Long-Term Results of Minimally Invasive Mitral Valvuloplasty: Insights From a 12-Year Single-Center Experience
Abstract
Purpose: The safety and long-term durability of minimally invasive mitral valvuloplasty (MIMVP) remain controversial. This study aimed to compare the perioperative and long-term outcomes of minimally invasive mitral valve surgery (MIMVS) and conventional sternotomy.
Methods: This study included 476 patients who underwent mitral valve surgeries at our institution between January 2011 and December 2023. Patients were classified according to whether they underwent sternotomy: the nonsternotomy (NS: 271 cases) and sternotomy (S: 205 cases) groups. Perioperative and long-term outcomes were compared between the two groups.
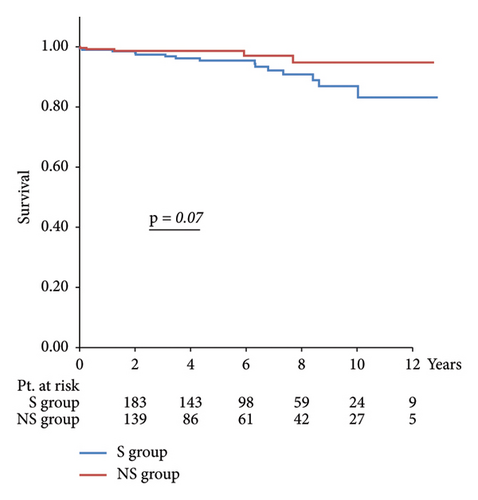
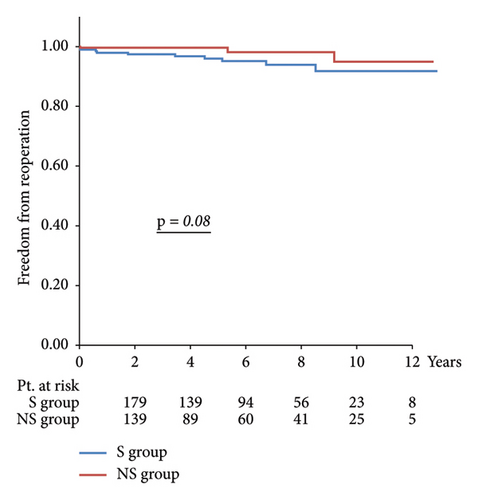
Results: The NS group had a lower preoperative age and EuroScore II. In the S group, the left ventricular ejection fraction was lower, while the left ventricular end-systolic diameter and left atrial diameter were larger. Operative time, cardiopulmonary bypass time, and aortic cross-clamp time were longer in the NS group. Postoperative atrial fibrillation, more transfusion, and increased length of hospital stay were more frequent in the S group. The 10-year freedom from reoperation and 10-year survival rates in the NS and S groups were 98.1% vs. 93.6% (p = 0.07) and 94.8% vs. 86.9%, respectively (p = 0.08), with no significant differences.
Conclusion: MIMVP demonstrates noninferior perioperative and long-term outcomes compared with conventional sternotomy.
1. Introduction
Minimally invasive mitral valvuloplasty (MIMVP) has been reported to offer benefits over conventional median sternotomy surgery in terms of cosmesis and early return to social activities [1, 2]. However, tendencies toward prolonged aortic cross-clamp times and higher morbidity have also been reported [3], making it difficult to establish its safety comprehensively.
In this study, we compared the perioperative and long-term outcomes of minimally invasive mitral valve surgery (MIMVS) and conventional sternotomy.
2. Methods
This retrospective observational study was approved by the institutional ethics committee, and the need for individual consent was waived.
Among the mitral valve surgeries performed at our institution between January 2011 and December 2023, 476 cases were included, excluding scheduled mitral valve replacement, aortic valve surgery, left ventricular reconstruction, ventricular septal defect, and atrial septal defect. Of these, patients who did not undergo sternotomy were classified into the nonsternotomy (NS) group, while those who underwent sternotomy were classified into the sternotomy (S) group. We compared the perioperative and long-term outcomes between the two groups.
2.1. Surgical Technique
Absolute contraindications of minimally invasive approaches include thoracic aortic aneurysm and the presence of a porcelain aorta, whereas relative contraindications include coronary artery disease and aortic valve regurgitation. All surgeries were performed under general anesthesia, and lung separation was achieved using a double-lumen endotracheal tube. A 3- or 4-cm incision was made along the fourth intercostal space in the anterior axillary line; in addition, to prevent postoperative pain, a rib spreader was not used; instead, a dual-ring wound protector was employed, and an intercostal nerve block was performed with ropivacaine. A port was created slightly posteriorly, in line with the main incision, and a 3D endoscope was inserted. In addition, an operating port with a 5-mm diameter was created at the third intercostal space (Figure 1). Details have been documented in previously published papers. [4].
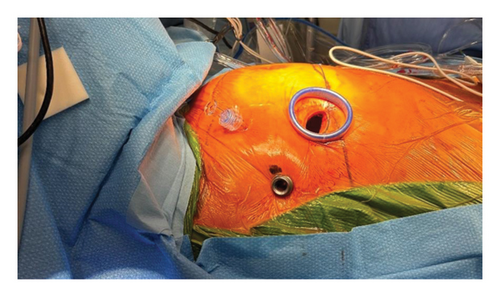
Canulation was performed via cut-down at the right groin under transesophageal echocardiography guidance using the right common femoral artery and right common femoral vein. In addition, in larger patients, a cannula was added from the right internal jugular vein to enhance venous drainage from the upper body. Aortic cross-clamping was performed, and myocardial protection was achieved by the antegrade delivery of a mixture of blood and crystalloid solution.
Annuloplasty was consistently performed for mitral valve repair in all cases. In instances of anterior leaflet prolapse, artificial chords were used, whereas resection, suturing, or plication was the primary techniques used for posterior leaflet pathology. In addition, if degeneration affected multiple scallops, artificial chords were used for posterior leaflet pathology. For cases with atrial fibrillation, we performed a maze procedure using cryoablation concurrently with left atrial appendage closure, if possible. Moreover, for moderate or severe tricuspid regurgitation, tricuspid valve repair with ring annuloplasty was performed simultaneously. At the end of the surgery, one chest drain was placed, with the tip positioned in the oblique sinus, passing through the base of the lung.
2.2. Statistical Analysis
Continuous data are presented as means ± standard deviations or medians (interquartile ranges), while categorical data are expressed as proportions. Statistical analyses were performed using the IBM SPSS Statistics 25 (IBM, Armonk, NY, USA). Continuous data were compared using the t-test or the Mann–Whitney U test based on whether they had a normal distribution, and categorical data were compared using the chi-square test. Cumulative survival rates were estimated using the Kaplan–Meier method, and comparisons were made using the generalized log-rank test. Statistical significance was set at p < 0.05.
3. Results
Table 1 shows the patients’ preoperative characteristics. The NS group had lower age and EuroScore II. The left ventricular ejection fraction was lower in the S group, and the left ventricular end-systolic diameter and left atrial diameter were larger in the S group. In addition, a history of cardiac surgery was significantly more common in the S group.
| Characteristics | NS group N = 271 |
S group N = 205 |
p value |
|---|---|---|---|
| Age | 58 ± 13 | 65 ± 13 | < 0.001 |
| Male sex | 169 (62%) | 128 (62%) | 0.986 |
| Body surface area (mm2) | 1.66 ± 0.2 | 1.61 ± 0.2 | 0.003 |
| Body mass index (kg/m2) | 22.1 ± 3.2 | 22 ± 3.5 | 0.756 |
| Hypertension | 114 (42%) | 97 (47%) | 0.254 |
| Diabetes mellitus | 24 (8.9%) | 30 (15%) | 0.049 |
| Dialysis | 1 (0.4%) | 6 (2.9%) | 0.046 |
| NYHA III-IV | 38 (14%) | 58 (28%) | < 0.001 |
| Atrial fibrillation | 55 (20%) | 89 (43%) | < 0.001 |
| Previous cardiac surgery | 2 (0.7%) | 9 (4.4%) | 0.012 |
| Urgent operation | 5 (1.8%) | 5 (2.4%) | 0.751 |
| EuroSCORE II (%) | 2.2 ± 5.0 | 4.5 ± 4.5 | < 0.001 |
| Infectious endocarditis | 4 (1.5%) | 2 (1.0%) | 0.703 |
| Echocardiographic features | |||
| Left ventricular ejection fraction (%) | 65 ± 9.4 | 58 ± 15 | < 0.001 |
| Left ventricular end-systolic diameter | 34 ± 5.6 | 36 ± 9.0 | < 0.001 |
| Left atrial dimension | 44 ± 7.5 | 47 ± 11 | < 0.001 |
- Abbreviation: NYHA = New York Heart Association.
The pathologies of the mitral valve are shown in Table 2. According to the Carpentier classification, Type 2 was more prevalent in the NS group, while Types 1 and 3b were more common in the S group. In additionally, among Type 2 lesions, posterior leaflet prolapse was significantly more frequent in the NS group, while no significant differences were observed in anterior leaflet lesions or both leaflet lesions.
NS group N = 271 |
S group N = 205 |
||
|---|---|---|---|
| Carpentier classification | |||
| Type I | 19 (7%) | 42 (20%) | < 0.001 |
| Type II | 247 (91%) | 146 (71%) | < 0.001 |
| Type IIIa | 2 (0.7%) | 5 (2.4%) | 0.146 |
| Type IIIb | 1 (0.4%) | 11 (5.3%) | 0.001 |
| Degenerative mitral regurgitation | |||
| Posterior leaflet prolapse | 163 (60%) | 96 (47%) | 0.004 |
| Anterior leaflet prolapse | 47 (17%) | 28 (14%) | 0.275 |
| Bileaflet prolapse | 37 (14%) | 24 (12%) | 0.529 |
Table 3 shows the perioperative outcomes. Concomitant procedures, such as the Maze procedure and tricuspid valve repair, were significantly more frequent in the NS group. The ring size was significantly larger in the NS group, and the use of artificial chords was also significantly more common in the NS group. Furthermore, complete rings were significantly more prevalent in the S group. Surgical time, cardiopulmonary bypass time, and aortic cross-clamp time were all significantly longer in the NS group. In addition, transfusion was significantly lower in the NS group, while postoperative atrial fibrillation and length of hospital stay were significantly higher in the S group. There were no significant differences in postoperative mitral regurgitation, cerebral infarction, acute renal failure, or in-hospital mortality rates. Furthermore, there were no occurrences of aortic injury or unilateral pulmonary edema in the NS group.
| Surgical outcomes | NS group N = 271 |
S group N = 205 |
p value |
|---|---|---|---|
| Operative time (min) | 254 ± 77 | 239 ± 69 | 0.028 |
| Cardiopulmonary bypass duration (min) | 168 ± 56 | 136 ± 52 | < 0.001 |
| Cross-clamp duration (min) | 115 ± 41 | 102 ± 42 | 0.001 |
| Endoscopy | 183 (68%) | 0 | |
| Partial sternotomy | 0 | 31 (15%) | |
| Concomitant procedures | |||
| Maze | 81 (30%) | 95 (46%) | < 0.001 |
| Left atrial appendage closure | 55 (20%) | 35 (17%) | 0.374 |
| Tricuspid valve repair | 30 (11%) | 89 (43%) | < 0.001 |
| Tricuspid valve replacement | 0 | 2 (1.0%) | 0.185 |
| Surgical technique | |||
| Annuloplasty | 263 (97%) | 196 (96%) | 0.402 |
| Complete ring | 67 (25%) | 95 (46%) | < 0.001 |
| Partial ring | 194 (72%) | 101 (49%) | < 0.001 |
| Ring size (mm) | 31.4 ± 2.9 | 30.7 ± 2.9 | 0.008 |
| Resection | 125 (46%) | 91 (44%) | 0.707 |
| Artificial chords | 94 (35%) | 53 (26%) | 0.039 |
| Conversion to mitral valve replacement | 7 (2.5%) | 8 (3.9%) | 0.398 |
| Postoperative outcomes | |||
| Post MR (degree) | 0.7 ± 0.7 | 0.7 ± 0.7 | 0.487 |
| In-hospital mortality | 1 (0.4%) | 1 (0.5%) | 0.843 |
| 30-day mortality | 1 (0.4%) | 1 (0.5%) | 0.843 |
| Postoperative AF | 73 (27%) | 91 (44%) | < 0.001 |
| Mechanical ventilation time (hours) | 3.4 ± 3.9 | 4.0 ± 5.5 | 0.148 |
| Hospital stay (days) | 9 (7–11) | 12 (10–14) | < 0.001 |
| Bleeding requiring reoperation | 2 (0.7%) | 6 (2.9%) | 0.080 |
| Transfusions | 49 (18%) | 63 (31%) | 0.001 |
| Acute renal failure | 4 (1.5%) | 1 (0.5%) | 0.396 |
| Cerebral infarction | 3 (1.1%) | 3 (1.5%) | 0.730 |
| Acute subdural hematoma | 5 (1.8%) | 0 | 0.073 |
| Mediastinitis | 0 | 1 (0.5%) | 0.431 |
| Readmission | 9 (3.3%) | 8 (3.9%) | 0.735 |
- Abbreviation: MR = mitral regurgitation.
Regarding long-term outcomes, the 10-year freedom from reoperation was 98.1% in the NS group and 93.6% in the S group (p = 0.07), and the 10-year survival rates were 94.8% in the NS group and 86.9% in the S group (p = 0.08), with no significant differences between the two groups.
Long-term outcomes are illustrated in Figure 2, with a 10-year reoperation avoidance rate of 98.1% in the NS group compared with 93.6% in the S group and a 10-year survival rate of 94.8% in the NS group versus 86.9% in the S group, with no significant differences observed between the two groups.
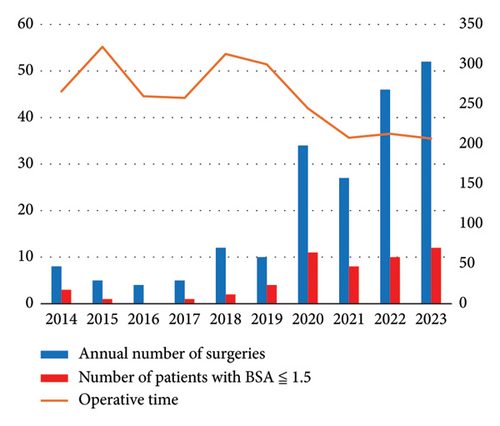
Furthermore, for a single surgeon, the annual average operation time has significantly decreased, and the annual number of surgeries tended to increase since 2020, when the cumulative number of surgeries exceeded 50 cases. Moreover, there has also been an increasing trend in procedures performed on smaller patients with a body surface area of less than 1.5 m2 since that year (Figure 3).
4. Discussion
Since Carpentier first reported MIMVS via right minithoracotomy in 1996 [5], this approach has been increasingly used safely and reproducibly, particularly given the advances in 3D endoscopy and robot-assisted surgery [6–8]. The consensus statement by the International Society for Minimally Invasive Cardiothoracic Surgery, based on a systematic review and meta-analysis conducted by Cheng et al. [9], indicated that MIMVS showed no significant difference from conventional sternotomy procedures in terms of in early and long-term mortality rates. Moreover, along with the similar morbidity rates, MIMVS also showed trends towards reduced blood transfusion, postoperative atrial fibrillation, sternal infections, ventilation time, intensive care unit stay, and hospitalization duration. However, it was also associated with a potentially higher incidence of stroke and aortic injury [10].
Our results were generally consistent with those of previous reports; however, no increase in the incidence of aortic injury or cerebral infarction was observed in MIMVP. Although no significant difference was found, we observed acute subdural hematoma (ASDH) in five cases (1.8%). None of these cases involved evident head trauma, and we attributed the occurrence of these hematomas to minor damage to the bridging veins due to fluctuations in intracranial pressure during cardiopulmonary bypass or slight body movements during surgery. The incidence of ASDH after cardiac surgery is approximately 0.1%, with a higher likelihood reported in females and in those with lower platelet counts during cardiac surgery [11, 12]. All of our patients who experienced ASDH were female, although their platelet counts were within the normal range. Among the four patients, only one underwent surgical evacuation of the hematoma, while the others were managed conservatively; none of the patients exhibited neurological deficits at discharge. To prevent ASDH and excessive intracranial pressure, we performed additional venous drainage via the right internal jugular vein. In addition, we promptly performed head computed tomography scans in cases where patients exhibited headaches or neurological symptoms to ensure early diagnosis.
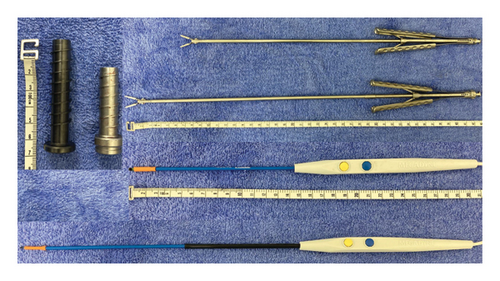
When performing MIMVP, achieving perfect reconstruction requires a high level of skill due to the limited visibility and narrow operative field. The learning curve for surgeons is often represented by the cumulative number of surgeries, with many reports suggesting that a technical threshold exists between 75 and 125 cases [13, 14]. In our institution, the current main surgeon began performing MIMVP in 2014, and since exceeding a cumulative total of 50 surgeries in 2020, the average annual operation time has fallen below 250 min, with an increasing trend in the annual number of surgeries. Particularly, Asians are often smaller in stature compared with Westerners [15], and expanding the indications for smaller patients has become a significant factor in increasing the annual number of surgeries. We evaluate the distance from the skin to the pleura and other factors preoperatively, and we adjust the lengths of the endoscopic port, electrocautery, and forceps according to the variations in body size (Figure 4). In addition, in smaller patients with a narrow common femoral artery, there may be cases where sufficient blood flow from cardiopulmonary bypass cannot be secured; therefore, we make it a practice to use the contralateral common femoral artery or the subclavian artery as the arterial cannulation site.
The main limitations of this study are its retrospective nature, it being conducted at a single institution, and the relatively small number of cases and events, which impose constraints. In addition, there is a selection bias between the S and NS groups.
5. Conclusion
MIMVP demonstrates noninferior perioperative and long-term outcomes compared with conventional sternotomy. However, more than 50 cases are required for proficiency in the technique, and further accumulation of cases, as well as improvements in techniques and instruments, will be necessary moving forward.
Ethics Statement
This study was approved by the Institutional Review Board—Ethics Committee at Nagoya Heart Center (Reference no. NHC-2024-1007-10). The need for individual consent was waived by the review board.
Consent
The need for informed consent was waived by the review board.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this study.
Open Research
Data Availability Statement
The data supporting the findings of this study can be obtained from the corresponding author upon reasonable request.