Synthesis of 2-Halo-3-Formylindoles via Vilsmeier–Haack Haloformylation Reaction of Oxindole
Abstract
A series of 2-halo-3-formylindole molecules were synthesized via Vilsmeier–Haack haloformylation reaction of N-Boc-oxindole with POCl3 or POBr3 and DMF, which are free from the use of catalyst and solvent. Moreover, through several simple postprocessing steps, corresponding adducts can be readily transformed to Tenidap analog intermediate. The feasibility of gram-scale experiments proved that the methodology has the potential to be scaled. In addition, a possible reaction mechanism was proposed based on control experiments.
1. Introduction
Formyl, as an important reactive group [1–3], can mainly be achieved through the formation of C-C bonds, such as the Vilsmeier–Haack reaction [4], Gattermann–Koch reaction [5, 6], and Reimer–Tieman reaction [7] [8–12]. Of all reactions, the Vilsmeier–Haack formylation reaction is widely used in organic synthesis and can be used to introduce formyl groups upon electron-rich aromatic hydrocarbons, heterocyclic aromatic hydrocarbons, electron-rich olefins, and other compounds [13–17]. However, despite many advantages of this reaction (mild conditions and easy operation), the selectivity of reaction sites is entirely uncontrollable. These competing regioselectivity pathways can substantially complicate product isolation by generating structurally analogous byproducts, while concomitant side reactions may further compromise purification efficiency through the formation of polar decomposition species [18, 19].
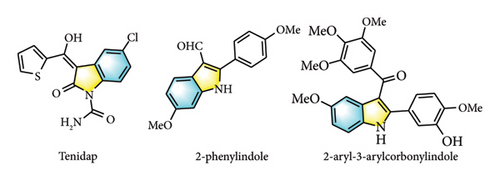
Indole frameworks are prevalent pharmacophore motifs found in biologically active compounds such as the nonsteroidal anti-inflammatory drug Tenidap [20–22], microtublin inhibitor 2-phenylindole [23–25], and antitumor drug 2-aryl-3-aryl corbonyl indole (Figure 1) [26, 27]. For indole derivatives, indole haloformylation is an important step to synthesize them and can introduce specific functional groups, change the chemical properties of indole molecules, and expand their applications in various fields [28–30]. The synthesis of 2-chloro-3-formylindoles involves three major routes (Scheme 1). (i) Palladium-catalyzed chlorination reaction of 1H-indole-3-carboxaldehyde with succinimide chloride [31], (ii) electrocatalytic formylation of 2-chloro indole with trimethyl amine [32], and (iii) Vilsmeier–Haack chloroformylation reaction of oxindole with phosphorus oxychloride and DMF catalyzed by pyridine [33, 34]. However, indole haloformylation reaction, as one of the steps in the synthesis of useful molecules, has not been systematically studied yet.
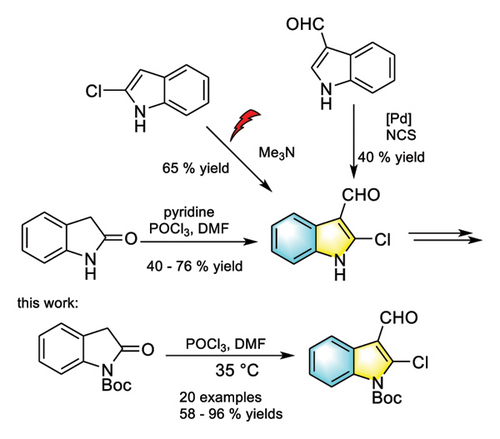
Oxindole, an electrophilic reagent, is prone to react with nucleophilic reagents, such as in Stetter reaction and Michael addition reaction [35–37]. Numerous compounds with specific structures and functions can be generated from these reactions [38, 39]. Oxindole can be isomerized into 2-hydroxyindole, which can undergo electrophilic reaction with electrophilic reagents at its C3 position. Based on the importance of 2-halo-3-formylindole in drug synthesis and our previous research work [40], we reported the solvent and catalyst-free Vilsmeier–Haack haloformylation reaction involving N-Boc-oxindole, which not only achieved the high yield synthesis of 2-halo-3-formylindole but also applied easily to multiple transformations.
2. Experimental Section
2.1. General Considerations
All the reagents including indolin-2-ones, phosphorus oxychloride (POCl3), N,N-dimethylformamide (DMF), and solvents were purchased from commercial sources and were used without further purification. N-Boc-oxindoles were synthesized by previous reported studies [41, 42]. All chemical shift values refer to δ TMS = 0.00 ppm or CDCl3 (δ [1H], 7.26 ppm; [13C], 77.2 ppm). Analytical TLC plates were viewed by UV light (254 nm). Column chromatographic purifications were performed on silica gel.
2.2. General Experiment Procedure
To an oven-dried 5-mL round-tube flask were added POCl3 1.6 mmol (146 μL) and DMF 1.0 mL. The suspension was stirred at 35°C for 0.5 h. Then, N-Boc-oxindoles 1a (0.2 mmol) were added. Then, the solutions were stirred at 35°C for 2 h. After the completion of the reaction, 1.0-mL H2O was added, then the solutions were stirred at 35°C for 1 h. And the resulting mixture was extracted with ethyl acetate. The combined organic layer was washed with brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The pure product was obtained by flash column chromatography on silica gel (petroleum ether:ethyl acetate = 9:1) as the eluent.
2.3. Experiment Procedure on Gram Scale Reaction
To an oven-dried 250-mL round-bottomed flask were added POCl3 80 mmol (7.3 mL) and DMF 50 mL. The suspension was stirred at 35°C for 0.5 h. Then, N-Boc-oxindole 1a (10 mmol) were added. Then, the solutions were stirred at 35°C for 3 h. After the completion of the reaction, 50-mL H2O was added, then the solutions were stirred at 35°C for 1 h. And the mixture was extracted three times with ethyl acetate, each time with 30 mL. The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The pure product 2a was obtained by flash column chromatography on silica gel (petroleum ether:ethyl acetate = 15:1) as the eluent.
3. Results and Discussion
We started our investigation by reacting oxindole with POCl3 (two equivalate) and DMF (1 mL) at room temperature for 2 h. Similar with previous reported studies [43, 44], only 30% yield product obtained (Table 1 Entry 1). It is known in the literature that unprotected nitrogen atoms give a formylation reaction [45]. Therefore, the use of a protective group was the most important factor in increasing the yield. As shown in Table 1 Entry 2, the yields of the product increased to 65% when changing the substrate to N-Boc-oxindole (1a), which superior to other protective groups (for details, see SI Table S1). For the reaction time, 2 h is the optimal option (Entry 2), whether to extend or shorten time cannot lead to better yields (Entries 3 and 4) (for details, see SI Table S2). When increasing the amount of POCl3 from 0.4 mmol to 1.6 mmol, the isolated yields improved to 96% (for details, see SI Table S3). The screening of reaction temperature showed that the reactivity decreased with the fall of temperature. For instance, when the temperature lowered to 20°C, the product yields decreased to 45% (Entry 7). While the reaction temperature increased to 50°C, the product showed poor stability, so no monitoring of the target product detected (Entry 8) (for details, see SI Tables S4 and S5). The yields of the product were decreased to 43% when adding (COCl)2 instead of POCl3 in the same condition (Entry 9). While 10% mol LiNTf2 used as additive in optimal conditions, the yields decreased to 38% (Entry 10) (for details, see SI Tables S6 and S7). The yields reached 73% in 1-mL CHCl3 as solvent (Entry 11). Other additives and solvents did not have better results (for details, see SI Table S8).
 |
|||||
| Entry | R | Chlorinated reagent (X mmol) | Time (h) | Temp. (°C) | Yielda (%) |
|---|---|---|---|---|---|
| 1 | H | POCl3 (0.4 mmol) | 2 | 35 | 30 |
| 2 | Boc | POCl3 (0.4 mmol) | 2 | 35 | 65 |
| 3 | Boc | POCl3 (0.4 mmol) | 1 | 35 | 44 |
| 4 | Boc | POCl3 (0.4 mmol) | 3 | 35 | 67 |
| 5 | Boc | POCl3 (0.8 mmol) | 2 | 35 | 73 |
| 6 | Boc | POCl3 (1.6 mmol) | 2 | 35 | 96 |
| 7 | Boc | POCl3 (1.6 mmol) | 2 | 20 | 45 |
| 8 | Boc | POCl3 (1.6 mmol) | 2 | 50 | NDb |
| 9 | Boc | (COCl)2 (1.6 mmol) | 2 | 35 | 43 |
| 10c | Boc | POCl3 (1.6 mmol) | 2 | 35 | 38 |
| 11d | Boc | POCl3 (1.6 mmol) | 2 | 35 | 73 |
- aIsolated yield.
- bND = no product detected.
- cLiNTf2 (10 mol%) was added as additive.
- d1-mL CHCl3 as solvents was added.
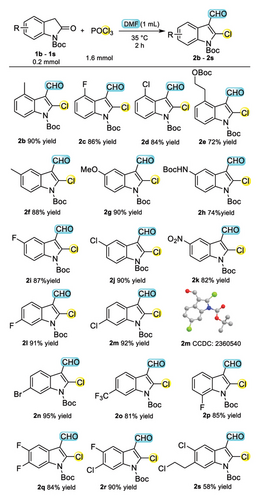
With the optimized conditions in hand, the scope for this Vilsmeier–Haack haloformylation reaction protocol was then investigated with N-Boc-oxindole. Overall, various reactants with different substitution patterns were found compatible to provide the corresponding products with moderate to excellent yields (58%–96%), and they are listed in Scheme 2. To our delight, the 4-methyl-substituted N-Boc-oxindole could also be tolerated, generating the corresponding chloroformylation product 2b with 90% yields. We next explored two types of haloarenes, 4- fluoric- and chloro-substituted N-Boc-2-chloro-3-formylindole (2c-2d) afforded with good isolated yields of 86% and 84%, respectively, demonstrating no obvious electrical and steric effects. Then, 4-alkyl and 5-BocNH-substituted adducts (2e, 2h) decreased yields to 72% and 74%, respectively, for steric hindrance. Five- donating- and withdrawing-electron substituted N-Boc-oxindole (2f-2g and 2i–2k) achieved good to excellent yields (82%–90%), and the results showed that the electrical properties and volume had affected the reactivity slightly. The target products can be obtained with excellent yields when halo substituted on the 6- site of N-Boc-oxindole (2l–2n). Notably, the structure of 2m was confirmed by single-crystal X-ray diffraction analysis (CCDC No. 2360540). Besides, 6-CF3 and 7-F-substituted substrate could also be tolerated under standard conditions with good yields (2o-2p). In addition, multisubstituted substrate can obtain the target products (2q-2r) with good yields as well (84% and 90%).Remarkably, 5-Cl-6-(2-chloroethyl) can also react smoothly with only 58% yields (2s).
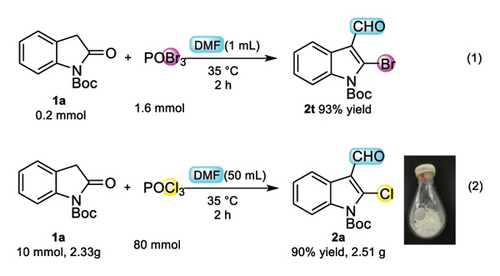
Then, we turned our attention to bromoformylation to extend the scope of application. N-Boc-2-bromo-3-formylindole (2t) can be obtained with 93% yields when taking POBr3 instead of POCl3 (Scheme 3 (1)). Gram-scale (10 mmol) reactions were conducted under standard conditions to demonstrate the practicality of the current method. The desired products were obtained with satisfactory yields, manifesting that this protocol serves as a practical approach for synthesizing N-Boc-2-chloro-3-formylindole (Scheme 3 (2) 90% yields).
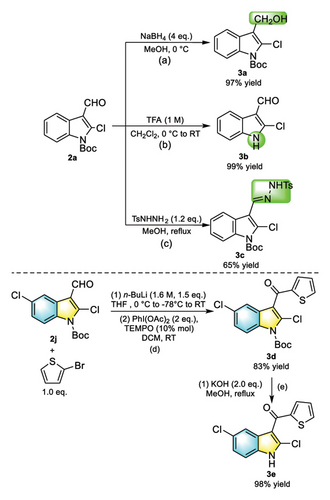
To evaluate the synthetic potential of the present reaction, various transformations were established, as shown in Scheme 4. Reduction of Compound 2b with NaBH4 provided hydroxy-substituted Product 3a in 97% yields (a). The deprotection of 2b was carried out with trifluoroacetic acid, delivering Product 3b in excellent result (99% yields) (b). Corresponding Derivative 3c was obtained with 65% yield after the treatment with TsNHNH2 (c). Furthermore, Compound 2j can undergo electrophilic addition reaction with 2-bromothiophene and n-BuLi, and Product 3d was afforded with 83% yield after the oxidation of PhI(OAc)2 [46]. Compound 3d can be converted to 3e with 98% yields by deprotection of KOH in methanol.
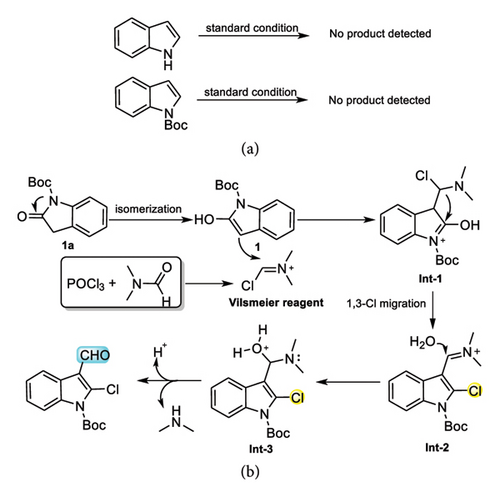
To obtain insight into the mechanism of reaction, a series of control experiments were conducted (Scheme 5 (a)). Under standard conditions, neither indole nor N-Boc-indole can react to form the corresponding target product. These experimental results showed that the carbonyl of indolone plays a crucial role in the formylation process. Finally, based on previous study [47–49], the plausible reaction mechanism was proposed. As shown in Scheme 5 (b), N-Boc-oxindole 1a rapidly isomerizes to N-Boc-2-hydroxyindole 1 under acidic conditions, and then 1 undergoes the electrophilic addition reaction with Vilsmeier reagents (generated by POCl3 with DMF), leading to Int-1, and in turn 1,3-Cl shift resulted in the formation imine cation intermediate Int-2. After addition reaction of water and Int-2, Int-3 generated. Finally, dimethylamine and H+ were removed from Int-3 and then the target 2-chlroindole-3-aldehyde product was produced.
4. Conclusions
In conclusion, this work has demonstrated that Vilsmeier–Haack haloformylation reaction of N-Boc-oxindole react with POCl3 or POBr3, and DMF can synthesize 2-halo-3-formylindole. This developed protocol is catalyst and solvent free. Meanwhile, the reaction conditions are mild, environmentally friendly, and efficient. The corresponding adducts can be readily transformed to different products by simple postprocessing steps. In addition, gram-scale experiments proved that the system has the potential to be scaled. Further studies on the exploration of the current method in organic synthesis and extension to other substrates are ongoing in our laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Peng Zhao conceived and directed the project. Mingyu Jiang, Jiahui Li, Shiyi Tang, and Pengluo Wei conducted the experimental work and data analysis. Peng Zhao wrote the manuscript. Juan Feng and Hua Zhang were involved in writing – review and editing.
Funding
The research was supported by the Natural Science Foundation of Sichuan ∗ 2022NSFSC1350, North Sichuan Medical College Talent Fund ∗ CBY21-QD19 ∗ CBY21-QD11, Special Fund for Strategic Cooperation in Science and Technology in Nanchong ∗ 22SXQT0397, and The College Students’ Innovative Entrepreneurial Training Plan Program in Sichuan Province ∗ S202410634052.
Acknowledgments
We are grateful for the financial support from the Natural Science Foundation of Sichuan (2022NSFSC1350), the North Sichuan Medical College Talent Fund (Nos. CBY21-QD19 and CBY21-QD11), Special Fund for Strategic Cooperation in Science and Technology in Nanchong (No. 22SXQT0397), and the College Students’ Innovative Entrepreneurial Training Plan Program in Sichuan Province (No. S202410634052).
Open Research
Data Availability Statement
The data supporting this article have been included as part of the Supporting Information.




