Homograft Root Replacement Does Not Provide Superior Outcomes in Invasive Aortic Valve Endocarditis Compared With Prosthetic Valve Conduits
Abstract
Background: Surgical dogma advocates for the use of homograft in invasive aortic valve endocarditis due to a perceived advantage in the prevention of recurrent infection. However, conclusive data to support this strategy are lacking. This study evaluated outcomes of root replacement in invasive aortic valve endocarditis using homografts or prosthetic-valved conduits.
Methods: A retrospective review of a single U.S. academic center’s aortic database identified 150 patients who underwent aortic root replacement for invasive aortic valve endocarditis from 2002 to 2022. Patients undergoing the Ross procedure or aortic valve replacement without root replacement were excluded from the study. Patients were divided into two groups based upon the type of valved conduit implanted. Preoperative characteristics, postoperative morbidity, reintervention for recurrence of infection, and short- and long-term survival were compared between the two groups.
Results: There were 70 patients who underwent a homograft root replacement (homograft), and 80 patients who received either a bioprosthetic or mechanical-valved conduit (prosthetic). The mean age of patients was 53.3 ± 15.6 and 21.3% were female. The overall incidence of preoperative stroke and aortic root abscess was 42% and 71%, respectively. There was no difference between the two groups in age, gender, end-stage renal disease, cardiogenic shock, and aortic root abscess. The prosthetic group had a higher incidence of preoperative stroke (prosthetic 52% vs. homograft 25%, p = 0.02). The incidence of preoperative prosthetic valve endocarditis was 30% for the cohort and significantly higher in the homograft group (p = 0.02). Reoperative sternotomy was 78.7% among the groups with a higher likelihood among the homograft group. Cardiopulmonary bypass and cross clamp times were shorter in the prosthetic group (p < 0.05). There was no difference in postoperative stroke or renal failure between the two groups. The 30-day mortality for the entire cohort was 20.1% and was increased in the homograft group (homograft 25.7% vs. prosthetic 16.3%, p = 0.15). At 7 years follow-up, survival was 62% in the prosthetic group and 53% in the homograft group. The need for reintervention due to recurrence of infection was 3.2% for the entire series and equivalent (homograft 3.5%, vs. prosthetic 4.2%, p = 0.82) between the groups.
Conclusions: The use of homograft for root replacement does not provide significant improved short- or long-term outcomes compared with prosthetic-valved conduits in invasive endocarditis. In this patient population, these data refute the necessity for a more complex procedure using homograft in these high-risk patients and conduit selection should be tailored to individual anatomy and surgeon-specific experience.
1. Introduction
Elective surgery of the ascending aortic, aortic arch, and aortic root can be safely performed with excellent outcomes for patients undergoing elective repair in the setting of primary sternotomy or reoperation in high volume aortic centers [1–3]. However, the surgical risk profile significantly increases for patients undergoing root replacement for invasive endocarditis of the native or previously replaced valve. In addition, the presence of an aortic root abscess identified with preoperative imaging further increases both the complexity of surgical repair and the perioperative risks with mortality rates ranging from 10% to 20%. The basis of this increase is due to the pathogen’s destructive nature of the periaortic tissues and the downstream involvement of multiple organ systems in the preoperative setting. Furthermore, the complex nature of the surgical reconstruction leads to extended cardiopulmonary bypass and myocardial ischemia times, which have been shown to negatively impact postoperative outcomes [4].
The use of cryopreserved allograft (homograft) has traditionally been accepted as the preferred valved conduit for root replacement in the setting of invasive aortic valve endocarditis due to the perceived advantage of decreased rates of reinfection and its ability to provide additional tissue that may be absent after surgical debridement. Previous studies have demonstrated varying results for homograft root replacement in the setting of invasive endocarditis with 30 days mortality ranging from 4% to 25% [5–8]. Conclusive data to support this strategy are lacking as previous studies comparing the use of homograft to bioprosthetic or mechanical-valved conduits in the setting of root replacement for endocarditis have demonstrated no difference in outcomes [9–11].
It is well recognized that homograft root replacement is performed by a highly select cohort of surgeons routinely and due to its technical complexity typically extends cardiopulmonary bypass and myocardial ischemia times for isolated root reconstruction. Furthermore, the availability of homograft conduits vary across institutions. Given the lack of conclusive data demonstrating an advantage of the homograft in the setting of invasive aortic valve endocarditis, we hypothesized that equivalent outcomes could be achieved with bioprosthetic or mechanical-valve conduits. The goal of this study is to evaluate the impact of conduit selection on operative mortality, perioperative morbidity, freedom from reinfection, and long-term survival in patients undergoing root replacement for invasive aortic valve endocarditis.
2. Patients and Methods
This study was approved by the Emory University Institutional Review Board (eIRB 22795, date of approval: 07/29/2009). The need for individual patient consent was waived given that all data were retrospectively collected. We retrospectively queried a single U.S. academic center’s aortic database to identify 150 patients who underwent aortic root replacement for invasive aortic valve endocarditis from 2002 to 2022. Patients undergoing the Ross procedure or aortic valve replacement without root replacement were excluded from the study. Patients were divided into two cohorts based upon the type of valved conduit implanted. There were 70 patients who underwent root replacement with a cryopreserved allograft valved conduit (homograft) and 80 patients who received either a bioprosthetic or mechanical-valved conduit (prosthetic). Conduit selection was at the individual surgeon’s discretion based upon the individual case.
Demographic information, past medical and surgical history, perioperative data, reintervention for recurrence of infection, and short- and long-term follow-up data were compared between the groups. The primary outcome was all-cause mortality. Secondary outcomes were reintervention for recurrent infection, postoperative stroke, and renal failure. Operative mortality was defined as occurring within 30 days of the index hospitalization.
All procedures were performed through median sternotomy with cardiopulmonary bypass. Cannulation and cerebral protection strategy were based upon the extent of the anticipated aortic reconstruction. Conduit selection was made at the time of operation on a case-by-case basis. Prosthetic conduit implantation included premanufactured mechanical or bioprosthetic-valved conduits or surgeon constructed mechanical, stented bioprosthetic, or stentless bioprosthetic-valved conduits. Suture technique included simple interrupted, ventricular based horizontal mattress with and without pledgets, and aortic-based horizontal mattress with pledgeted sutures and was determined at the discretion of the operating surgeon at the time of repair. Aortic annular reconstruction was performed selectively using bovine pericardium.
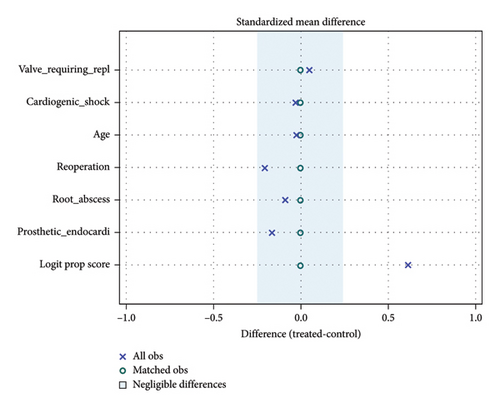
Continuous demographic characteristics and baseline clinical variables were summarized using the mean ± standard deviation (or median [1st quartile–3rd quartile], as appropriate). Categorical variables were reported as frequencies (percentage). To correct the preoperative imbalance between the homograft and prosthetic groups, patients were matched based on prosthetic endocarditis, root abscess, reoperation, age, and secondary valve intervention, resulting in 53 matched pairs. The propensity score matching was done using the greedy nearest neighbor matching. Matching was preformed based on six variables: additional valve requiring replacement, cardiogenic shock, age, reoperation, root abscess, and prosthetic endocarditis (Figure 1). The two groups were also compared using the intraoperative and postoperative variables. For continuous variables, two-sample t-tests were performed. For categorical variables, chi-square tests were conducted. Finally, the Kaplan–Meier method was used to estimate survival probabilities for each group as well as draw survival curves. When the event of interest was reoperation, Gray’s method was used to compare groups, as death needed to be accounted for as a competing risk (Supporting Information) [12]. Cumulative incidence functions were constructed to estimate the probability of reoperation on invasive aortic valve endocarditis. Tests of hypotheses were two-sided with a significance level of 0.05. Data for major baseline and outcome variables were complete and no data manipulation necessary for managing missing data. All data analyses were performed using SAS 9.4 statistical software (SAS Institute Inc, Cary, NC).
3. Results
Of the 150 patients included in this study, 70 patients had homograft replacement and 80 patients had prosthetic-valved conduits. Within the prosthetic group, 22.5% received mechanical-valved conduits, the remaining 77.5% received conduits constructed of stented or stentless bioprosthetic valves. Overall mean follow-up time is 2.44 ± 3.43 years.
Baseline demographics for the unmatched group showed no differences in age, gender, hypertension, chronic lung disease, end stage renal disease requiring dialysis, diabetes, peripheral arterial disease, cardiogenic shock, severe aortic insufficiency, or the presence of root abscess at the time of surgery (Table 1). The mean age of patients was 53.2 ± 15.3 and 21.9% were female. End-stage renal disease was present in 13.0% of the patients. Hypertension, chronic lung disease, diabetes, and peripheral arterial disease were present in 76.7%, 23.2%, 26.0%, and 9.0% patients, respectively. Aortic root abscess was identified at the time of surgery in 70.1% of the patients. For the unmatched cohort, cerebrovascular accident was more common within the patients who received prosthetic conduits (52.0, 25.0; p = 0.02). Reoperative sternotomy was required in 78.7% of all patients and was more common in the homograft cohort (63.5, 90.0; p = 0.002). The incidence of preoperative prosthetic valve endocarditis was 71.0% for the unmatched cohort and significantly higher in the homograft group (63.5, 80.0; p = 0.02).
| Variable | All (155) | Prosthetic (85) | Homograft (70) | p value |
|---|---|---|---|---|
| Age | 53.17 ± 15.29 | 52.01 ± 15.86 | 54.59 ± 14.56 | 0.30 |
| Gender (female) | 34 (21.94%) | 20 (23.53%) | 14 (20.00%) | 0.6 |
| Hypertension | 119 (76.77%) | 63 (74.12%) | 56 (80.00%) | 0.39 |
| CVA | 33 (42.31%) | 26 (52.00%) | 7 (25.00%) | 0.02 |
| Chronic lung disease | 36 (23.23%) | 12 (14.12%) | 24 (34.29%) | 0.003 |
| Dialysis (ESRD) | 19 (13.01%) | 12 (14.46%) | 7 (11.11%) | 0.55 |
| Diabetes | 40 (25.97%) | 19 (22.62%) | 21 (30.00%) | 0.30 |
| Peripheral arterial disease | 14 (9.03%) | 7 (8.24%) | 7 (10.00%) | 0.70 |
| Reoperation | 122 (78.71%) | 59 (69.41%) | 63 (90.00%) | 0.002 |
| Prosthetic endocarditis | 110 (70.97%) | 54 (63.53%) | 56 (80.00%) | 0.02 |
| Root abscess | 110 (70.97%) | 57 (67.06%) | 53 (75.71%) | 0.24 |
| Cardiogenic shock | 13 (8.39%) | 6 (7.06%) | 7 (10.00%) | 0.51 |
| Secondary valve intervention | 24 (15.48%) | 15 (17.65%) | 9 (12.86%) | 0.41 |
| Aortic insufficiency (severe) | 18 (12.08%) | 9 (10.71%) | 9 (13.85%) | 0.6 |
| CPB time | 261.50 (215.00,305.50) | 246.50 (202.00,296.00) | 271.00 (228.00,321.00) | 0.02 |
| Aortic crossclamp (min) | 210.00 (184.50,244.50) | 201.50 (175.00,237.00) | 218.00 (195.00,250.00) | 0.08 |
| Postop perm stroke | 8 (6.40%) | 5 (7.04%) | 3 (5.56%) | < 0.99 |
| Pneumonia | 12 (8.96%) | 8 (10.26%) | 4 (7.14%) | 0.53 |
| Renal failure (new dialysis) | 12 (8.96%) | 8 (10.26%) | 4 (7.14%) | 0.53 |
| Reoperation for bleeding | 24 (17.91%) | 14 (17.95%) | 10 (17.86%) | 0.99 |
| Postop duration (days) | 9.00 (6.00, 14.00) | 10.00 (7.00, 18.00) | 8.50 (6.00, 13.00) | 0.005 |
| 30-day morality | 30 (19.35%) | 12 (14.12%) | 18 (25.71%) | 0.07 |
| Long-term mortality | 45 (29.03%) | 18 (21.18%) | 27 (38.57%) | 0.02 |
| Reintervention | 9 (5.8%) | 5 (5.9%) | 4 (5.7%) | 0.73 |
| Infection | 5 (3.2%) | 3 (3.5%) | 3 (4.2%) | 0.82 |
| Structural failure | 4 (2.9%) | 2 (2.4%) | 1 (1.4%) | 0.67 |
| Death and valve events | 52 (33.55%) | 22 (25.88%) | 30 (42.86%) | 0.03 |
- Note: Quantitative variables are summarized using the mean ± standard deviation (or median [first quartile, third quartile], as appropriate). Categorical variables are reported using frequency (percentage of the group).
Patients were matched categorically for patients over the median age of 55.0 years, prosthetic endocarditis, presence of aortic root abscess, reoperation, and secondary valve intervention, resulting in 53 matched pairs. Within the matched pairs, there was no significant difference between age, gender, hypertension, cerebrovascular accident, end-stage renal disease, diabetes, peripheral arterial disease, presence of aortic root abscess, prosthetic endocarditis, reoperation, or concomitant valve surgery (Table 2). Chronic lung disease was more prevalent among the homograft group (15.1, 35.9; p = 0.01), while preoperative severe aortic insufficiency was more common in the prosthetic group (50.1, 30.6; p = 0.02).
| Variable | All (106) | Prosthetic (53) | Homograft (53) | p value |
|---|---|---|---|---|
| Age (> 55) | 50 (47.17%) | 25 (47.17%) | 25 (47.17%) | < 0.99 |
| Gender (female) | 23 (21.70%) | 15 (28.30%) | 8 (15.09%) | 0.1 |
| Hypertension | 81 (76.42%) | 39 (73.58%) | 42 (79.25%) | 0.49 |
| CVA | 23 (39.66%) | 17 (50.00%) | 6 (25.00%) | 0.06 |
| Chronic lung disease | 27 (25.47%) | 8 (15.09%) | 19 (35.85%) | 0.01 |
| Dialysis (ESRD) | 10 (10.20%) | 5 (9.62%) | 5 (10.87%) | < 0.99 |
| Diabetes | 27 (25.71%) | 11 (21.15%) | 16 (30.19%) | 0.29 |
| Peripheral arterial disease | 9 (8.49%) | 3 (5.66%) | 6 (11.32%) | 0.49 |
| Reoperation | 96 (90.57%) | 48 (90.57%) | 48 (90.57%) | < 0.99 |
| Prosthetic endocarditis | 90 (84.91%) | 45 (84.91%) | 45 (84.91%) | < 0.99 |
| Root abscess | 76 (71.70%) | 38 (71.70%) | 38 (71.70%) | < 0.99 |
| Secondary valve intervention | 10 (9.43%) | 5 (9.43%) | 5 (9.43%) | < 0.99 |
| Aortic insufficiency (severe) | 42 (41.18%) | 27 (50.94%) | 15 (30.61%) | 0.02 |
| CPB time (min) | 263.00 (218.00, 300.00) | 244.00 (199.00, 283.00) | 271.00 (233.00, 307.00) | 0.09 |
| Aortic crossclamp (min) | 210.00 (187.00, 243.00) | 200.50 (175.00, 235.00) | 217.00 (195.00, 249.00) | 0.46 |
| Postop perm stroke | 5 (5.81%) | 3 (6.82%) | 2 (4.76%) | < 0.99 |
| Pneumonia | 7 (7.61%) | 6 (12.24%) | 1 (2.33%) | 0.12 |
| Renal failure (new dialysis) | 6 (6.52%) | 4 (8.16%) | 2 (4.65%) | 0.68 |
| Reoperation for bleeding | 16 (17.39%) | 10 (20.41%) | 6 (13.95%) | 0.42 |
| Postop duration (days) | 9.00 (6.00, 13.00) | 10.00 (7.00, 15.00) | 8.00 (6.00, 11.00) | 0.006 |
| 30-day morality | 20 (18.87%) | 8 (15.09%) | 12 (22.64%) | 0.32 |
| Long-term mortality | 31 (29.25%) | 11 (20.75%) | 20 (37.74%) | 0.05 |
| Death and valve events | 35 (33.02%) | 13 (24.53%) | 22 (41.51%) | 0.06 |
- Note: Quantitative variables are summarized using the mean ± standard deviation (or median [first quartile, third quartile], as appropriate). Categorical variables are reported using frequency (percentage of the group).
Cardiopulmonary bypass and crossclamp times were shorter in the unmatched prosthetic group (p < 0.05), but there was no difference identified after matching.
3.1. Early Outcomes
Thirty-day mortality for the entire cohort was 19.4% and was increased in the homograft group (prosthetic 14.1% vs. homograft 25.7%, p = 0.07) (Figure 2). Postoperative stroke was 6.4% for the entire cohort and not significantly different between groups (p < 0.99). Renal failure requiring new postoperative hemodialysis was 8.9% for the entire cohort and not significantly different between groups (p = 0.53).
Short-term mortality for the matched cohort was 18.9% overall and remained statistically insignificant for the matched cohort (prosthetic 15.1% vs. homograft 22.6%; p = 0.32). Permanent stroke, pneumonia, reoperation for bleeding, new onset dialysis, and length of stay were equivalent among the groups.
3.2. Late Clinical Outcomes
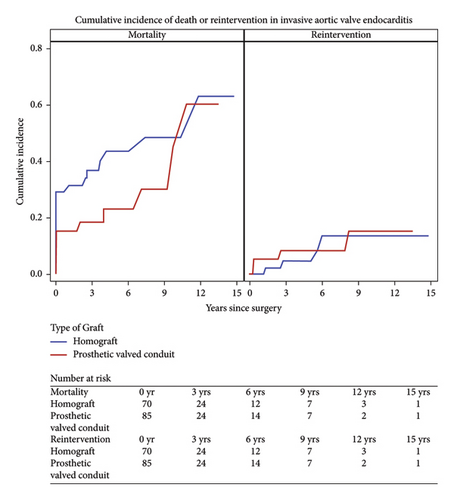
Long-term survival was 70.8% among the matched cohort and favored the prosthetic group (79.3, 62.3; p = 0.05). At 7-year follow up, survival was 62% in the prosthetic group and 53% in the homograft group (Figure 3).
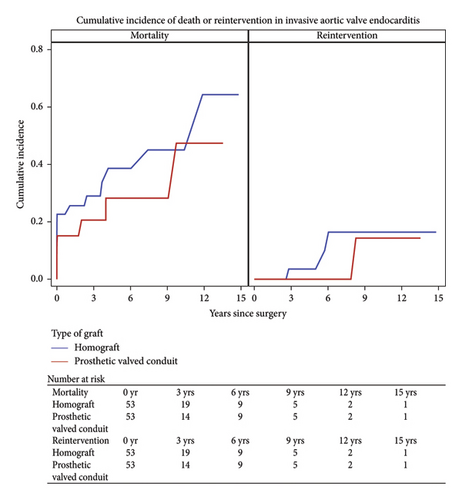
The overall freedom from reintervention was 94.2%. Within the prosthetic group, five patients (5.9%) required reoperation of which three (3.5%) required reoperation for reinfection, of which two patients presented with reinfection from a new pathogen. The average interval for reoperation for the bioprosthetic group was 3.1 years. Four patients within the homograft group (5.7%) required reoperation. Recurrent endocarditis was the indication for three of these patients in which one patient had recurrence of the original pathogen. The average interval for reoperation in the homograft group was 3.9 years.
4. Comments
This study reviewed a single center’s experience of aortic root replacement in the setting of invasive endocarditis over a 20-year period. We compared patients receiving homograft conduit to prosthetic-valved conduit. Conduit selection was determined on an individual basis at the time of operation. Aortic root abscess was defined as the presence of pus at the time of surgery and present in 70.97% of the patients. Despite this finding, conduit selection did not differ among this subgroup of patients. Reconstruction with homograft was more prevalent among patient with prosthetic endocarditis requiring redo sternotomy. Overall, early and late mortality did not differ between the groups in this study, however trended toward favoring the prosthetic group over time (Figures 4 and 5). Reinfection rates were equivalent over the follow-up period.
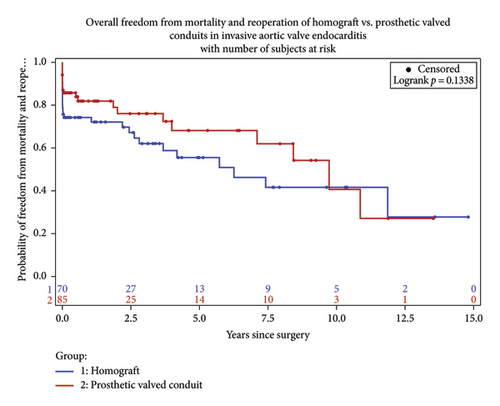
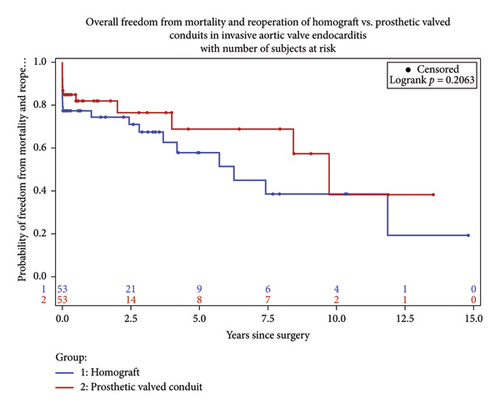
Early mortality for aortic root intervention in the setting of invasive endocarditis ranges as high as 10%–20%. Over the years, several studies have shown nonsuperiority of homograft replacement with regards to short- and long-term survival and recurrence of infection [9–11]. Traditional training has favored the avoidance of prosthetic material within an infected field due to the perceived benefit of reduced future rates of infection. In addition, complete debridement of the infected annular tissue with identification of the offending pathogen and appropriate antibiotic therapy is a mainstay in treatment. However, aggressive debridement of infected annular tissue oftentimes results in large defects of denuded tissue, which may lend itself to the pliability of a homograft or surgeon constructed stent less bioprosthetic-valved conduit. Alternatively, the rigidity of a stented bioprosthetic or mechanical conduit makes these options less appealing in patients with severe annular destruction. In addition, advancements in molecular tissue assessment for previously unidentified or culture negative pathogens may be useful in identifying offending pathogens and tailoring antibiotic therapies. PCR tissue evaluation has higher sensitivity and specificity in identifying pathogens that may have already been treated with long standing antibiotic therapy or pathogens not identified with traditional blood cultures or gram staining preoperatively [13]. We contend that aggressive annular debridement and pathogen identification coupled with targeted antibiotic therapy are as important as conduit selection and should be effectively utilized for each case to terminate the offending pathogen and limit microbial resistance.
There are limited data regarding aortic root replacement in the setting of invasive endocarditis. Kim et al. recently found no significant benefits of homografts compared with prosthetic valves with regards to reinfection or survival. However, this study was not limited to aortic root replacement and included subgroup of patients that underwent isolated AVR as a less extensive approach in this higher risk cohort [10]. The closest comparison study was a comprehensive retrospective review performed by Jasser et al., which looked specifically at aortic root replacement in the setting of invasive endocarditis with similar results to what we have demonstrated in this study. Over a 10-year period, they performed aortic root replacement of 134 patients. Of those patients, 67.2% had prosthetic endocarditis requiring reoperation and 82.1% were found to have root abscess at the time of surgery. Their group implanted 73.1% prosthetic-valved conduits and 26.9% homografts and found no difference in late mortality between the groups [11].
The use of homograft for root replacement in this study did not provide superior short- or long-term outcomes compared with prosthetic-valved conduits in the setting of invasive endocarditis. The data call into question the absolute need for a homograft replacement in this high-risk population, which is often times a more complex repair and not widely available to all surgeons. Although structural valve deterioration was low within this study, a future reoperation for valve deterioration may lend itself favorable to a nonhomograft conduit given the severe calcification associated with homografts and the complex nature of homograft explantation. Svensson et al. published a comprehensive review of 25-year data comparing conduit selection for elective aortic root replacement. Their findings demonstrated the safety and efficacy of each method. Notably, this study showed lower postoperative gradients among patient’s receiving homograft conduits; however, this group also showed a higher likelihood of severe AI at long-term follow-up. Conduit selection in the setting of invasive endocarditis should be based on patient anatomy and the degree of annular destruction at the time of operation. Surgeon specific experience and conduit availability should guide repair technique for these patients.
Limitations of this study include the retrospective nature of a single centers experience. In addition, given the general infrequent nature of this patient population, this study is underpowered to make any conclusive statements with regards to conduit selection. The sterility of the surgical field at the time of operation also confounds the interpretation of this study. Although conduit selection appeared to be equivalent amongst groups in the setting of annular abscess, homografts may have been more frequently selected in patient with ongoing active infections and annular destruction. Finally, this experience is confined to a high volume aortic center and may not translate to lower volume centers. For these reasons, further studies are needed to definitively assess conduit selection in this patient population.
Ethics Statement
This study was approved by the Emory University Institutional Review Board (eIRB 22795, date of approval: 07/29/2009). Need for individual patient consent was waived given that all data were retrospectively collected.
Disclosure
Abstract presented at 59th Society of Thoracic Surgeons Annual Meeting, January 21–23, 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All listed authors have made a significant contribution to the research in the manuscript, approved its claims, and agreed to be an author. Specifically, the authors have contributed equally to this manuscript in the following areas.
1. Woodrow J. Farrington: provided substantial contributions to the conception or design of the work, the acquisition, analysis, and interpretation of data and drafting the work and reviewing it critically for important intellectual content.
2. Xiaoying Lou: provided substantial contributions to the acquisition, analysis, and interpretation of data and drafting the work and reviewing it critically for important intellectual content.
3. Jonathan R. Zurcher: provided substantial contributions to the analysis and interpretation of data and drafting the work and reviewing it critically for important intellectual content.
4. Edward P. Chen: provided substantial contributions to the conception or design of the work, the acquisition, analysis, and interpretation of data and drafting the work and reviewing it critically for important intellectual content.
5. William Brent Keeling: provided substantial contributions to the conception or design of the work, the acquisition, analysis, and interpretation of data and drafting the work and reviewing it critically for important intellectual content.
6. Bradley G. Leshnower: provided substantial contributions to the conception or design of the work; the acquisition, analysis, and interpretation of data and drafting the work and reviewing it critically for important intellectual content.
Furthermore, all authors give final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
There was no specific funding for this project.
Supporting Information
Code is provided used for calculating competing risks and related Kaplan Meier curves.
Open Research
Data Availability Statement
The underlying data are available on request from the corresponding author.