 | 42.3 ± 35.5 | 0.451 |
| Acceleration (rad/s2) | ||||||
| 25 ± 5% period | 336.2 ± 114.3 | 212.4 ± 102.8 | 224.7 ± 168.2 | 186.4 ± 101.8 | 198.9 ± 103.2 | 0.003 |
- Note: One-way repeated analysis of variance among the triggered signals is presented with p-values (2-tailed).
- Abbreviations: EMG-A, assistive arm EMG-induced activation; EX, external sound; GRIP-A, grip on the assistive hand; GRIP-N, grip on the nonassistive hand; rad/s, radians per second; rad/s2, radians per second squared; VOICE, voice.
The users’ perceived exertion, perceived assistive force, timeliness, cooperation, learnability, and preference for these triggered signals are reported in Table 5. The Bonferroni post-hoc test indicated that GRIP-N had the lowest preference for activating the wearable robot arm compared with the EMG-A (p = 0.212), GRIP-A (p = 0.471), and EX (p = 0.154). There was no significant preference for the GRIP-N or VOICE.
| Subjective assessment | Evaluating (Mean ± SD) | p-Value | ||||
|---|---|---|---|---|---|---|
| EMG-A | GRIP-A | GRIP-N | VOICE | EX | ||
| Perceived exertion (%) | 27.9 ± 11.0 | 32.5 ± 12.2 | 29.9 ± 11.5 | 26.0 ± 12.3 | 25.3 ± 12.3 | 0.387 |
| Perceived assistive force (%) | 33.6 ± 25.6 | 35.0 ± 24.7 | 29.3 ± 21.7 | 36.4 ± 22.1 | 42.1 ± 21.2 | 0.108 |
| Timeliness (score 0 ± 3) | 0.14 ± 0.36 | −0.14 ± 0.66 | 0.08 ± 1.27 | 0.79 ± 1.31 | 0.29 ± 0.83 | 0.108 |
| Cooperation (%) | 64.3 ± 27.7 | 60.7 ± 28.7 | 55.7 ± 26.5 | 57.1 ± 30.0 | 65.0 ± 18.7 | 0.587 |
| Learnability (%) | 70.0 ± 29.6 | 68.6 ± 30.6 | 62.1 ± 23.6 | 61.4 ± 30.1 | 70.0 ± 20.0 | 0.260 |
| Preference (%) | 70.0 ± 28.6 | 65.7 ± 30.8 | 50.0 ± 27.5 | 55.0 ± 27.4 | 66.4 ± 20.2 | 0.007 |
- Note: One-way repeated analysis of variance among the triggered signals is presented with p-values (2-tailed). Bold used to highlight significant values.
- Abbreviations: EMG-A, assistive arm EMG-induced activation; EX, external sound; GRIP-A, grip on the assistive hand; GRIP-N, grip on the nonassistive hand; VOICE, voice.
According to the additional subjective feedback, most of the participants found all signals easy to use to activate the wearable robot arm. However, two males and two females reported confusion about using GRIP-N to initiate movement on the opposite side while wearing the robotic arm, particularly regarding the timing after gripping. One female participant felt shy about saying “Go” aloud to command the robotic arm, while two other females and one male noted potential embarrassment in using voice commands in real-life situations, although they found them acceptable in a private experimental setting. Additionally, one male participant stated that focusing on the start signal in the EX condition made him anxious and possibly led to unnatural movement.
4. Discussion
The constant torque control of a wearable robot arm, estimating assistive forces at 50% of the compensatory load from 15% MVCBB, effectively reduced %MVCBB activity during elbow flexion when operated by the participants (EMG-A, GRIP-A, GRIP-N, and VOICE) and through external signals (EX). The participants delivered the perceived wearable robot arm assistance at 30%–40% while lifting a 15% compensating weight dumbbell. Same as reported by Muraki et al. [21], providing assistive forces during elbow flexion lifting 15% MVCBB with a wearable robot arm could decrease BB and TB activities compared to unassisted conditions.
Focusing on the initial phases of elbow flexion, the participants tended to provide a lower %MVCBB and %MVCTB at the start and 25% of the period before the start time when activating the wearable robot arm by GRIP-N. According to previous studies [24–26], users insist on muscle preparation to act earlier in assistive force generation, particularly in intralimb movement. Prior to an initiated movement, the brain processes cues to organize stability, adaptability, efficiency, and coordination [27, 28]. Starting the wearable robot arm using the GRIP-N cues, which involve interlimb coordination, might require a more proactive operation. Lack of proactive productivity refers to the reduced ability to estimate movements to preserve smoother, more coordinated, and effortless goal-directed movements [29].
Under the GRIP-N condition, the range of %MVCBB from start to peak developed greater activities than under EMG-A and GRIP-A, possibly because of the grip handle weights. The unbalanced weights of the grips challenge the proprioceptive feedback, affecting torque estimation at the elbow joint and energy expenditure [30]. The lighter weight triggers a heavier weight, which may disrupt the neuromuscular coupling that controls unbalanced weight, complicating bilateral connections or interlimb transfer skills [31]. As noted by Choi et al. [32], loading free weight on the nonassistive hand in bilateral elbow flexion increased the overshoot ratio compared with that on the assistive hand. Reflected in GRIP-N, the grip-activated elbow flexor synergies on the nonassistive hand, like a free-weight handle, while they managed the load, potentially owing to reduced bilateral neural connections in response to unbalanced weight.
Along with the GRIP-N results, the VOICE indicated lower %MVCBB and %MVCTB values at the start time. Using a voice to initiate the wearable robot arm increases the coordination challenges from asynchronous sensory inputs to create a unified response. Compared to direct feedback linked to the primary action, these diverse inputs increase the complexity of anticipating timing and effort, leading to insufficient information for unrelated tasks [33, 34]. Adding voice feedback requires task-switching or dual-task skills, potentially increasing the cognitive load during information processing to initiate an action [35]. A high cognitive load can affect the limited capacity of working memory, hindering both proactive and reactive strategies [36]. In scenarios involving wearable robots, attempts to boost proactive mechanisms without sufficient cognitive information can inhibit reactive control, adaptability, and cooperation with devices [34, 37, 38].
Moreover, the participants rated GRIP-N and VOICE as having less cooperation with the wearable robot arm, indicating lower preferences. GRIP-N and VOICE were not directly linked to assistive arm elbow flexion, leading users to perceive these inputs as less cooperative. Elaborating on the joint angular movement, GRIP-N started elbow flexion with a lower velocity but rapidly increased in acceleration during the mid-uplift phase. Generally, muscles require sustained preparation to cooperate effectively with devices and achieve purposeful joint kinetics and kinematics. Rapid tasks with high joint angular acceleration can increase the need for proactive mechanisms to prevent muscle injury, especially if the body is not adequately prepared for such movements [39]. Although GRIP-N and VOICE may be suitable for individuals with low hand and arm function, using these signals can lead to feelings of uncooperativeness and result in unsynchronized elbow flexion with the wearable robot arm.
Given these findings, an alternative triggering mechanism may help enhance cooperation with the wearable robot arm. Unlike GRIP-N and VOICE, GRIP-A demonstrated a stronger preactivation response in this study, as seen in the greater %MVCBB and %MVCTB at 25% before the start to the actual start time. This suggests that preemptive engagement with the arm through GRIP-A facilitated a more synchronized and intentional movement pattern. Intentional movement refers to a satisfactory decision to act, involving an improved executive action plan [40]. Compared with the participants’ responses, GRIP-A addressed superior preferences over GRIP-N and VOICE conditions. Intralimb movement cooperation with the wearable robot arm is essential for users to align their positions and movement expectations with their intent to act. As described by Suzuki et al. [41], the user’s intention, estimated by biomechanical signals, significantly reflects the intralimb control of the HAL suit. Therefore, the user’s intended actions enable natural cooperation with the wearable robot arm.
Interestingly, initiating the wearable robot arm with EMG-A increased the TB activity to the greatest %MVCTB among other signals. As per Gribble et al. [42], an excessive antagonist (TB) counteracts an overactive agonist (BB), causing the movement to become jerky and less precise. Proven by the angular moment, EMG-A invented the highest joint angular acceleration during mid-initial elbow flexion. Covered by co-contraction, the activation using EMG-A addressed the highest ratio in the late initial phase, which also found the peak TB value. These circumstances affirmed that primary muscle contraction via the BB in EMG-A was greater than that in the other signals. Utilizing EMG signals to control the elbow flexion device caused overactivation of the BB, triggering compensatory contraction of the TB to balance excessive activity. This mechanism may enhance elbow flexion performance without harming the BB [43]. However, this adjustment increases energy expenditure and places greater demand on neuromuscular function, ultimately leading to prolonged fatigue during movement [44].
As acknowledged by participants and recorded EMG activities, users expressed clear preferences for EMG-A and GRIP-A but raised concerns regarding the usability and cooperation of GRIP-N, VOICE, and EX. The feedback signified that users prioritized minimizing cognitive load during tasks by selecting simple activation methods that aligned with their individual abilities and task demands. Ideally, we designed trigger methods with distinct functionalities: EX relied on conservative activation, EMG-A employed advanced assistive control, VOICE facilitated device commands and enhanced robot learnability, while GRIP-A and GRIP-N were intended for intuitive human control with minimal signal interference. While healthy young adults interacted easily with EMG-A and GRIP-A, other methods may be more suitable for diverse users, such as GRIP-N for unilateral upper limb dysfunction and VOICE for total limb dysfunctions or broader robotic control.
Practically, EMG signals proved the feasibility of operating the wearable robot. However, several limitations of EMG control were discussed in the context of human adaptability [45] in cases where users had unstable muscle signals [46] and human acceptability, such as failure to personalize the setting and muscle twitches concern [47]. Additionally, force signals are useful for exoskeleton gloves to augment the grip power for performing grip tasks, such as the Bioservo Ironhand [48] and stroke survivors [49] with lower muscle strain and individualized calibration concerns. Both biomechanical and bioelectrical signals enriched cognitive-friendly interfaces by reducing excessive attention, planning, or task-switching, which also refined user-centered design approaches that prioritize ease of learning and long-term usability.
Notably, grip forces contribute to a cost-effective ergonomic design because balanced force adjustments may help reduce proprioceptive disruptions. Additionally, adaptable user settings across lower-cost sensors may enhance long-term user adaptability. The flexible film force-sensitive resistor (FSR) sensor is easy to integrate into devices without requiring user-specific customization or complex application processes at any stage of use, which may also improve device longevity and user acceptance experience. This method only requires ensuring that activation is directly related to the intended action to avoid increasing cognitive load.
Our findings highlight the importance of grip force triggers in user-cooperative control mechanisms in assistive technology to enhance usability, simplify signal activation, and improve cost-effectiveness. This suggests that future devices should incorporate personalized calibration using biomechanical signals, particularly grip force triggers for an upper limb wearable robot. Future implementations of switch-based trigger methods should focus on integrating biomechanical signals directly with primary actions rather than linking to unrelated actions. The GRIP-A condition demonstrated superior results, highlighting the need for further investigation into real-world tasks such as gripping and lifting objects, as well as examining assistive force adjustments in relation to trigger forces.
Despite the strengths and contributions of this study, some limitations must be acknowledged. We adopted a switch-based control wearable robot arm using low-torque control, resulting in participants perceiving minimal assistive force. Further studies are needed to explore higher assistive forces or alternative control methods to improve cooperability. Additionally, the outcomes of this study are limited to a specific scenario of nonrepeated, natural-speed elbow flexion performed by healthy young adults. To deepen these results, further studies on a wider range of participants, particularly those with limited arm and hand function, are required, as preferences and cooperation with the robot may vary depending on user needs. Ultimately, preferences and other subjective evaluations should be extensively examined.
5. Conclusion
This study explored signals that trigger a wearable robot arm, focusing on grip force from the assistive hand (GRIP-A). EMG signals enhanced cognitive intention, facilitating smoother activation of the wearable robot arm, especially during the initial phase of elbow flexion. Moreover, force signals through GRIP-A helped maintain the TB amplitude and the joint moment, accelerating elbow flexion and resulting in smoother, more controllable biceps contraction. Therefore, implementing GRIP-A to activate the wearable robot arm effectively minimized BB activities and reduced the overantagonist of the TB effect.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by JSPS KAKENHI (Grant Numbers JP21H04898 and JP24H00725).
Acknowledgments
We would like to thank JSPS KAKENHI for their financial support of this study. We would also like to express our sincere gratitude to Mr. Daichi Kusumoto for his support in the 3D printing process.
Open Research
Data Availability Statement
The data that do not compromise personal confidentiality will be available upon request.




