Effect of Modified Remote Ischemic Preconditioning on Perioperative Outcomes of CABG Patients With CPB
Abstract
Objective: To investigate the effect of modified remote ischemic preconditioning (MRIC) on perioperative outcomes in patients undergoing coronary artery bypass grafting (CABG) on cardiopulmonary bypass (CPB).
Methods: This study included 118 patients who planned to undergo CABG surgery at the Affiliated Hospital of Xuzhou Medical University. These patients were randomly divided into the MRIC group (n = 40), remote ischemic preconditioning (RIPC) group (n = 39), or control group (n = 39). The MRIC group received 3 cycles of 5 min ischemia/5 min reperfusion on the left upper limb at 2 days, 1 day, and 2 h preoperatively. The RIPC group received RIPC 2 h preoperatively, while the control group did not receive ischemic preconditioning. The STS score of patients was calculated according to the coronary angiography results and clinical data for risk stratification. The serum concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP), creatine kinase MB (CK-MB), high-sensitivity cardiac troponin-T (hs-cTnT), and creatinine (Cr) of patients were recorded at postoperative 0, 12th, 24th, 48th, 72th h , and seventh days for each patient. Major adverse cardiac events (MACEs) in the hospital were recorded.
Results: A total of 118 participants were included. The overall MACE incidence was 83.4%. A total of 36 MACE cases (92.3%) occurred in the control group, 28 cases (70.0%) in the MRIC group (RR: 0.75; 95% CI: 0.61–0.95), and 35 cases (89.7%) in the RIPC group (RR: 0.97; 95% CI: 0.84–1.12). Compared to the control group, MRIC and RIPC groups had lower concentrations of CK-MB at postoperative 0 and 12th h (p < 0.05); MRIC group had lower concentrations of hs-cTnT at postoperative 12th h (p < 0.05). The MRIC group had a higher concentration of NT-proBNP at postoperative 24th, 48th, and 72th h (p < 0.05). The differences in the concentration of Cr among the three groups were not statistically significant (p > 0.05); There was no statistically significant difference in the effects of MRIC on the indexes of the low-risk patients and the medium-high-risk patients (p > 0.05).
Conclusion: (1) MRIC has cardioprotective effects and reduces the occurrence of postoperative MACE. (2) MRIC could not reduce the concentrations of NT-proBNP and Cr postoperatively. (3) MRIC showed no significant difference in myocardial protection in patients with different STS score risk stratifications.
1. Introduction
Although there has been substantial improvement in atherosclerotic cardiovascular disease (ASCVD) outcomes in recent decades, ASCVD remains the leading cause of morbidity and mortality globally [1]. Currently, the main treatment modalities for CAD are drug therapy, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG). Coronary artery bypass surgery (CABG) is preferable to PCI in patients with multivessel disease in combination with diabetes [2]. Although CABG effectively reduces the mortality of CVD, perioperative complications such as major adverse cardiac event (MACE), pulmonary and renal impairment, cardiac arrhythmias, and low cardiac output syndrome still affect the efficacy of CABG [3], and inflammatory factors suppress the cardiac function after extracorporeal circulation after CABG under cardiopulmonary bypass (CPB), affecting clinical prognosis [4, 5].
To address this issue, clinicians are conducting studies to mitigate perioperative trauma. Murry et al. found that coronary artery ligation of dogs subjected to four cycles of 5-min ischemia and 5-min reperfusion preconditioning resulted in significant cardiac resistance to subsequent sustained ischemic injury and a significant reduction in the size of myocardial infarct [4]. This phenomenon is known as ischemic preconditioning (IPC). Subsequent studies have shown that remote limb ischemia–reperfusion still had cardiac protective effects [6], and thus remote IPC (RIPC) was proposed. The protective effects of various types of RIPC have been confirmed in animal experiments [7]. However, the results were disappointing in clinical trials. Many clinical trials have evaluated the cardioprotective effects of RIPC in PCI and cardiac surgery. Some studies have confirmed that RIPC reduces postoperative troponin and creatine kinase MB (CK-MB) concentrations in patients [8–10], whereas others have not reached similar conclusions [11]. In particular, two large clinical trials did not yield positive results regarding markers of myocardial injury or clinical outcomes [12, 13]. Finally, meta-analyses confirmed that RIPC could reduce postoperative troponin release without clinical benefit to overall outcomes [14, 15]. Therefore, there is no consensus on the results of basic and clinical studies on RIPC. Ischemic conditioning has little direct clinical application in cardiac surgery.
The number of cycles, the duration of each ischemic sequence, and the application site of RIPC are considered to affect the clinical effect [16]. Bódi et al. found that repeated RIPC or postconditioning improved survival in a dose-dependent manner [17]. Studies have shown that increasing the number of RIPC sessions reduces myocardial infarct size and adverse left ventricular remodeling. This suggests that multiple iterations of RIPC or postconditioning may confer additional cardiac protection compared to a single dose of RIPC.
Currently, the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the Society of Thoracic Surgeons mortality risk score (STS score) are the more authoritative risk assessment scores for cardiac surgery [18]. Fewer studies have evaluated the efficacy of RIPC after CABG in patients with a high risk of EuroSCORE score [19]. There are currently no studies evaluating RIPC for CABG in patients with different risk stratifications of STS score.
We designed a procedure to perform RIPC at several time points. This experiment aimed to investigate the perioperative cardioprotective effect of modified RIPC (MRIC) in patients undergoing CABG with CPB, and whether the use of different stimulation amounts of RIPC affects its effect. Besides, subgroups of different risk stratification are further analyzed, thus providing a theoretical basis for clinical application.
2. Methods
2.1. Study Population
This study is a single-center prospective, randomized controlled trial. 120 patients were included in the present study, which was conducted from January 2022 to December 2022. The study was approved by the Medical Ethics Committee with the consent of the patients and their guardians (ethics committee acceptance number: XYFY2021-KL320-01).
Inclusion criteria were as follows: (1) aged ≥ 18 years, (2) elected to undergo CABG surgery under CPB, and (3) agreed to participate in the study and signed an informed consent form. Exclusion criteria were as follows: (1) patients with cardiac enzymes and N-terminal pro-B-type natriuretic peptide (NT-proBNP) above the upper limit of normal; (2) comorbid valvular surgery; (3) comorbidities with severe hematological or rheumatological diseases in active stages, recent infections, or other diseases affecting the systemic inflammatory response; (4) patients with severe hepatic or renal insufficiency; (5) contraindications to RIPC, such as peripheral vascular disease or physical disability; and (6) patients who have failed to complete the RIPC operation in accordance with the regulations.
2.2. Intervention Measures
People were randomly divided into three groups based on the remote ischemic conditioning procedure: the MRIC group (received 3 cycles of 5 min ischemia/5 min reperfusion on the left upper limb at 2 days, 1 day, and 2 h before surgery), the RIPC group (2 h before surgery), and the control group (no treatment).
2.3. Information Collection
General information about the patients was collected and recorded: gender, age, BMI, history of smoking and alcohol consumption, history of diabetes mellitus, hypertension, hyperlipidemia, history of previous myocardial infarction, PCI, and cardiac surgery. Upper limb venous blood specimens were taken at 6:00 a.m. on the day after the study subjects were admitted to the department. The specimens were sent to the laboratory for the evaluation of the following items: routine blood, liver and kidney function, electrolytes, cardiac enzymes, coagulation function, brain natriuretic peptide, blood lipids, and blood glucose. The results of the relevant examinations and drug use of all the selected patients were recorded. Record whether in-hospital MACE events occurred in patients, specifically including the following variables: cardiovascular death, myocardial infarction, stroke, sudden cardiac arrest, cardiac tamponade, recurrent angina, symptomatic heart failure, ventricular arrhythmias (frequent ventricular ectopy, ventricular tachycardia, and ventricular fibrillation), new-onset atrial fibrillation, high-degree atrioventricular block, and clinically indicated target-vessel coronary revascularization.
2.4. Surgical Operations
Central venous pressure, electrocardiogram, and nasopharyngeal temperature were recorded continuously. Anesthesia was induced by one of the following: sufentanil 7 μg/kg, etomidate 0.3 mg/kg (maximum dose 20 mg), rocuronium bromide 0.8 mg/kg, dezocine 5 mg, palonosetron 75 μg, and midazolam 0.05 mg/kg; anesthesia maintenance: propofol 1-2 mg/kg or dexmedetomidine 0.5 μg/kg/h, sevoflurane 1% inhaled, and rocuronium 25 mg/h pumped. All patients were operated on by the same team, and after the establishment of extracorporeal circulation, blood cardioplegia fluid was used to protect the myocardium, while the patient’s saphenous vein was taken to construct a coronary artery bypass graft, and after the graft was anastomosed, the extracorporeal circulation was stopped. After the resumption of the beat, milrinone 0.5–1 μg/kg/min and nitroglycerin 0.2–1 μg/kg/min were administered, with specific doses adjusted according to the patient’s blood pressure and heart rate.
2.5. STS Scores
Patient’s STS scores were calculated from their clinical data using a web-based calculation tool (https://riskcalc.sts.org/stswebriskcalc/calculate), and the population was categorized into low-risk and intermediate–high risk (low risk: STS < 4%; intermediate risk: 4 ≤ STS ≤ 8%; and high-risk: STS > 8%).
2.6. End Points
Primary outcome: To assess the cardioprotective effect of RIPC on postoperative cardioprotection by examining the differences in the concentrations of CK-MB, high-sensitivity cardiac troponin-T (hs-cTnT), and NT-proBNP among the three groups of patients undergoing CABG.
Secondary outcomes: (1) To examine creatinine (Cr) concentrations in the three groups of patients to compare the effect of RIPC on postoperative renal injury in patients. (2) To estimate the effect of RIPC on the short-term prognosis of patients according to the occurrence of postoperative MACE. (3) To stratify patients by STS score and to evaluate the effect of RIPC on the outcome of patients with different risks according to the examination results and clinical outcomes.
2.7. Statistical Analysis
The statistical analyses were performed using GraphPad Prism 10. The normality of the data was tested using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were presented as mean ± standard deviation (SD); non-normal variables were reported as median (interquartile range). Data were analyzed by ANOVA or the Kruskal–Wallis test. Categorical variables were described as the number of cases (%), and the chi-square test was used for comparison between groups. The differences in CK-MB, hs-cTnT, NT-proBNP, and Cr among the three groups at each postoperative time point were analyzed by two-way repeated-measures ANOVA followed by Tukey’s post hoc test. p < 0.05 was considered statistically significant.
3. Result
3.1. Main Characteristics of the Participants
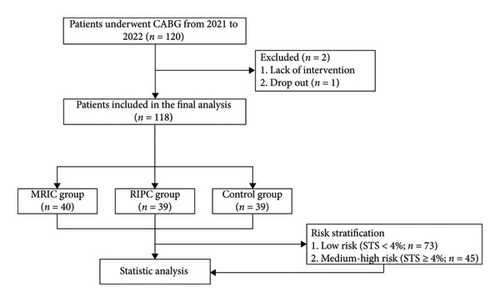
The flowchart is shown in Figure 1. 118 participants were randomly assigned to the MRIC group (40 patients), the RIPC group (39 patients), and the control group (39 patients). The average age of the participants was 64.44 ± 8.03 years, and 85 participants (72%) were male. The participants in the three groups had similar demographic characteristics, including gender, age, BMI, diabetes, hypertension, drug application, blood results, and surgical characteristics. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (FS) were lower in the MRIC group than in the rest of the groups (p < 0.05) (Table 1).
MRIC (N = 40) |
RIPC (N = 39) |
Control (N = 39) |
p value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Female (n, %) | 29 (72.5%) | 29 (74.4%) | 27 (69.2%) | 0.878 |
| Age (years) | 66.0 [60.8; 68.2] | 66.0 [59.0; 71.0] | 66.0 [58.5; 70.0] | 0.978 |
| BMI (kg/m2) | 24.1 [22.4; 27.8] | 25.0 [22.8; 27.2] | 24.4 [22.5; 25.6] | 0.585 |
| Smoking (n, %) | 9 (22.5%) | 10 (25.6%) | 3 (7.69%) | 0.094 |
| Diabetes (n, %) | 19 (47.5%) | 13 (33.3%) | 13 (33.3%) | 0.325 |
| Hypertension (n, %) | 24 (60.0%) | 21 (53.8%) | 15 (38.5%) | 0.144 |
| Previous PCI (n, %) | 6 (15.0%) | 3 (7.69%) | 0 (0.00%) | 0.040 |
| Prior myocardial infarction (n, %) | 7 (17.5%) | 8 (20.5%) | 12 (30.8%) | 0.340 |
| Ultrasonic cardiogram results | ||||
| LVEF (%) | 54.5 [48.8; 63.0] | 60.0 [57.0; 64.0] | 62.0 [56.5; 64.0] | 0.012 |
| LA (mm) | 37.5 [36.0; 41.0] | 37.0 [34.0; 41.0] | 36.0 [34.0; 38.5] | 0.094 |
| IVS (mm) | 9.00 [9.00; 10.0] | 10.0 [9.00; 10.0] | 9.00 [9.00; 10.0] | 0.095 |
| LVDd (mm) | 50.0 [47.0; 53.0] | 48.0 [45.0; 51.0] | 48.0 [44.5; 52.0] | 0.073 |
| FS (mm) | 28.5 [25.0; 34.0] | 32.0 [30.0; 34.5] | 33.0 [29.5; 35.0] | 0.006 |
| E/A | 0.946 | |||
| < 1 | 13 (32.5%) | 13 (33.3%) | 14 (35.9%) | |
| > 1 | 27 (67.5%) | 26 (66.7%) | 25 (64.1%) | |
| Blood results | ||||
| HDL (mmol/L) | 0.86 [0.81; 1.08] | 0.91 [0.76; 1.02] | 0.94 [0.80; 1.20] | 0.409 |
| LDL (mmol/L) | 2.38 [1.83; 2.98] | 2.11 [1.63; 2.58] | 1.80 [1.39; 2.38] | 0.057 |
| TG (mmol/L) | 1.40 [1.04; 1.94] | 1.53 [0.98; 1.69] | 1.38 [1.03; 1.91] | 0.936 |
| TC (mmol/L) | 3.90 [3.21; 4.85] | 3.75 [3.20; 4.44] | 3.70 [3.12; 4.50] | 0.757 |
| Surgical characteristics | ||||
| Number of grafts, n (%) | 0.582 | |||
| 1 | 2 (5.00%) | 2 (5.13%) | 4 (10.3%) | |
| 2 | 2 (5.00%) | 5 (12.8%) | 2 (5.13%) | |
| 3 | 36 (90.0%) | 32 (82.1%) | 33 (84.6%) | |
| Length of surgery time (min) | 346 [310; 381] | 330 [285; 355] | 300 [258; 352] | 0.063 |
| Location of culprit lesion, n (%) | ||||
| LAD | 38 (95.0%) | 35 (89.7%) | 36 (92.3%) | 0.635 |
| LCX | 34 (85.0%) | 32 (82.1%) | 27 (69.2%) | 0.191 |
| RCA | 28 (70.0%) | 33 (84.6%) | 30 (76.9%) | 0.302 |
- Note: IVS, interventricular septum thickness; LCX, left circumflex; LVDd, left ventricular end-diastolic diameter; MRIC, modified remote ischemic preconditioning; RIPC, remote ischemic preconditioning; TG, triglyceride. Values in bold represent significant differences (p < 0.05).
- Abbreviations: BMI, body mass index; FS, fractional shortening; HDL, high-density lipoprotein; LA, left atrium; LAD, left anterior descending; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery; TC, total cholesterol.
3.2. Comparison of Serum CK-MB, hs-cTnT, NT-proBNP, and Cr Concentrations
There were differences in the concentration of CK-MB (F = 15.63, p < 0.001), hs-cTnT (F = 4.16, p < 0.001), NT-proBNP (F = 22.80, p < 0.001), and Cr (F = 4.99, p < 0.001) at six time points.
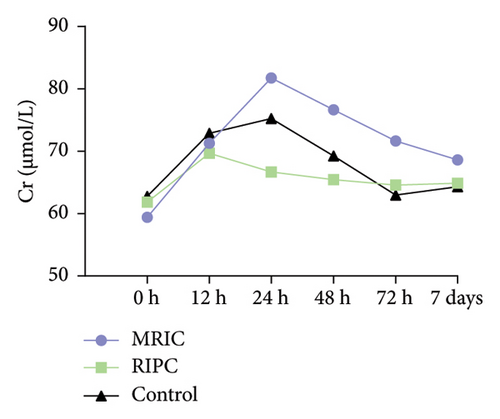
There were differences in the concentration of CK-MB (F = 7.66, p < 0.001), hs-cTnT (F = 7.56, p < 0.001), and NT-proBNP (F = 38.02, p < 0.001) among the three groups. Compared to the control group, MRIC and RIPC groups had lower concentrations of CK-MB at postoperative 0 and 12th h (p < 0.05). MRIC had a lower concentration of hs-cTnT at postoperative 12th h than the control group (p < 0.05). The concentrations of NT-proBNP in the MRIC group were higher than those in the RIPC and control groups at all postoperative time points. The differences in concentrations of Cr among the three groups at all postoperative time points were not statistically significant (p > 0.05) (Figure 2).
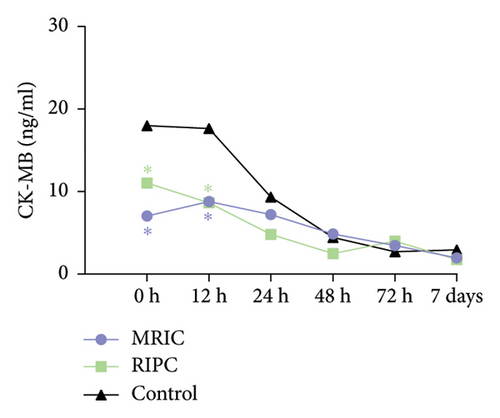

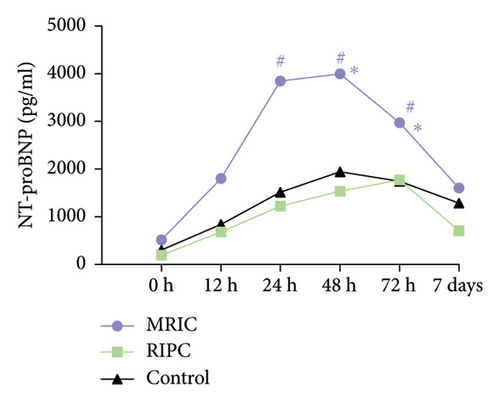
3.3. Comparison of Postoperative MACE in Patients
The overall MACE incidence was 83.4%. A total of 36 MACE cases (92.3%) occurred in the control group, 28 cases (70.0%) in the MRIC group (RR: 0.75; 95% CI: 0.61–0.95), and 35 cases (89.7%) in the RIPC group (RR: 0.97; 95% CI: 0.84–1.12). MRIC reduced the incidence of MACE compared to the RIPC and control groups (p < 0.05), with no statistically significant differences seen in specific adverse event comparisons (Table 2).
MRIC (N = 40) |
RIPC (N = 39) |
Control (N = 39) |
p value | |
|---|---|---|---|---|
| MACE (n, %) | 28 (70.0%)∗# | 35 (89.7%) | 36 (92.3%) | 0.013 |
| Angina (n, %) | 4 (10.0%) | 5 (12.8%) | 5 (12.8%) | 0.881 |
| VA (n, %) | 20 (50.0%) | 26 (66.7%) | 24 (61.5%) | 0.302 |
| NOAF (n, %) | 1 (2.50%) | 2 (5.13%) | 4 (10.3%) | 0.305 |
| SCA (n, %) | 1 (2.50%) | 1 (2.56%) | 1 (2.56%) | 1.000 |
| CT (n, %) | 1 (2.50%) | 1 (2.56%) | 0 (0.00%) | 1.000 |
| Cardiac death (n, %) | 1 (2.50%) | 1 (2.56%) | 2 (5.13%) | 0.845 |
- Note: MRIC, modified remote ischemic preconditioning; RIPC, remote ischemic preconditioning. Values in bold represent significant differences (p < 0.05).
- Abbreviations: CT, cardiac tamponade; MACE, major adverse cardiovascular event; NOAF, new-onset atrial fibrillation; SCA, sudden cardiac arrest; VA, ventricular arrhythmia.
- ∗p < 0.05 versus control.
- #p < 0.05 versus RIPC.
3.4. Hazard Stratification
According to the STS score, the study subjects were divided into low-risk stratum (STS < 4) and medium-high-risk stratum (STS ≥ 4), among which there were 73 cases in the low-risk stratum and 45 cases in the medium-high-risk stratum. In the low-risk stratum, there were 26 cases in the MRIC group, 23 cases in the RIPC group, and 24 cases in the control group; in the medium-high-risk stratum, there were 14 cases in the MRIC group, 16 cases in the RIPC group, and 15 cases in the control group.
For low-risk and middle-high-risk stratified subjects, the results of repeated measurement analysis of variance showed that there was no significant difference in CK-MB and hs-cTnT concentrations among the three groups at each time point after operation (p > 0.05).
In the low-risk stratification, the total incidence of MACE was lower in the MRIC group than in the other two groups (p < 0.05), and the occurrence of ventricular arrhythmias was not statistically significant. The total incidence of MACE and the occurrence of ventricular arrhythmias were not statistically significant in the medium- and high-risk strata (Table 3).
| Low risk | Medium-high risk | |||||||
|---|---|---|---|---|---|---|---|---|
| MRIC group | RIPC group | Control group | p value | MRIC group | RIPC group | Control group | p value | |
| (N = 26) | (N = 23) | (N = 24) | (N = 14) | (N = 16) | (N = 15) | |||
| MACE | 18 (69.2%)∗# | 21 (91.3%) | 22 (91.7%) | 0.049 | 10 (71.4%) | 14 (87.5%) | 14 (93.3%) | 0.259 |
| VA | 12 (46.2%) | 15 (65.2%) | 13 (54.2%) | 0.407 | 8 (57.1%) | 11 (68.8%) | 11 (73.3%) | 0.674 |
- Note: MRIC, modified remote ischemic preconditioning; RIPC, remote ischemic preconditioning. Values in bold represent significant differences (p < 0.05).
- Abbreviations: MACE, major adverse cardiovascular event; VA, ventricular arrhythmia.
- ∗p < 0.05 versus control.
- #p < 0.05 versus RIPC.
4. Discussion
CABG is a classic and important treatment for coronary artery revascularization. However, CABG is more traumatic than interventional treatment, and in addition to myocardial and organ damage in the perioperative period, postoperative inflammatory reactions are more pronounced, which affects the therapeutic efficacy of the treatment to a different extent.
The myocardial protective effect of RIPC has been demonstrated in many animal studies [20, 21]. However, satisfactory results have not been achieved in clinical trials. A study by Lomivorotov et al. showed that RIPC did not improve the concentrations of hemodynamic and cardiac enzymological indices in patients after CABG [22], and a meta-analysis also showed that RIPC did not have an improvement in postoperative clinical outcomes [23], but a meta-analysis that included 15 randomized controlled studies showed that RIPC reduced the release of troponin after CABG [24]. Additional experiments showing whether RIPC reduces renal impairment after CABG have also been inconclusive [11, 25]. However, all of these experiments used RIPC as a one-time protective measure. Considering that the effect of RIPC is closely related to the production of multiple endogenous factors, researchers have begun to focus on improved RIPC strategies. In recent years, how to improve the treatment of remote ischemia has received extensive attention. RIPC revealed biphasic protection [26]. The early protection stage appeared immediately after remote ischemic treatment, lasting for 2-3 h. The second protection stage lasted for 48–72 h after 12–24 h of preconditioning. Pryds et al. found that long-term RIPC therapy, although it did not improve ejection fraction in patients with chronic heart failure, increased skeletal muscle function, lowered blood pressure and NT-proBNP, and had anti-inflammatory and antiremodeling effects in patients with high NT-proBNP concentrations [27, 28]. This suggests that multiple repetitions of RIPC may provide additional cardioprotection compared to traditional single RIPC. Therefore, in this study, modified RIPC was applied to CAD patients undergoing CABG under elective CPB. It was observed that MRIC could effectively reduce the release of CK-MB in the immediate postoperative period and the release of hs-cTnT at postoperative 12th h in CAD patients undergoing CABG. However, it did not have a significant protective effect in lowering the postoperative concentration of NT-proBNP and renal injury in the postoperative period.
It is a common phenomenon that myocardial injury leads to the increase of myocardial enzymes after CABG, and the concentration of myocardial enzymes may be related to the prognosis of patients after CABG. A long-term follow-up study showed that CK-MB was an independent predictor of long-term mortality after CABG [29]. The peak concentration of hs-cTnT measured within 24 h after CABG was correlated with MACE, 30-day mortality, MI, and ICU stay > 48 h [30–32]. Previous studies have shown that the increase of NT-proBNP concentration after CABG is related to the occurrence of postoperative atrial fibrillation, early cardiac dysfunction, major adverse events, and postoperative length of stay [33, 34]. In this study, the MRIC and RIPC groups had a lower concentration of CK-MB than the control group at postoperative 0 and 12th h (p < 0.05). For the concentration of hs-cTnT, the overall trend of the MRIC group and the RIPC group was lower than that of the control group, although it was statistically significant only at 12th h after operation. Our results show that the role of RIPC in the second protective window is clear. At postoperative 12th h, MRIC and RIPC had lower CK-MB and hs-cTnT concentrations compared to the control group, although only in the MRIC group were both significantly different. This seems to suggest a stronger myocardial protective effect of MRIC. The lack of effect of distal IPC for longer periods after surgery may be due to a gradual attenuation of its effect. Considering the surgery time, the first protection window has passed after the end of the surgery, and cardiac reperfusion caused by CPB may cause secondary myocardial damage after the operation, which is not during the first protection window of RIPC, so it is not possible to observe whether it benefits obviously. In addition, there may be varying degrees of direct intraoperative damage to the heart, which could lead to a potential risk of bias.
Interestingly, IPC seems to have a negative effect on the concentration of NT-proBNP. MRIC group has a higher NT-proBNP concentration compared to the other two groups. Few previous studies have shown that RIPC can increase the concentration of NT-proBNP, and there is no relevant mechanism research at present. However, our preoperative auxiliary examination analysis shows that the left ventricular function was lower in the MRIC group of study subjects compared to the other two groups, and this baseline difference may be the main factor causing the higher concentration of NT-proBNP in the MRIC group. The effect of RIPC on postoperative NT-proBNP concentrations after CABG needs further study, but it should be applied with caution in patients with impaired left heart function.
Acute kidney injury (AKI) is a common complication in patients undergoing cardiac surgery, and it has a significant impact on operative mortality, ICU transition time, and length of hospital stay, with postoperative AKI occurring in approximately 20% of cardiac surgery patients [35]. There are no clinical studies showing that RIPC reduces the incidence of AKI after CABG, and meta-analyses have only shown a reduction in contrast kidney injury after PCI [36]. The meta-analysis only shows that RIPC can reduce contrast-induced renal injury after PCI, but it has no obvious effect on reducing renal injury after CABG. The results of this study showed no statistically significant differences in postoperative Cr concentrations among the three groups. In this study, the majority of the subjects had near-normal concentrations of postoperative Cr, which was an important reason for the lack of significant differences in the results. Moreover, the glomerular filtration rate and imaging tests were not observed in this study, so a single index may make the results unrepresentative. Therefore, in subsequent studies, more indicators that can reflect renal function impairment can be included to further evaluate the role of RIPC in renal injury or perfusion after CABG.
In addition, compared to the other two groups, the incidence of MACE in hospitals in the MRIC group was significantly reduced, which suggested that MRIC had a positive effect on improving the short-term prognosis after CABG. The higher MACE incidence in the control group may be attributed to the inclusion of frequent premature ventricular contractions (PVCs) and short runs of ventricular tachycardia in the MACE statistics, which could have contributed to the observed higher rate. Frequent PVCs and short runs of ventricular tachycardia have an adverse impact on prognosis, especially in patients with underlying heart disease or impaired cardiac function, who are at higher risk. This underscores the positive effect of MRIC in reducing ventricular arrhythmias. Moreover, patients in the MRIC group had poorer left heart function, but they had the lowest incidence of MACE, reinforcing the positive effect of MRIC in the postoperative period of CABG. In this study, there is no statistical difference in the occurrence of embodied MACE, and some of them are considered as low probability events, which need to be confirmed by large-scale clinical research.
This study did not confirm that MRIC or RIPC had an additional protective effect on medium-high-risk study subjects. Considering the presence of confounding factors such as reoperation, blood transfusion, and rehydration therapy in a small number of patients due to individual differences and the small number of high-risk study subjects included in the study resulted in a small difference in the overall risk. This may be an important reason for the lack of positive results, and further studies should be conducted in the future with larger sample sizes.
This study has some limitations, which are as follows: (1) This experiment was a single-center study with a small sample size and the results lacked some representativeness. (2) The observation index is relatively single and easily influenced by other factors. (3) The out-of-hospital adverse events and long-term prognosis of the study subjects were not followed up.
5. Conclusion
In conclusion, MRIC has cardioprotective effects and reduces the occurrence of postoperative MACE. MRIC could not reduce NT-proBNP and Cr concentrations after CABG. There is no significant difference in myocardial protection of MRIC in patients with different STS scores.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Defeng Pan, Hong Zhu, Yuanyuan Luo, Qi Sun, Xiaotong Wang, and Guodong Wang participated in the design and execution of the study and drafted the manuscript. Chunyan Huan, Minjia Guo, and Wanling Wu performed the data analysis. Yongbo Hou, Guoxiang Wang, and Jie Liu collected the samples and performed the experiments. All authors read and approved the final manuscript. Qi Sun and Xiaotong Wang contributed equally to this work.
Funding
This research was supported by the Xuzhou Municipal Science and Technology Bureau ∗ KC20097.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
Data are available from the first author upon request.