Comparison of the Energy Properties of Sunflower Stalk Fibers for Solid Biofuel Production
Abstract
The possibility of producing solid biofuels from sunflower stalks, an agricultural residue that is currently underused, has been investigated. By cutting the tissues along the sunflower stalks and mechanical grinding, samples were obtained, namely, sunflower stalk rind (SSR), sunflower stalk intermediate (SSI), and sunflower stalk pith (SSP), and by grinding sunflower stalks, as a whole, sunflower stalk general (SSG) sample was obtained. Different chemical compositions of sunflower stalk samples, according to the cellulose, hemicellulose, and lignin, influenced their thermal decomposition behavior. The lignin contents were found to be 19.8 wt.% in the SSR, 16.6 wt.% in the SSI, 3.8 wt.% in the SSP, and 15.8 wt.% in the SSG samples. The results were useful to forecast different calorific values of samples—higher for SSR, SSI, and SSG compared to the SSP sample. According to the results of comprehensive thermal analysis, the thermal decomposition of all samples occurred in five stages, different in their temperature ranges for every individual sample and mass loss of substances. By DTA curves comparison, the highest calorific value for the SSB sample, lower for the SSI and the SSG samples, and the lowest for the SSP sample were established. Calorific values determined by the calorimetric method were 18.2 MJ/kg for the SSR, 14.7 MJ/kg for the SSІ, 7.4 MJ/kg for the SSP, and 14.2 MJ/kg for the SSG samples. The results of the experimental determination of ash content showed 8.8 wt.% for SSR, 7.6 wt.% for SSI, 16.1 wt.% for SSР, and 10.2 wt.% for SSG samples. Due to the adequate calorific value, but the excessive level of ash content, the rind, intermediate segments of sunflower stalks, and sunflower stalks as a whole were recommended as components of solid composite fuel based on woody biomass.
1. Introduction
Nowadays, a transition from fossil fuels to alternative energy is a global, socioeconomic, and environmental challenge, and the search for new alternative energy sources is an urgent worldwide issue. Utilizing a diverse array of biomass for energy generation is significant for various reasons. This includes environmental benefits such as replacing fossil fuels to reduce greenhouse gas emissions, the main contributors to global climate change, and minimizing pollution that endangers human health and the stability of the planet’s ecosystems [1]. Additionally, there are political and economic advantages, such as enhancing a country’s energy self-sufficiency [2, 3]. Therefore, to increase the share of biomass energy in the global energy mix, it is necessary to involve new biomass sources.
Agri-biomass residues, as a type of biomass, have great potential to be used as a source of lignocellulosic material due to their rapid renewability, environmental friendliness, and high calorific value. They should be considered as an attractive and promising energy source for the near future [4–7]. These residues can be converted into various forms of energy, such as heat, electricity, and transportation fuels, using several methods. Moreover, such a solution will also solve the problem of agricultural waste disposal.
Sunflower (Helianthus annus L.) is a fast-growing, high-yielding crop grown primarily for its seeds and oil. Currently, the global sunflower seed production stands at 56.7MMT, and the major producing countries are Russia, Ukraine, Argentina, China, USA, Tanzania, Uganda, Zambia, Romania, Bulgaria, Turkey, Hungary, France, and India. Over 27 million hectares of sunflowers are planted worldwide [8, 9]. At the same time, the high average above-ground biomass yield of sunflowers of 45-75t biomass/ha increases after-harvest residue accumulation [10, 11]. Sunflower stalks are not disposed of sustainably in significant quantities, which make them an area of interest. They are often crushed for use as natural fertilizer and animal feed, or burned directly in the fields, which leads to the loss of biomass resources, increased environmental pollution, and numerous respiratory problems. Therefore, deep processing of sunflower stalks into highquality and highly competitive products is needed.
Researchers often provide information on the preliminary separation of sunflower stalks into rind (bark) and pith before using them for production [12, 13]. Stalk rind is primarily used as a source of natural fibers for the production of paper [14], thermoplastic composites [12, 15, 16], and particleboards [17, 18]. The pith of sunflower stalks serves as a raw material for the extraction of pectin and glucose [19], and fibers are a source for the manufacture of insulation boards [20, 21], solar water desalination, and evaporator devices [22, 23]. However, these solutions solve the problem of rationally disposing of only an insignificant portion of sunflower stalks. Researchers [24] have observed that sunflower stalks, as a whole object, contain approximately 30% cellulose and about 67% volatiles and have a high heating value (HHV) of around 14 MJ/kg, indicating the feasibility of biomass conversion into liquid and gaseous biofuels [25–32]. While harvesting, sunflower stalks have a high moisture content, which creates challenges for storage and processing into solid biofuels. The optimal moisture content for biomass used in solid biofuel production is between 4% and 12%. When the moisture content is outside this range, the quality of the briquettes is compromised. Due to the high energy costs associated with drying as a pretreatment step, it is crucial that we apply the well-studied filtration drying method to dehydrate sunflower stems [33, 34], which makes it possible to reduce energy costs and intensify the process, as also observed in the drying of other types of plant materials [35–37]. Given the significant average diameter of the sunflower stalk ranging from 2 to 7 cm and its complex tissue structure in the cross section, whole sunflower stalks and their separated tissues have attracted our interest as lignocellulosic raw materials for producing solid biofuel such as pellets, briquettes, and granules.
Important processes during pyrolysis events can be summarized as follows: removal of water molecules from biomass, degradation of hemicellulose, cellulose, and lignin. It is essential to study the peculiarities of thermal decomposition processes during the combustion of the whole sunflower stalk and its tissues separately, which predictably differ in the temperature ranges of individual stages of destruction and mass losses of substances according to the chemical compositions of the objects under study. According to European standards for solid biofuels, EN ISO 17225, several quality classes of solid biofuels are defined based on the origin and source of the material in terms of quality and chemical–physical properties. Therefore, it is necessary to determine the calorific value and ash content of the fuel as these parameters indicate its quality.
Thus, the work aimed to study the features of the thermal decomposition process, calorific value, and ash content of sunflower stalk tissues and stalk as a whole object.
2. Experimental
2.1. Materials and Methods
2.1.1. Materials
Sunflower (Helianthus annus L.) stalks, collected from the Lviv region of Ukraine after seed harvesting, were cleaned out of leaves, sand, and dust. Taking into account the arrangement of tissues from the periphery to the center in the cross-section of the sunflower stalk (Figure 1(a) [38]), microscopic observation showed that the outer tissues are composed of small thick-walled cells (Figure 1(b)) while the pith (parenchyma tissue) consisting of large thin-walled cells (Figure 1(c)).
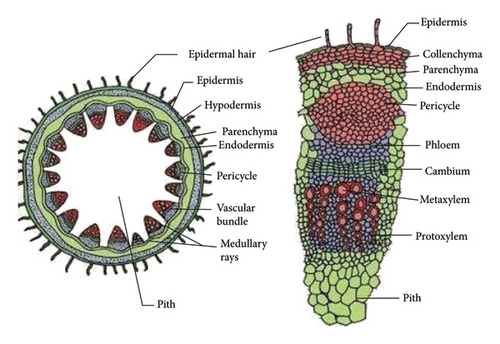
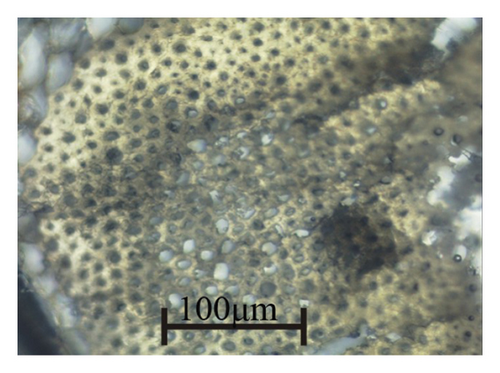
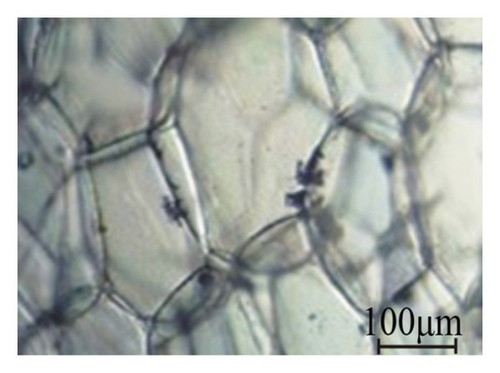
By cutting the tissues along the stalks and grinding them in a jaw crusher to particles of 0.01–5 mm in size, samples were prepared: sunflower stalk rind (SSR), which consists mainly of the epidermis and hypodermis tissues; sunflower stalk intermediate (SSI), which consists primarily of the parenchyma, the endodermis, the pericycle, and vascular bundles; and sunflower stalk pith (SSP), which consists of the parenchyma as well as medullary rays. SSR, SSI, and SSP samples are shown in Figures 2(a), 2(b), and 2(c), respectively. By grinding sunflower stalks as a whole in the jaw crusher to the same size of particles, a sunflower stalk general (SSG) sample was prepared.



To realize the drying of SSR, SSI, SSP, and SSG samples to a moisture content of 4%–12%, we used a filtration drying method. The dried SSR, SSI, SSP, and SSG samples were sieved, and the medium-size fraction with particles of 0.63–1.25 mm in size was used for further research, as its content in the total fraction of each sample was the largest (about 29.9%–32%).
2.2. Methods
2.2.1. Lignin and Holocellulose Content Determination
Holocellulose content (cellulose and hemicellulose) in sunflower stalk samples was estimated using the method described in [41], according to which 3 g of the sample was submitted to the solution of the distilled water (240 mL) with NaClO2 (5 g) and CH3COOH (2 mL) and the obtained mixture was kept at 70°C for 1 h to oxidize the lignin and another 1 h to obtain holocellulose.
2.2.2. Comprehensive Thermal Analysis
To evaluate the influence of temperature on the thermal decomposition of SSR, SSI, SSP, and SSG samples, comprehensive thermal analysis was used, which includes thermogravimetry (TG), differential TG (DTG), and differential thermal analysis (DTA). It was carried out using a Q-1500 derivatograph of the Paulik–Paulik–Erdey system connected to a personal computer [42]. The samples (with particles of 0.63–1.25 mm in size) were heated in an air atmosphere. The sample heating rate was 5°C·min−1. The weight of all samples was 50 mg. Aluminum oxide was served as a reference substance.
2.2.3. Calorific Value and Ash Content Determination. X-Ray Fluorescence (XRF) Analysis of Sunflower Stalk Ash Samples
The calorific values of SSR, SSI, SSP, and SSG samples were determined using a precision bomb calorimeter B-08-MA equipped with an isothermal (±0.015°C) shell [43]. The energy equivalent of the calorimetric system (W) was determined by combustion of the reference benzoic acid of the K-1 brand (the content of the main component is 99.995 ± 0.001 mol.%; combustion energy under standardized conditions is 26,434.4 ± 0.6 J/g). The samples were burned in an atmosphere of oxygen, previously purified from CO2 and H2O, in a calorimeter with a self-sealing bomb. For combustion, samples in the form of briquettes with prepressed cotton threads were placed in thin-walled quartz cups. The total heat amount released in the experiment was equal to W⋅ΔΤ. During the sample combustion, the temperature change (ΔΤ) was determined with an accuracy of ±1 10−4°C. The calorific values of the samples under bomb conditions at a temperature of 25.15°C were found considering the heat of combustion of the thread and the heat required for the soot to burn off. To ensure the reproducibility of the results, duplicates of tests were performed. To determine the ash content, 1 g of the material was carbonized in an oven at 550°C for 3 h (according to ISO 18122 (E) standard «Solid biofuels-determination of ash content») in a porcelain, previously standardized at 550°C for 4 h. It is assumed that the low ashing temperature prevents the evaporation of alkali metals [44]. XRF of sunflower stalk ash samples was performed on the Elvax Light SDD analyzer.
3. Results and Discussion
3.1. Lignin and Holocellulose (Cellulose and Hemicellulose) Contents in the Samples
Lignin and holocellulose (cellulose and hemicellulose) contents in SSR, SSI, SSP, and SSG samples were investigated experimentally according to the described above methods. Replicate experiments were conducted to determine the accuracy and reliability of the results. The results were obtained on five samples of the same material (SSR, SSI, SSP, and SSG, respectively). The tests showed close results for each tested material; therefore, the means according to the lignin and holocellulose (cellulose and hemicellulose) contents in the rind, intermediate, and pith segments of sunflower stalks, and sunflower stalks, as a whole object, were calculated. Obtained values showed agreement with results reported by other authors [12, 45–49] according to the distribution of the mentioned components in sunflower stalk tissues and sunflower stalks as a whole (Table 1). Minor variations in the cellulose, hemicellulose, and lignin contents reported in the literature, and experimental data can be explained, from one side, by characteristics of sunflower genotype, dissimilarities in the growing location, maturity or harvesting period (consistent with the results obtained by authors [6, 50], which reported variations in the content of these components across late, medium, mid-early, and early maturity rice genotypes), and from another side—by application of different analysis methods. According to the findings, the pith derived from the sunflower stalk differs significantly from other stalk fiber compositions. Specifically, it contains low levels of hemicelluloses and lignin but has a high concentration of pectin (23.2%, compared to only 5.6% in the rind [51]).
| Experimental results | ||||
|---|---|---|---|---|
| Samples | Cellulose (wt.% d.m.) ∗ | Hemicellulose (wt.% d.m.) | Lignin (wt.% d.m.) | References |
| SSR | 39.4 | 28.5 | 19.8 | — |
| SSI | 37.2 | 25.4 | 16.6 | |
| SSP | 34.2 | 6.3 | 3.8 | |
| SSG | 35.3 | 20.4 | 15.8 | |
| Existing literature results | ||||
| Stalk tissues | Cellulose (wt.% d.m.) | Hemicellulose (wt.% d.m.) | Lignin (wt.% d.m.) | References |
| Stalk rind | 41 | 32 | 17 | [45] |
| 48 | Not specified | 14 | [12] | |
| Stalk pith | 45.4 | Not specified | 3.2 | [45] |
| Not specified | 4.4 | 3.2 | [46] | |
| 31.5 | Not specified | 2.5 | [12] | |
| Whole stalks | 34.2–40.7 | 9.8–21.3 | 18.4–23.3 | [47] |
| 32.6 | 20.7 | 13.2 | [48] | |
| 33.5 | 21.7 | 14.3 | [49] | |
- ∗d.m.: dry matter, value expressed on a dry basis.
Different chemical compositions of sunflower stalk samples, according to the cellulose, hemicellulose, and lignin (which have the highest decomposition temperature), may directly influence their thermal decomposition behavior [52] and inorganic compounds—the ash formation in the final stages. Taking into consideration that lignin has a higher calorific value than cellulose and hemicellulose [53], the lignin contents of 19.8 wt.% for SSR, 16.6 wt.% for SSI, and 15.8 wt.% for SSG samples made it possible to predict the higher levels of the calorific values of the SSR, SSI, and the SSG samples compared with the SSP sample with poor lignin content (3.8 wt.%) as well as low hemicellulose level.
3.2. Comprehensive Thermal Analysis Results
Comprehensive thermal analysis, known as a reliable, low cost, and fast method for investigating the thermal decomposition of biomass in an oxidizing (combustion) medium, was used. The results for sunflower stalk samples in the form of thermograms are shown in Figures 3, 4, 5, and 6. The thermograms of three replicate experiments of each sample showed an overlap.
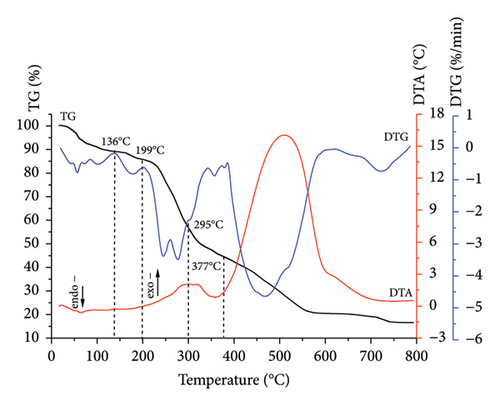
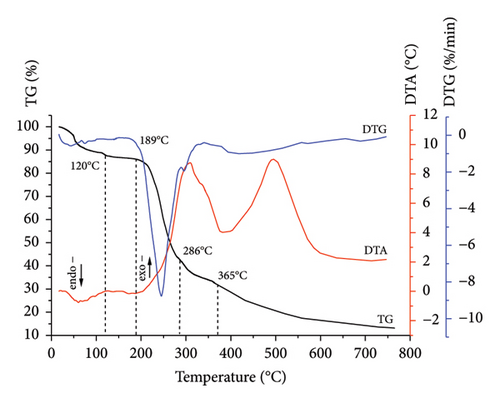
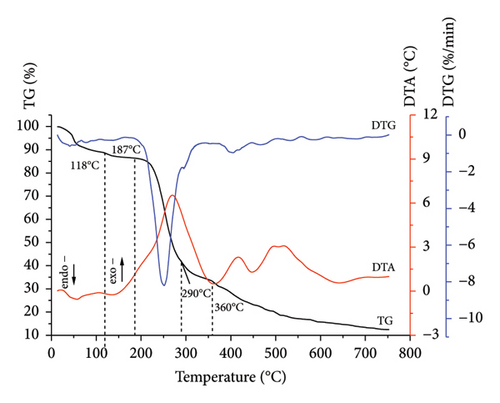
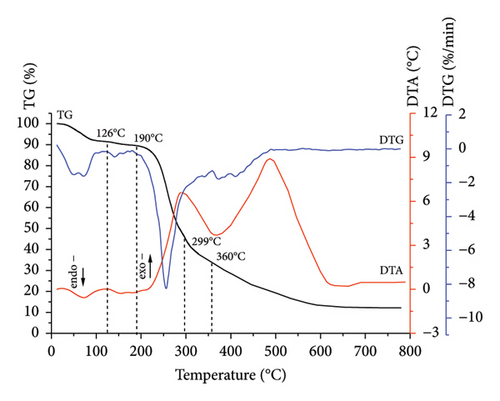
Thermograms (Figures 3, 4, 5, 6) show that the thermal decomposition of SSR, SSI, SSP, and SSG samples occurs in five stages, which differ in temperature ranges and component mass losses (Table 2). Processes identified during the thermal decomposition of biomass include water removal, hemicellulose decomposition, cellulose degradation, and lignin breakdown.
| Samples | Degradation stage | Temperature range (°С) | Mass loss (%) |
|---|---|---|---|
| SSR | І | 20–136 | 10.8 |
| ІІ | 136–199 | 3.4 | |
| ІІІ | 189–295 | 29.1 | |
| IV | 295–377 | 12.3 | |
| V | 377–750 | 28.0 | |
| SSI | І | 20–120 | 12.2 |
| ІІ | 120–189 | 1.7 | |
| ІІІ | 189–286 | 42.8 | |
| IV | 286–365 | 10.3 | |
| V | 365–750 | 24.6 | |
| SSP | І | 20–118 | 11.2 |
| ІІ | 118–187 | 2.3 | |
| ІІІ | 187–290 | 44.9 | |
| IV | 290–360 | 8.4 | |
| V | 360–750 | 9.1 | |
| SSG | I | 20–125 | 8.6 |
| II | 126–190 | 1.9 | |
| III | 190–298 | 43.7 | |
| IV | 298–360 | 12.0 | |
| V | 360–750 | 17.0 | |
According to the comprehensive thermal analysis data (Table 2), at the first stage of thermal decomposition, which occurred in the low temperatures region, the initial mass loss of samples was observed due to the evaporation of adsorbed moisture (10.8% for SSR, 12.2% for SSI, 11.2% for SSP, and 8.6% for SSG). This process was accompanied by the appearance of the extremum on the DTG curves and shallow endothermic effects on the DTA curves.
At the second stage of thermal decomposition, slight mass losses of all samples were observed due to the release of constitutional water [42]. The processes were accompanied by the shift of the DTA curves toward the region of endothermic effects.
At the third stage, the greatest decomposition of all samples occurred. The intensive mass loss of the SSR sample (29.1%) in the temperature range of 189°C–295°C is caused by endothermic processes of hemicellulose pyrolysis and cellulose destruction with a sharp decrease of the degree of its polymerization and the beginning of lignin decomposition. In the same temperature range, exothermic thermal oxidation processes of the SSR components were actively developing, as evidenced by the deviation of the DTA curves into the region of exothermic effects. A double extremum was observed on the DTG curve of the SSR sample in the temperature range of 189°C–295°C, with maxima at temperatures of 244°C and 277°C. The first extremum was, obviously, due to the pyrolysis of hemicellulose, and the second one was due to the deep destructive processes of cellulose.
The highest degradation of SSI and SSP samples occurred within temperature ranges of 189°C–286°C and 187°C–290°C, respectively. In contrast to the SSR sample, for SSI and SSP samples, more intensive mass loss was observed (42.8% and 44.9%, respectively) due to the higher amount of less thermostable components. Thermo-oxidative processes were accompanied by the appearance of a sharp extremum on the DTG curves, with a maximum at temperatures of 244°C for the SSI sample and 250°C for the SSP sample. Intensive thermal oxidation processes of the SSG components occurred within a temperature range of 190°C–298°C corresponding to the intensive mass loss (43.7%) with the maximum at a temperature of 255°C.
The mass loss for SSI and SSP samples at the fourth stage was 10.3% (in the temperature range of 286°C–365°C) and 8.4% (in the temperature range of 290°C–360°C), respectively. This stage for SSI and SSP samples was accompanied by the appearance of a rapid exothermic effect on the DTA curves corresponding to the occurrence of deep thermo-oxidative processes and the combustion of decomposition products. Unlike SSI and SSP samples, the exothermic effect for the SSR sample was less rapid, which indicated a higher content of more thermostable components in this sample. The mass loss for the SSR sample was 12.3% in the temperature range of 295°C–377°C, caused by deep destructive and thermal oxidation of destruction residues. The mass loss for the SSG sample was 12.0% in the temperature range of 298°C–360°C.
At the fifth stage, the mass loss of 28.0% for the SSR sample in the temperature range of 377°C–750°C was observed, caused by the combustion of the pyrolytic residue. Similar processes occurred in SSI and SSP samples. The mass loss for the SSI sample was 24.6% in the temperature range of 365°C–750°C, and 9.1% for the SSP sample in the temperature range of 360°C–750°C. The fifth stage of thermal decomposition for all samples was accompanied by an exothermic effect on the DTA curves. The appearance of a rapid exoeffect on the DTA curve for the SSR sample in the temperature range of 377°C–750°C was caused by the highest contents of cellulose and lignin in it. The mass loss for the SSG sample was 17.0% in the temperature range of 360°C–750°C, which corresponds to the appearance of a rapid exothermic effect on the DTA curve.
The comparison of the DTA curves for SSR, SSI, SSP, and SSG samples is shown in Figure 7. It was concluded that the SSR sample had the highest calorific value among the sunflower stalk samples, due to the highest lignin and cellulose content. The combustion of the pyrolytic residue of this sample in the temperature range of 377°C–750°C was accompanied by the appearance of the most rapid exothermic effect on the DTA curve. The SSI sample had a somewhat lower calorific value compared with the SSR. The combustion of the pyrolysis products of this sample (at 189°C–365°C) was accompanied by the appearance of the most rapid exothermic effect on the DTA curve compared to others samples. However, the combustion of the pyrolytic residue (at 365°C–750°C) corresponded to the release of a smaller heat amount in comparison with the SSR sample. The SSP sample had the lowest calorific value, evidenced by the appearance of minor exo-effects that accompanied the combustion of the pyrolytic residue of the sample (at 360°C–750°C). The calorific value of the SSG sample was lower than those for SSR and SSI samples, but closer to the SSI sample.
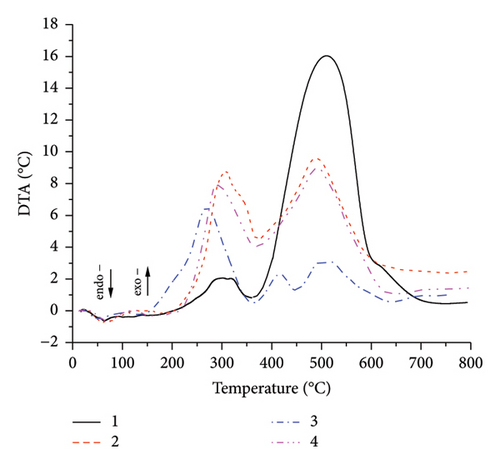
Aspen (Populus tremula) is a fast-growing and widely distributed tree in Ukraine, extensively utilized as an energy raw material. The thermal analysis of the aspen sample, comprising trunk wood from a ten-year-old aspen mixed with bark, was conducted under the same conditions as the sunflower stalk samples to compare fuel properties. The comparison of DTA curves for sunflower stalk and aspen samples is shown in Figure 8.

The appearance of the exothermic effect on the DTA curve of the aspen sample in the temperature range of 187°C–373°C corresponds to thermo-oxidative destruction and combustion of the destruction products of the sample. The exothermic effect in the temperature range of 373°C–564°C corresponds to the combustion of the pyrolytic residue of the aspen sample. The appearance of less significant exothermic effects on the DTA curve of the aspen sample indicates its lower calorific value compared with the sunflower stalk sample (SSG sample). Therefore, sunflower stalk biomass, as a whole object, can be considered as raw material for biofuel production.
3.3. Calorific Value and Ash Content Determination Results. XRF Analysis of Sunflower Stalk Ash Samples
Calorimetric studies were conducted on sunflower stalk samples, and the parameter necessary for the definition of their energetic content, namely, the calorific value, was determined by averaging five measurements (Table 3). The obtained data are in good agreement with the comprehensive thermal analysis results.
| Biomass as fuel type | Calorific values (MJ/kg d.m.) | Ash content (wt.% d.m.) |
|---|---|---|
| Herbaceous and agricultural biomass: | Mostly about 14–18 [54–58] | 4–12 [44, 57] |
| SSR sample | 18.2 | 8.8 |
| SSI sample | 14.7 | 7.6 |
| SSР sample | 7.4 | 16.1 |
| SSG sample | 14.2 | 10.2 |
| Wood and woody biomass: | About 15–19 [54, 55] | 0.5–7 [44] |
| Energy willow | 17.6 [42] | 1 [44] |
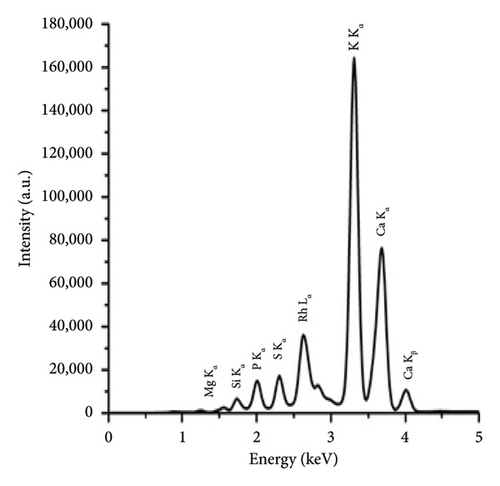
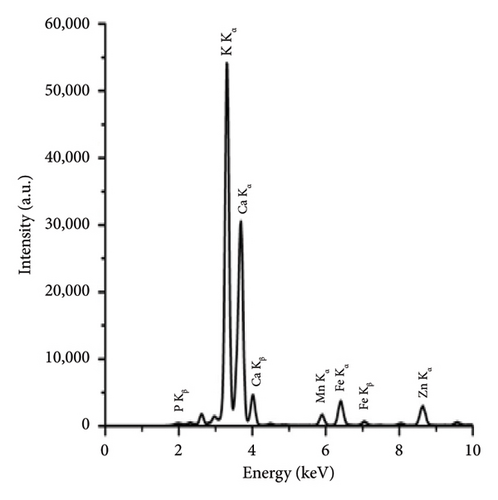
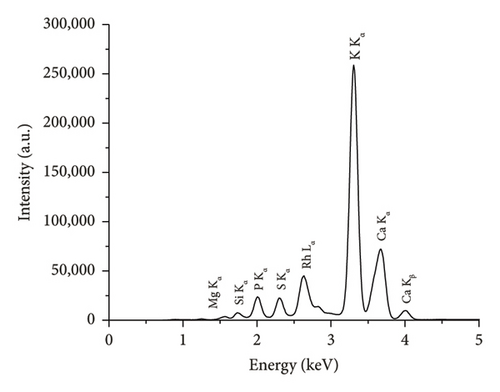
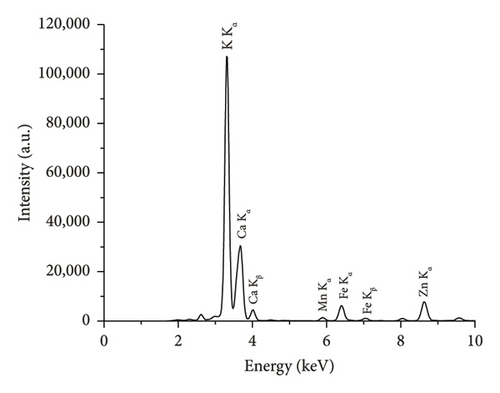
The comparison of the calorific values obtained for SSR, SSI, SSP, and SSG samples with herbaceous and agricultural biomass, as well as wood and woody biomass, is presented in Table 3. The highest lignin content in the SSR sample resulted in its highest calorific value being in good agreement with all types of biomass and even higher than the calorific value of energy willow used as a baseline. The calorific values of SSI and SSG samples were closer to herbaceous and agricultural biomass data. As expected, due to low lignin content, the сalorific value of the SSP sample was the lowest. The results of the experimental determination of ash content for SSR, SSI, and SSP samples, obtained by averaging five test results for each material, agree with herbaceous and agricultural biomass data (Table 3). The SSР sample showed the highest ash content caused by the highest content of inorganic substances. The ash chemical composition of sunflower stalk samples obtained through XRF analysis is presented in Table 4, and XRF spectra are shown in Figures 9, 10, and 11.
| Chemical composition of the ash for the SSR sample (wt.%) | Chemical composition of the ash for the SSI sample (wt.%) | Chemical composition of the ash for the SSP sample (wt.%) | |||
|---|---|---|---|---|---|
| MgO | 6.1 ± 0.1 | MgO | 5.1 ± 0.1 | MgO | 2.7 ± 0.1 |
| SiO2 | 4.1 ± 0.04 | SiO2 | 3.3 ± 0.03 | SiO2 | 0.4 ± 0.02 |
| P2O5 | 3.8 ± 0.02 | P2O5 | 4.4 ± 0.02 | P2O5 | 0.2 ± 0.01 |
| SO3 | 2.2 ± 0.01 | SO3 | 2.1 ± 0.01 | SO3 | 0.5 ± 0.01 |
| K2O | 38.0 ± 0.1 | K2O | 50.8 ± 0.01 | K2O | 28.6 ± 0.08 |
| CaO | 44.5 ± 0.3 | CaO | 33.1 ± 0.2 | CaO | 67.2 ± 0.1 |
| Fe2O3 | 0.5 ± 0.01 | Fe2O3 | 0.6 ± 0.01 | Fe2O3 | 0.2 ± 0.01 |
| Other oxides | 0.8 ± 0.01 | Other oxides | 0.8 ± 0.01 | Other oxides | 0.2 ± 0.01 |
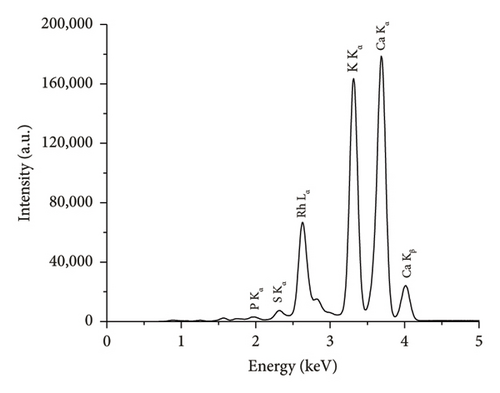
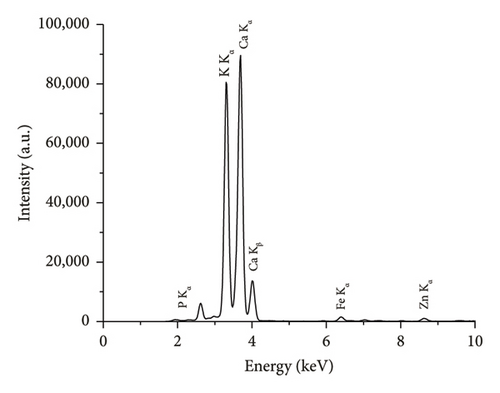
The rind, intermediate segments of sunflower stalks, and sunflower stalks as a whole object have the proper calorific values but excessive ash content. Therefore, they have been recommended as components for solid composite fuels based on woody biomass. Due to low calorific value and high ash content, sunflower stalk pith, on its own, is not suitable for solid biofuel production.
4. Conclusions
Higher calorific values were predicted for SSR, SSI, and SSG samples due to higher lignin contents (19.8 wt.%, 16.6 wt.%, and 15.8 wt.%, respectively) compared to the SSP sample with only 3.8 wt.% of lignin.
According to the comprehensive thermal analysis results, the thermal decomposition of SSR, SSI, SSP, and SSG samples occurred in five stages, and the chemical compositions of the samples (the cellulose, hemicellulose, and lignin contents) directly influenced their thermal decomposition processes. Therefore, the decomposition stages slightly differed in the temperature ranges and mass losses. As observed upon comparing the DTA curves, the SSR sample had the highest calorific value, followed by SSI and SSG samples, while the SSP sample had the lowest.
Calorific value determined by a calorimetric bomb was 18.2 MJ/kg for SSR sample, 14.7 MJ/kg for SSI, 7.4 MJ/kg for SSP, and 14.2 MJ/kg for SSG. The obtained data agreed well with the results from the comprehensive thermal analysis.
The results of the experimental determination of ash content showed 8.8 wt.% for SSR, 7.6 wt.% for SSI, 16.1 wt.% for SSР, and 10.2 wt.% for SSG samples.
Thus, considering proper calorific values but excessive ash content, the rind, intermediate segments of sunflower stalks, and sunflower stalks, as a whole, have been recommended as components of solid composite fuel based on woody biomass. The proposed solution contributes to solving the problem of rational utilization of sunflower biomass and, in the case of optimal proportions of mixing fuel components, allows for obtaining solid biofuels with quality indicators that meet European standards. Due to its low calorific value and high ash content, sunflower stalk pith is not suitable for solid biofuel production as an independent object.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was obtained for this study.
Open Research
Data Availability Statement
All related data are mentioned in the manuscript with references.




