Prevalence and Predictors of Venous Thromboembolism Following Coronary Bypass Surgery
Abstract
Background: Venous thromboembolism (VTE) is a rare complication after coronary artery bypass surgery (CABG), leading to increased morbidity and mortality. There are no current societal guidelines directing prophylaxis. Utilizing a regional database, we sought to determine the prevalence of VTE and characterize regional center practices.
Methods: We identified all patients undergoing on-pump, isolated CABG (2010–2020). Patients on oral therapeutic anticoagulation or requiring mechanical circulatory support were excluded. Participating centers were surveyed to determine center level practices. Multivariable regression and hierarchical logistic regression were utilized for risk-adjusted outcomes and influence of center practices on VTE rates, respectively.
Results: Of 20,719 CABG patients, the overall prevalence of postoperative VTE was 1.3% (266/20,719). Patients developing VTE were more often female (30.1% vs. 23.4%, p = 0.01), had higher STS predicted risk of mortality (1.2% [0.7%, 2.2%] vs. 0.9% [0.5%, 1.7%], p < 0.001) and higher unadjusted operative mortality (4.1% vs. 1.0%, p < 0.001). Risk-adjusted analysis demonstrated pulmonary embolism as an independent predictor of mortality (OR = 3.4 [1.06, 11.0], p = 0.04). Increasing time from admission to surgery (OR = 1.05 [1.01, 1.09], p = 0.001), preoperative heparin use (OR = 1.47 [1.13, 1.90], p = 0.004), and intraoperative prothrombin complex concentrate (PCC) (OR = 4.85 [1.47, 15.96], p = 0.009) were predictors of VTE. Regional practices were mainly homogenous with no specific center-level protocol associated with decreases in VTE.
Conclusion: VTE following CABG is an infrequent postoperative complication with pulmonary embolism as an independent predictor of mortality. Increasing time from admission to surgery and intraoperative PCC may increase the risk of VTE.
1. Introduction
Venous thromboembolism (VTE) has been known to be a major contributor to morbidity and mortality after major surgery although in cardiac surgery has been previously identified as a rare event [1, 2]. However, a recent systematic review and meta-analysis by Ho et al. showed that the incidences of symptomatic deep vein thrombosis (DVT) and pulmonary embolism after cardiac surgery were higher than previously thought at 3.2% and 0.6%, respectively, with incidence of a fatal PE at 0.3% [3]. Despite these results, there is considerable variability in clinical practice due to a lack of societal guidelines and conflicting recommendations on pharmacologic prophylaxis following cardiac surgery [4–7].
Many of the identifiable risk factors for VTE including age, obesity, and prolonged ventilation are common in the cardiac surgical population, thus highlighting the potential benefit of VTE prophylaxis [8–10]. However, controversy persists as to the ideal modality to achieve this goal, be it mechanical, pharmacologic, or both while balancing the risk of postoperative bleeding [3, 5–7, 11].
The objective of this study was to utilize a regional collaborative of cardiac surgery centers to define contemporary practices in VTE prophylaxis and their impact on outcomes as well as to identify predictors of VTE following isolated coronary artery bypass surgery (CABG). We hypothesized that the presence of a center-level VTE prevention protocol would be associated with reduced rates.
2. Methods
2.1. Patients
The Virginia Cardiac Services Quality Initiative is a regional collaborative of 17 hospitals in the state of Virginia that captures each center’s Society of Thoracic Surgeons Adult Cardiac Surgery Database data. We identified all patients from these 17 centers undergoing isolated CABG between January 2010 and August 2022. Patients on preoperative oral therapeutic anticoagulation, undergoing emergent surgery, major concomitant procedures, circulatory or fibrillatory arrest, undergoing off-pump approach, or requiring any perioperative mechanical circulatory support were excluded. Outcomes were followed to 30 days following discharge from database availability. VTE was defined as any postoperative DVT or PE occurring in-hospital or within 30 days of index hospitalization. The diagnosis of PE required imaging confirmation such as via angiogram, computed tomography angiogram, or ventilation perfusion scan. This study was approved by the University of Virginia’s Institutional Review Board (#23305).
2.2. Survey
Given perceived variability in center VTE prophylaxis, all participating centers were surveyed to gain an understanding of their protocols for isolated CABG patients. A survey was distributed utilizing the electronic Qualtrics XM platform (Qualtrics International Inc., Seattle, WA, Provo, UT). Center respondents were instructed to respond as if answering questions pertaining to an isolated CABG patient with no indication for or against anticoagulation. Respondents consisted of surgeons and licensed independent practitioners of the ICU staff who were equipped with appropriate knowledge to answer the survey. Survey logic and required questions were utilized to ensure complete answers. Responses were matched to each institution’s corresponding STS ACSD data in a deidentified manner and represented as a categorical variable.
2.3. Statistical Analysis
Continuous variables were analyzed via the Wilcoxon rank sum test with expression as either the mean and standard deviation or median with interquartile ranges (IQRs). Categorical variables were expressed as absolute numbers with percentages and analyzed via chi-square testing or Fisher’s exact test for expected outcomes of less than 5 events. Logistic regression was utilized to determine independent predictors of VTE and risk-adjusted mortality. Modeling of VTE predictors was accomplished via stepwise selection with cutoff p value less than 0.2. Variables for multivariable modeling were chosen based on statistical significance on univariate analysis and known clinical contributors based on present literature. Survey data were analyzed utilizing a hierarchical logistic regression to account for center variability to determine center protocol effects on VTE. Median imputation was utilized for missing data with all missingness less than 5%. All statistical analyses were carried out using SAS Version 9.4 (SAS Institutive, Cary, NC), with a p value less than 0.05 determining significance.
3. Results
3.1. Population Characteristics
From 2010 until 2022, a total of 24,188 patients underwent CABG (Figure 1). Following exclusions, a total of 20,719 patients undergoing isolated CABG met inclusion criteria. The rate of VTE was 1.3% (266/20,719). DVT and pulmonary embolism accounted for 60.2% (160/266) and 39.8% (106/266) of VTE events, respectively. Most of these events occurred during the index hospitalization (69.5%, 185/266), with 32.0% (85/266) as a reason for readmission within 30 days.
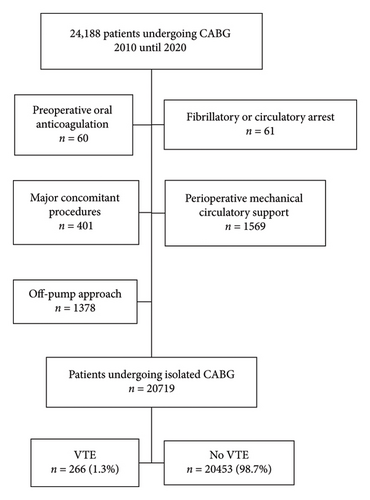
Patients developing VTE were older (67 vs. 65 years, p = 0.002) and more often female (30.1% vs. 23.4%, p = 0.01) with higher median predicted risk of mortality (PROM) (1.2% vs. 0.9%, p < 0.001). Acute coronary syndrome (ACS) was the presentation more often in VTE patients (45.9% vs. 39.9%, p = 0.047) including ST-elevated myocardial infarctions (STEMIs) (3.0% vs. 1.4%, p = 0.03). There was no difference between groups regarding preoperative antiplatelets like adenosine diphosphate receptor inhibitors (ADPi) (4.5% vs. 5.7%, p = 0.39) or glycoprotein IIb/IIIa inhibitors (GpIIb/IIIa) (2.3% vs. 1.8%, p = 0.54) although higher preoperative, unfractionated weight-based heparin was used within 48 h of surgery in VTE patients (47.0% vs. 35.1%, p < 0.001) (Table 1).
| Preoperative characteristics | VTE (n = 266, 1.3%) | No VTE (n = 20,453, 98.7%) | p value |
|---|---|---|---|
| Age (years) | 67 [59, 74] | 65 [59, 72] | 0.002 |
| Body mass index (kg/m2) | 29.7 [26.6, 34.0] | 29.0 [25.8, 33.1] | 0.008 |
| Sex (female) | 80 (30.1%) | 4790 (23.4%) | 0.01 |
| Diabetes | 119 (44.7%) | 9459 (46.3%) | 0.62 |
| Sleep apnea | 48 (18.1%) | 2701 (13.2%) | 0.02 |
| Chronic lung disease | 88 (33.1%) | 5314 (26.0%) | 0.009 |
| Atrial fibrillation or flutter | 6 (2.3%) | 303 (1.5%) | 0.30 |
| White blood cell count (10 k) | 7.9 [6.4, 9.8] | 7.6 [6.3, 9.2] | 0.03 |
| Platelet count (100 k) | 213 [173, 259] | 217 [186, 250] | 0.18 |
| Hematocrit (%) | 39 [34.5, 41.6] | 40 [36, 43] | < 0.001 |
| Albumin (g/dL) | 3.8 [3.4, 4.0] | 3.8 [3.6, 4.0] | 0.10 |
| Hemoglobin A1c (%) | 6.1 [5.6, 7.2] | 6.1 [5.7, 7.0] | 0.83 |
| INR | 1.0 [1.0, 1.1] | 1.0 [1.0, 1.1] | 0.004 |
| Total bilirubin | 0.5 [0.4, 0.7] | 0.5 [0.4, 0.9] | 0.73 |
| MELD | 7.5 [6.4, 8.4] | 7.5 [6.4, 8.3] | 0.61 |
| Preoperative creatinine (g/dL) | 1.0 [0.8, 1.12] | 1.0 [0.8, 1.2] | 0.90 |
| Ejection fraction (%) | 55 [45, 60] | 55 [48, 60] | 0.81 |
| Urgent status | 188 (70.7%) | 13,130 (64.2%) | 0.03 |
| Preoperative low molecular weight heparin | 25 (9.4%) | 2426 (11.9%) | 0.22 |
| Preoperative weight-based unfractionated heparin | 125 (47.0%) | 7171 (35.1%) | < 0.001 |
| Preoperative thrombin inhibitor | 1 (0.4%) | 14 (0.07%) | 0.18 |
| Aspirin use | 237 (89.1%) | 18,355 (89.7%) | 0.73 |
| ADP inhibitor use | 12 (4.5%) | 1172 (5.7%) | 0.39 |
| GPIIa/IIIb inhibitor use | 6 (2.3%) | 360 (1.8%) | 0.54 |
| IV nitrate use within 24 h | 26 (9.8%) | 1605 (7.9%) | 0.25 |
| Preoperative inotrope use | 3 (1.1%) | 101 (0.5%) | 0.15 |
| Prior myocardial infarction | 133 (50.0%) | 9093 (44.5%) | 0.07 |
| Myocardial infarction within 1 week | 87 (32.7%) | 5752 (28.1%) | 0.10 |
| Time from admission to surgery (days) | 3 [1, 5] | 2 [0, 4] | < 0.001 |
| Acute coronary syndrome presentation | 122 (45.9%) | 8151 (39.9%) | 0.047 |
| NSTEMI presentation | 57 (21.4%) | 3656 (17.9%) | 0.13 |
| STEMI presentation | 8 (3.0%) | 286 (1.4%) | 0.028 |
| Predicted risk of mortality | 1.2% [0.7%, 2.2%] | 0.9% [0.5%, 1.7%] | < 0.001 |
| Predicted risk of morbidity or mortality | 11.1% [7.7%, 17.1%] | 9.4% [6.5%, 14.4%] | < 0.001 |
When looking at intraoperative characteristics (Table 2), patients that developed VTE had similar cardiopulmonary bypass (94 [77, 116] vs. 91 [72, 86] minutes, p = 0.05) and cross clamp times (70 [53, 90] vs. 68 [52, 86] minutes, p = 0.21) although longer total operative times (239 [205, 292] vs. 231 [196, 271] minutes, p = 0.003). They were more likely to receive blood products in the operating room including fresh frozen plasma (5.6% vs. 2.7%, p = 0.004) and red blood cells (17.7% vs. 10.1%, p < 0.001). There was no difference in the utilization of Factor VII use between the groups (p > 0.05) although a higher rate of intraoperative prothrombin complex concentrate (PCC) in VTE patients (1.1% vs. 0.2%, p = 0.03).
| Postoperative outcomes | VTE (n = 266, 1.3%) | No VTE (n = 20,453, 98.7%) | p value |
|---|---|---|---|
| Cardiopulmonary bypass time (mins) | 94 [77, 116] | 91 [72, 113] | 0.05 |
| Cross clamp time (mins) | 70 [53, 90] | 68 [52, 86] | 0.21 |
| Total arterial revascularization | 17 (6.4%) | 1038 (5.1%) | 0.33 |
| Total operative time | 239 [205, 292] | 231 [196, 271] | 0.003 |
| Intraoperative cryoprecipitate | 7 (2.6%) | 303 (1.5%) | 0.12 |
| Intraoperative fresh frozen plasma | 15 (5.6%) | 559 (2.7%) | 0.004 |
| Intraoperative platelets | 2 (0.8%) | 104 (0.5%) | 0.40 |
| Intraoperative red blood cells | 47 (17.7%) | 2061 (10.1%) | < 0.001 |
| Intraoperative factor VII | 0 (0.0%) | 16 (0.1%) | > 0.99 |
| Intraoperative prothrombin complex concentrate | 3 (1.1%) | 48 (0.2%) | 0.03 |
3.2. Postoperative Outcomes
Table 3 shows the postoperative outcomes of patients with and without VTE. Patients that developed VTE were more likely to receive postoperative blood products including fresh frozen plasma (6.0% vs. 3.4%, p = 0.02) and red blood cells (40.2% vs. 21.0%, p < 0.001). They had longer mean ventilator hours (4.7 [4.7, 10.2] vs. 4.7 [4.1, 5], p < 0.001) and median ICU hours (97 [48, 191] vs. 47 [25, 74] hours, p < 0.001) with more frequent ICU readmission (19.6% vs. 2.9%, p < 0.001). There were more significant bleeding events in the VTE group (10.2% vs. 1.5%, p < 0.001) and a higher rate of reoperation for bleeding (3.0% vs. 1.3%, p = 0.02). Regarding major morbidity, patients with VTE had significantly higher rates of pneumonia (13.2% vs. 1.5%, p < 0.001), renal failure (10.2% vs. 1.8%, p < 0.001), and operative mortality (4.1% vs. 1.0%, p < 0.001). Length of stay (12 [7, 20] vs. 5 [4, 7] days, p < 0.001) was also longer in the VTE group as was hospital readmission (30.5% vs. 4.2%, p < 0.001).
| Postoperative outcomes | VTE (n = 266, 1.3%) | No VTE (n = 20,453, 98.7%) | p value |
|---|---|---|---|
| Postoperative creatinine (g/dL) | 1.2 [1.0, 1.7] | 1.1 [0.9, 1.4] | < 0.001 |
| Postoperative cryoprecipitate | 9 (3.4%) | 383 (1.9%) | 0.07 |
| Postoperative fresh frozen plasma | 16 (6.0%) | 695 (3.4%) | 0.02 |
| Postoperative platelets | 2 (0.8%) | 74 (0.4%) | 0.30 |
| Postoperative red blood cells | 107 (40.2%) | 4297 (21.0%) | < 0.001 |
| Initial intensive care unit hours | 73.4 [43.2, 151.5] | 46 [25, 73] | < 0.001 |
| Total intensive care unit hours | 96.7 [48, 191.1] | 46.5 [25.1, 74.3] | < 0.001 |
| Postoperative ventilator hours | 4.7 [4.7, 10.2] | 4.7 [4.1, 5] | < 0.001 |
| Reintubation | 23 (8.7%) | 184 (0.9%) | < 0.001 |
| Readmission to intensive care unit | 52 (19.6%) | 589 (2.9%) | < 0.001 |
| Postoperative heparin induced thrombocytopenia (HIT) | 3 (1.1%) | 5 (0.02%) | < 0.001 |
| Postoperative HIT thrombosis | 2 (0.8%) | 2 (0.01%) | 0.001 |
| Deep sternal wound infection | 2 (0.8%) | 49 (0.2%) | 0.14 |
| Anticoagulation related bleeding event | 20 (7.5%) | 46 (0.2%) | < 0.001 |
| Any bleeding event | 27 (10.2%) | 314 (1.5%) | < 0.001 |
| Reoperation for bleeding | 8 (3.0%) | 266 (1.3%) | 0.02 |
| Any reoperation | 15 (5.6%) | 334 (1.6%) | < 0.001 |
| Postoperative cardiac arrest | 13 (4.9%) | 185 (0.9%) | < 0.001 |
| Postoperative permanent stroke | 6 (2.3%) | 223 (1.1%) | 0.08 |
| Postoperative atrial fibrillation | 84 (31.6%) | 4255 (20.8%) | < 0.001 |
| Prolonged ventilation | 74 (27.8%) | 1062 (5.2%) | < 0.001 |
| Postoperative pneumonia | 35 (13.2%) | 315 (1.5%) | < 0.001 |
| Postoperative renal failure | 27 (10.2%) | 359 (1.8%) | < 0.001 |
| Postoperative dialysis | 21 (7.9%) | 200 (1.0%) | < 0.001 |
| Conduit harvest site infection | 3 (1.1%) | 40 (0.2%) | 0.01 |
| Dialysis after discharge | 8 (3.0%) | 61 (0.3%) | < 0.001 |
| Length of stay (days) | 12 [7, 20] | 5 [4, 7] | < 0.001 |
| Operative mortality | 11 (4.1%) | 199 (1.0%) | < 0.001 |
| Discharge to facility | 104 (39.1%) | 3486 (17.0%) | < 0.001 |
| Readmission | 81 (30.5%) | 868 (4.2%) | < 0.001 |
3.3. Risk-Adjusted Associations
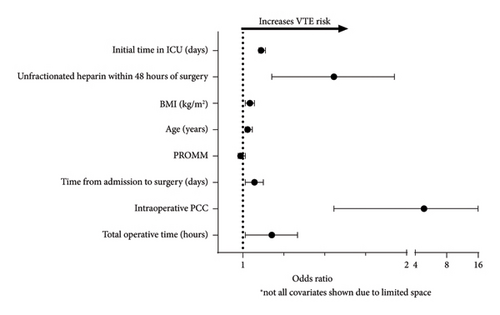
A multivariable logistic regression analysis was created utilizing a stepwise model (Table 4, Figure 2). Predicted risk of morbidity or mortality (PROMM) was included in the model to account for baseline risk. Preoperative unfractionated weight-based heparin use (OR = 1.47 [1.13, 1.90], p = 0.004), total operative time (OR = 1.13 [1.01, 1.26], p = 0.033), increasing time from admission to surgery (OR = 1.05 [1.01, 1.09], p = 0.009), age (OR = 1.02 [1.01, 1.04], p = 0.001), BMI (OR = 1.03 [1.01, 1.05], p = 0.012), and intraoperative PCC use (OR = 4.85 [1.47, 15.96], p = 0.009) were all found to be predictive of VTE.
| C statistic = 0.70 | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Total operative time (hours) | 1.13 | [1.01, 1.26] | 0.033 |
| Intraoperative PCC | 4.85 | [1.47, 15.96] | 0.009 |
| STEMI presentation | 1.69 | [0.82, 3.51] | 0.16 |
| Time from admission to surgery (days) | 1.05 | [1.01, 1.09] | 0.009 |
| Intraoperative RBC transfusion | 1.32 | [0.92, 1.90] | 0.13 |
| PROMM | 0.99 | [0.98, 1.01] | 0.37 |
| Age (years) | 1.02 | [1.01, 1.04] | 0.001 |
| BMI (kg/m2) | 1.03 | [1.01, 1.05] | 0.012 |
| Sleep apnea | 1.36 | [0.98, 1.89] | 0.067 |
| Unfractionated heparin within 48 h of surgery | 1.47 | [1.13, 1.90] | 0.004 |
| Initial time in ICU (days) | 1.08 | [1.07, 1.10] | < 0.001 |
Logistic regression was performed including PROMM, STS postoperative morbidities and complications, and last DVT or PE to determine whether VTE contributed independently operative mortality (Table 5). Pulmonary embolism was found to be independently associated with 30-day mortality (OR = 3.41 [1.06, 11.00], p = 0.04).
| C statistic = 0.85 | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Predicted risk of mortality | 1.10 | [1.07, 1.13] | < 0.001 |
| Pulmonary embolism | 3.41 | [1.06, 11.0] | 0.039 |
| Pneumonia | 3.77 | [2.35, 6.03] | < 0.001 |
| Reoperation for bleeding | 3.98 | [2.13, 7.43] | < 0.001 |
| Acute renal failure | 17.04 | [12.05, 24.09] | < 0.001 |
| Deep sternal wound infection | 2.68 | [0.67, 10.79] | 0.16 |
| Permanent stroke | 6.42 | [3.74, 11.01] | < 0.001 |
| Deep vein thrombosis | 1.09 | [0.46, 2.56] | 0.85 |
3.4. Survey Data
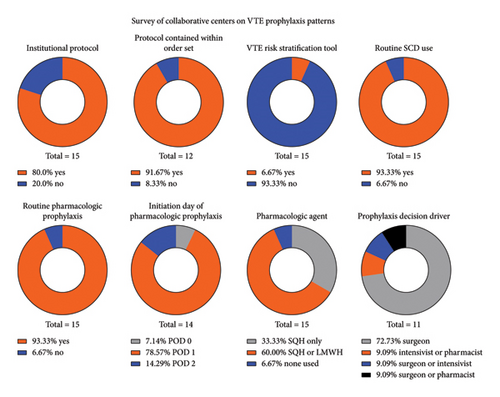
The response rate to the survey on VTE prophylaxis practices was 88% (15/17) (Supporting Figure 1). Out of all respondents, 80% (12/15) possessed a protocol for the management of VTE prophylaxis following cardiac surgery, and 92% (11/12) of these institutions had their protocol contained within the postoperative order set. Of the respondents, 93% (14/15) utilized routine pharmacologic prophylaxis in the form of either weight-based subcutaneous unfractionated heparin or weight-based low molecular weight heparin, with initiation on postoperative day one in 79% (11/14) of those centers. The most utilized anticoagulant amongst centers was either subcutaneous heparin or low molecular weight heparin with variability between the two at 60% of centers. Roughly 33% utilized only subcutaneous heparin without use of low molecular weight heparin. Surgeons most often drove variability in the initiation of chemoprophylaxis in 82% (9/11) of the centers as compared with intensivists or pharmacists (Figure 3).
3.5. Hierarchical Logistic Regression Utilizing Survey Responses of Center Protocols
Survey responses were matched to each center’s respective data and included in a multivariable model of postoperative VTE (Table 6). Patients from nonrespondent centers were excluded from this analysis. A hierarchical logistic regression was employed to account for center variability and practice with utilization of PROMM for individual patient risk adjustment. Only PROMM was found to be associated with VTE (OR = 1.03 [1.02, 1.04], p < 0.001) while other center practices such as institutional protocols and routine pharmacologic prophylaxis were not significant (p > 0.05).
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Presence of institutional protocol | 2.37 | [0.93, 6.00] | 0.07 |
| Routine chemical prophylaxis | 0.83 | [0.22, 3.19] | 0.79 |
| PROMM | 1.03 | [1.02, 1.04] | < 0.001 |
| Early mobility protocol present | 0.73 | [0.41, 1.30] | 0.29 |
4. Discussion
Through utilization of a regional collaborative capturing 99% of adult cardiac surgeries in Virginia, we found that in patients undergoing isolated CABG, the risk of postoperative VTE was 1.3%. VTE was significantly associated with morbidity and mortality and despite risk adjustment, PE remained a significant predictor of operative mortality. Uniquely, this study assessed regional practices regarding VTE prophylaxis and matched database outcomes to center protocols to determine whether institutional practices influenced rates of postoperative VTE. We uncovered homogeneity in institutional practices with no specific practice affecting the rate of VTE.
VTE was significantly associated with morbidity and pulmonary embolism was found to be associated with mortality, a finding similar to Khoury et al. [12]. The rate of VTE is our study was low although like other large studies with rates of 0.74%–1.75% [2, 8, 9, 12]. Studies utilizing routine screening following cardiac surgery have demonstrated a much higher rate of DVT compared to present literature. Schwann et al. found the incidence of DVT to be 13% when patients received routine screening ultrasounds prior to discharge despite maximally aggressive prophylaxis postoperatively [13]. An additional study by Ambrosetti et al. also suggested a higher DVT rate of 17.4% among patients entering cardiac rehab after CABG [10]. These patients underwent routine, serial DVT ultrasounds with half of these occurring in the contralateral leg from the saphenous vein harvest site. These data suggest that VTE may be underdiagnosed and not present itself during the index hospitalization unless prompted for evaluation by symptomatology. This is also confirmed by the fact that close to a third of patients in our study developed VTE as a reason for readmission, reinforcing the potential delay in presentation. Strategies aimed at postdischarge VTE prophylaxis may serve as an area for quality improvement similar to what is done for oncologic patients undergoing lung or esophageal cancer resection [14].
Multiple mechanisms promote thrombin generation, increase fibrinogen concentration, and reduce fibrinolysis in the postoperative period creating a prothrombotic state starting day one after cardiac surgery [3, 11]. Despite this heightened risk for VTE after cardiac surgery, there may be hesitation to initiate pharmacologic prophylaxis due to the high rate of bleeding complications [10, 15]. To account for baseline bleeding risk, we excluded patients on therapeutic oral anticoagulation or on any mechanical circulatory support during their hospital stay. In the present study, patients that developed VTE had higher perioperative blood product use and increased rates of significant bleeding events resulting in potential pharmacologic prophylaxis delays. Previous studies have demonstrated no significant increase in the rate of adverse bleeding events after cardiac surgery with utilization of pharmacologic VTE prophylaxis although well-powered clinical trials are lacking [2, 16].
Intraoperative PCC use was independently associated with VTE and the prevalence of VTE among those receiving PCC was 6%, several times higher than the overall prevalence of 1.2% in our population. PCC is most often utilized for urgent reversal of oral vitamin K antagonists although all patients on preoperative warfarin or DOAC were excluded from the study. Therefore, its use was off-label. Studies on its use in coagulopathy after cardiac surgery have suggested that PCC may increase the risk for thromboembolic events [17, 18]. In patients receiving intraoperative PCC, once the concern for active bleeding has subsided, early initiation of pharmacologic VTE prophylaxis may be warranted and perhaps ultrasound screening for DVT prior to discharge.
Interestingly, patients receiving unfractionated weight-based heparin within 48 h before surgery had higher rates of VTE. These patients represented ACS presentations that require therapeutic heparin infusions. Several studies have demonstrated that myocardial infarction increases the risk of subsequent VTE, especially in the first month after diagnosis [19–21]. In our study, increasing time from admission to date of surgery significantly increased the risk of VTE, even after controlling for baseline risk factors. Perhaps, patient activity restrictions in the hospital due to risk of coronary events may have limited mobility and increased VTE risk. In addition to their acute coronary event, this may increase their risk for VTE above the suggested decrease in risk from receiving heparin products preoperatively. For those patients presenting with stable coronary disease not requiring any active inpatient treatment prior to revascularization, discharge to home ahead of their operation may be beneficial in decreasing their risk of VTE.
Data on the use of VTE prophylaxis after cardiac surgery remain mixed. The American College of Chest Physicians recommends mechanical over pharmacologic prophylaxis in the uncomplicated cardiac surgery patient, with addition of pharmacologic prophylaxis only in those patients developing nonhemorrhagic complications. The European Association for Cardio–Thoracic Surgery instead recommends mechanical and pharmacologic prophylaxis on postoperative day one for all patients following cardiac surgery, regardless of risk categorization [5, 6]. We hypothesized that center practices would be variable given lack of firm, societal recommendations. The most common practice among our centers was routine initiation of pharmacologic VTE prophylaxis in the form of either subcutaneous unfractionated heparin or low molecular weight heparin on postoperative day one. Despite the lack of current societal guidelines directing therapy, most centers had similar practices suggesting that institutions understand VTE as a severe complication and take preventative actions. We did not demonstrate presence of an institutional protocol, routine pharmacologic prophylaxis, early mobility protocols, or sequential compression device usage as significantly protective from VTE.
This study has several limitations. As a retrospective examination of clinical registry data, there remains risk of unmeasured confounding. Due to database limitations, we cannot distinguish asymptomatic from symptomatic VTE events or the location of the clots, which may have resulted in management changes. We also do not possess data on the technique of saphenous vein harvesting and the effect; this may have had on development of DVT. Postoperative chemoprophylaxis is not captured in the database nor is sequential compression device use and early mobility which may play a factor in the rate of VTE at some institutions. Through survey of institutions, we attempted to strengthen this analysis by capturing what typical prophylactic strategies occurred postoperatively at the center level. However, we cannot ensure that these practices neither occurred at the patient level nor at the timing of when institutional protocols were implemented during the study period.
5. Conclusions
The incidence of VTE after CABG is a rare complication although associated with significant morbidity and mortality. Given a lack of consensus on appropriate prophylaxis measures following cardiac surgery and the morbidity and mortality presented by VTE events, additional, prospective case-controlled studies are warranted. In addition, since a significant proportion of VTE events occurred after discharge prompting readmission, future studies evaluating the safety and efficacy of postdischarge pharmacologic prophylaxis may be warranted.
Disclosure
This work was presented at the American Association for Thoracic Surgery 103rd Annual Meeting the Los Angeles Convention Center, Los Angeles, CA, USA.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded in part by a grant under Award Number , 2UM HL088925 as well as by the National Heart, Lung, and Blood Institute (grant T32 HL007849).
Supporting Information
Supporting Figure 1: Survey questions sent to collaborative centers on VTE prophylaxis practices.
Open Research
Data Availability Statement
The Society of Thoracic Surgeons Adult Cardiac Surgery database data used to support the findings of this study are restricted in order to protect patient and hospital privacy. The data used to support the findings of this study are available from the corresponding author upon reasonable request.