GC–MS and FTIR Characterization of Bioactive Compounds in Aqueous Extracts of Terminalia laxiflora Root and Mitragyna inermis Stem Bark
Abstract
The contribution of medicinal plants in healthcare delivery has been acknowledged due to the numerous plant chemicals with health-promoting properties. In Northern Ghana, where health resources are scarce coupled with endemic poverty, most traditional folks rely heavily on plants as a source of treatment. Among some of the common plants employed for the treatment of malaria, diarrhea, vomiting, stomachache, wounds, and epilepsy are Terminalia laxiflora root and Mitragyna inermis stem bark. Despite the claimed efficacy and potency of these medicinal plants, little is known about the bioactive compounds in these plants. The current study aimed to characterize the phytochemical constituents in the aqueous extracts of Terminalia laxiflora root and Mitragyna inermis stem bark. Fourier transform infrared spectroscopy and gas chromatography coupled with mass spectrometry were employed for this study. The FTIR analysis revealed the presence of alkene, carbonyl, carboxylic acids, hydroxyl groups, alkyl halides, ethers, and esters. The GC-MS analysis also revealed the presence of seventeen (17) different phytochemical constituents including trans-13-octadecanoic acid, methyl ester, 9-octadecanoic acid, (E)-, hexadecenoic acid, n-hexadecenoic acid, phenol, 4-heptyl- 2-hydroxy-1-(hydroxymethyl)ethyl ester, 9-octadecenamide, (Z)- and 9-octadecenoic acid (Z), and 3-dihydroxyphene. These compounds have supported the treatment of various diseases in humans as natural compounds. The above-characterized bioactive compounds might be responsible for the traditional folks’ claimed efficacy of these medicinal plants. The study findings will contribute to the existing knowledge and also provide scientific data for future research. Further studies should isolate and purify these compounds as lead compounds for possible drug development.
1. Introduction
Plants were the main source of therapy for a variety of illnesses prior to modern medical procedures, and many herbal cures are still in use today [1]. Plants have long been recognized for their potential as valuable repositories of natural compounds that possess therapeutic properties, and this recognition has led to their integral role in the development of modern pharmaceuticals [2]. Phytochemicals provide health benefits beyond macro- and micronutrients [3]. The World Health Organization (WHO) has estimated a 75%–80% of the world population reliance on traditional practitioners for their primary healthcare [2]. The search for plants with therapeutic activities has long been crucial to human health, and there is a pressing need for natural remedies given the widespread belief that they are products of fundamental evolutionary behavior [4].
More than four thousand phytochemicals have been characterized based on their chemical, physical, and defensive properties [5]. Changes in temperature, precipitation, sunlight, and other environmental variables can directly impact phytochemical prevalence and concentrations [6]. These phytochemical constituents have demonstrated notable pharmacological activities including acetylcholinesterase inhibition, cytotoxic, antifungal, antiparasitic, and antiproliferative properties [7, 8].
The importance of having molecules accessible to cure or at least alleviate diseases was recently highlighted by the COVID-19 pandemic [9]. The indigenous people within Northern Ghana use Mitragyna inermis stem bark and Terminalia laxiflora roots for the treatment of wounds, stomach upsets, diarrhea and vomiting. Notwithstanding the claimed abundance of phytochemical constituents in medicinal plants used by traditional folks, little is known about the chemical compounds present in these plants. Characterizing the chemical compounds in Mitragyna inermis stem bark and Terminalia laxiflora roots used by traditional folks is crucial to understanding the relationship between phytochemicals in plants and their claimed biological activities [10]. Several methods are employed in the characterization and identification of bioactive compounds in plants’ crude extracts, the commonly used methods are Fourier infrared spectroscopy and gas chromatography (GC) coupled with mass spectrometry (MS).
The technique of GC is highly beneficial in isolating volatile components from a mixture [11]. GC heats a liquid sample to vaporization upon injection and converts them into pulses of pure compounds. The compounds are then passed through a long, narrow stationary phase, a coiled column covered with a nonpolar or polar material. Specific chemicals are expelled at distinct intervals throughout the sample’s passage. The mass of every component is ascertained by means of ionization and fragmentation of the molecules. The generated MS spectra are then run through a variety of databases that include mass spectra of well-known substances. At a certain confidence level, the chemical structure linked to a particular scan may be ascertained by comparing data points [12].
FTIR analysis revealed characteristic functional groups associated with bioactive compounds, confirming the presence of specific chemical constituents. The identified bioactive compounds have potential pharmacological significance, supporting the medicinal uses of Terminalia laxiflora and Mitragyna inermis. These identified phytochemical constituents might be the rationale for the usage of these plants by Traditional folks in the Savannah belt of Northern Ghana. Future research should consider isolating these compounds for further biological screening.
2. Materials and Methods
2.1. Materials
The following equipment/chemicals were secured from the indicated sources: Water bath JBN12 (Cambridge Limited, England), fume chamber SGC097 (Gallenkamp, United Kingdom), rotary evaporator R-210 (Buchi, Switzerland), separating funnel AS 260.5 (Anumbra, United Kingdom), volumetric flask, PerkinElmer GC Clarus 580 GC, MS PerkinElmer (Clarus SQ 8 S) equipped with ZB-5HTMS, pH meter 8100 (Stratlab Limited, England), conical flask, stirring rod, spatula, wash bottle, electronic weighing balance JJ223/323, oven OB60, Whatman no. 42 filter paper (Powerfix IAN56288, model: S32585), hydrochloric acid 109,057 (Merck Specialties Private Limited, Germany), methanol 67-56-1 (Fisher Scientific, United Kingdom), potassium bromide 7758-02-3 (KBr), sulfuric acid 7664-93-9 (Sigma Aldrich, United Kingdom), methanol BDH2029-1GLP (VWR Prolabo Chemicals, France), chloroform 67-66-3 (BDH Limited, England), ethanol, helium gas, 5% concentrated sulfuric acid, and 20% sodium hydroxide 1310-73-2 (NaOH).
2.2. Methods
2.2.1. Plants and Parts
The plants’ parts used in the current study were the stem bark of Mitragyna inermis and the root of Terminalia laxiflora. These plants were obtained from the Savannah ecological zone, Ghana. The study was carried out in the towns of Larabanga and Kabampe in the Savannah region of Ghana. It is located in longitude -1°51131.18 to the east and latitude 9°12156.16 to the north. The plants were identified with the help of traditional healers in the Larabanga town and authenticated by a botanist at the Mole National Park. The Mitragyna inermis stem bark and Terminalia laxiflora root were retained at the Mole National Park herbarium with sample codes as MIS/002/2346/2023 and TLR/2347/2023 for Mitragyna inermis stem bark and Terminalia laxiflora root, respectively.
A descriptive map of the study area is shown in Figure 1.
2.3. Sample Preparation and Extraction
The identified samples were harvested and transported to the Dr. Hilla Limann Technical University laboratory, Wa, Upper west region, Ghana. The samples were washed under running water and air-dried for 16 days under room temperature. The dried samples were pulverized and transported to Professor Marion Martienssen’s Biotechnology Laboratory, Brandenburg University of Technology, Germany, for laboratory analysis.
Extraction of phytochemical constituents was performed using water. 100 g of pulverized plant sample was weighed into a cleaned extraction container and 1000 mL of double distilled water was added. The mixture was then placed on a mechanical shaker for 72 h at 180 rpm to ensure complete extraction. The extracts were filtered into porcelain crucibles and the solvent evaporated under a rotary evaporator. The crude extracts were then scooped and stored in a fridge until used.
2.3.1. Fourier Transform Infrared Spectroscopy
A JASCO FTIR-6300 Fourier transform infrared spectrometer equipped with a JASCO IRT-7000 Intron infrared microscope operating at a resolution of 4 cm−1 (JASCO, Tokyo, Japan) was used to record the IR spectrum of the aqueous extract of Mitragyna inermis stem bark and Terminalia laxiflora root. The FTIR analysis was conducted using potassium bromide (KBr) pellet (FTIR grade method in the ratio of 1 : 1000 according to the protocol of [13]). All analysis was conducted at the Brandenburg University of Technology, Cottbus, Germany.
2.3.2. Gas Chromatography–Mass Spectrometry
The aqueous extracts of the sample were subjected to GC–MS analysis at the Brandenburg University of Technology, Cottbus, Germany, using a PerkinElmer GC Clarus 580 GC interfaced to a MS PerkinElmer (Clarus SQ 8 S) equipped with ZB-5HTMS (5% diphenyl/95% dimethyl polysiloxane) fused capillary column (30 × 0.25 μm ID × 0.25 μm DF). The oven was set to start at 100°C (isothermal) for two minutes, then climb by 10°C/min to 200°C, then by 5°C/min to 280°C, and finally to hold at 280°C for 15 min. An electron ionization device with an ionization energy of 70 eV was used in the electron impact mode for GC–MS detection. As a carrier gas, helium gas (99.9999%) was employed at a steady flow rate of 1 mL/min. At 70 eV, mass spectra were recorded with a 1 s scan interval and an MS scan between 50 and 500 Da. The GC/MS running duration was 43 min overall, with a solvent delay of 0–3 min.
Turbo-Mass was the mass detector utilized in this investigation, and Turbo-Mass Version 6.1.0 was the software used to control the mass spectra and chromatograms. Mass-spectrum GC–MS interpretation was carried out with the National Institute of Standard and Technology (NIST) database, which has over 62,000 patterns [14].
3. Results and Discussion
3.1. Discussion
The Fourier transform infrared spectroscopy provides a high-quality analytical method for the determination of chemical components and structural clarification of compounds in plant extracts. It is a quick and nondestructive analysis for fingerprinting plant extracts or powders. The study was conducted within the Larabanga enclave of the Savannah region (Figure 1).
The FTIR spectrum of the current study revealed vibrational frequencies at 3254.20, 2928.64, 1609.2, 1554.74, 1405.10, 1341.95, 1260.37, 1101.12, and 780.04 cm−1 from aqueous extracts of Mitragyna inermis stem bark and Terminalia laxiflora root as presented in Tables 1 and 2. The vibrational frequencies (Figures 2 and 3) correspond to the presence of —O-H, N-H, C-H, C-Cl, C=C, nitrates, and silicates stretching, respectively. The N-H stretching may signify the presence of alkaloids and O-H stretching may indicate the presence of polyphenols, flavonoids, and terpene due to the C-H group [15].
| S/N | Peak value | Functional group | Peak intensity |
|---|---|---|---|
| 1 | 3237.09 | O-H broad | Strong |
| 2 | 1550.70 | C=O amide stretch | Strong |
| 3 | 1405.64 | C-O-H stretch | Strong |
| 4 | 1341.95 | S=O symmetric | Strong |
| 5 | 1260.37 | C-N stretch | Weak |
| 6 | 1159.39 | C-F stretch | Strong |
| 7 | 1049.95 | C-OH stretch | Strong |
| 8 | 928.70 | O-H bend | Weak |
| 9 | 764.48 | C-Cl stretch | Strong |
| 10 | 451.41 | C-I stretch | Strong |
| S/N | Peak value | Functional group | Peak intensity |
|---|---|---|---|
| 1 | 3254.20 | O-H broad | Strong |
| 2 | 1554.74 | C=O amide stretch | Strong |
| 3 | 1405.10 | C-O-H stretch | Strong |
| 4 | 1337.56 | NO2 stretch | Strong |
| 5 | 1101.12 | C-OH stretch | Stretch |
| 6 | 1048.31 | C-F stretch | Strong |
| 7 | 927.06 | O-H bend | Strong |
| 8 | 780.04 | C-Cl stretch | Strong |
| 9 | 452.86 | C-I stretch | Strong |

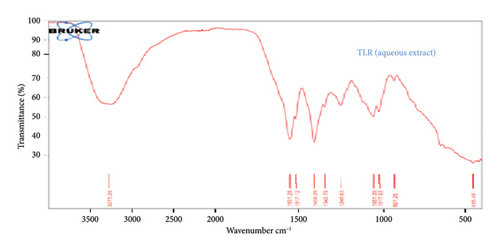
The claimed medicinal properties of these plants may be attributed to the distinctive functional groups, carboxylic acids, anhydride, alcohols, phenols, amines, amides, esters, and ethers, as shown in Figure 3. The findings in this current study show similar patterns compared to the findings of the authors in [15], who reported the presence of similar functional groups in Micrococca mercurialis.
A study conducted by the authors in [16] revealed that the fingerprint areas and functional groups of plant crude extracts had comparable band patterns that corresponded to significant phytochemical compounds [16].
Similar results published by the authors in [17] revealed relevant functional groups including C-N, COOH, C-F, and -OH in the ethanolic extract of Ichnocarpus frutescens [17]. The findings of the present study also shared some similarities with those of [18], who established that the functional groups obtained from dried plant extracts can be related to the presence of bioactive compounds in plants. The variations among the functional groups in the current study and previously published findings may be due to several factors as reported by the authors in [17].
GC in conjunction with MS was utilized to profile the bioactive compounds in the crude aqueous extracts of Terminalia laxiflora root and Mitragyna inermis stem bark.
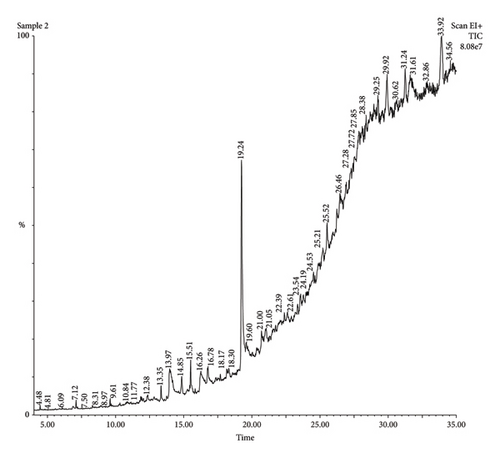
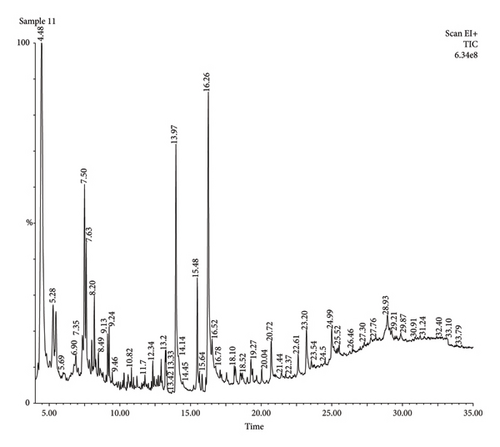
The findings presented in Figures 4 and 5 and Tables 3 and 4 revealed several bioactive compounds including bioactive compounds: 3-allyl-6-methoxyphenol, 1-tetradecene,1-(hydroxymethyl)-1,2-ethanediyl ester, methyl-, 4-heptyl-, Trans-13-octadecanoic acid, methyl ester, 9-octadecanoic acid, (E)-, hexadecenoic acid, 1-Heptane, 3-dihydroxyphene, acetic acid tert-butyldimethylsilyl ether, (9-octadecenamide, 5,5-Dimethyl-1,3-Diol, 13-hydroxy-4,4,6a,6b,8a,11,11,14b-octamethyldocosahydropicen-3-yl ester, 12, and 15-octadecatrienoic acid, 2,3-bis[propyl ester (trimethylsilyl)oxy], (Z, Z, Z)-, 2-propylpheno: 2-propylphenol, acetic acid,13-hydroxy-4,4,6a,6b,8a,11,11,14b-Octamethyldocosahydropicen-3-yl ester, tetranethyl-3,8,12,16-heptadecan-3,7,11,15-tetranyl), 10-hexadecenoic acid acid cis-veccenic, 2.2,4-trimethyl-3--cyclohexanol, 2,6-bis[oxy(tert-butyldimethylsilyl)]-, and tert-butyldimethylsilyl ester. These Bioactive compounds (Tables 3 and 4) have been recognized to possess antimicrobial qualities. The authors in reference [19] assessed 9-octadecanoic acid’s efficacy against Escherichia coli and Staphylococcus aureus [19]. Similar research findings reported by the authors in [20] revealed that 3-hydroxy-1-(hydroxymethyl) ethyl ester possesses antibacterial, anticancer, and antifungal properties [20]. The antihistaminic, anti-inflammatory, and antioxidant qualities of 1-tetradecene and 3-allyl-6-methoxyphenol have been shown in studies by [21].
| Peak number | Retention time | Peak value | Molecular weight | Name of the compound | Molecular formula |
|---|---|---|---|---|---|
| 1 | 13.97 | 8889 | 256.42 | n-Hexadecanoic acid | C16H32O2 |
| 2 | 14.85 | 119,669 | 493.2 | Stigmastan-3-ol,5-chloro-, acetate (3α,5α) | C31H53ClO2 |
| 3 | 15.51 | 18,073 | 296.5 | 10-Hexadecenoic acid, methyl ester | C19H36O2 |
| 4 | 16.26 | 18,782 | 282.5 | Cis-veccenic acid | C18H34O2 |
| 5 | 25.52 | 32,427 | 428.7 | 2.2,4-trimethyl-3-(3,8,12,16-tetramethyl-heptadecan-3,7,11,15-tetranyl)-cyclohexanol | C30H52O |
| 6 | 26.46 | 40,319 | 667.39 | Cyclononasiloxane, octadecamethyl | C18H54O9Si9 |
| 7 | 27.85 | 22,797 | 490.90 | 17-Pentatriacontene | C35H70 |
| 8 | 29.25 | 30,756 | 456.70 | α-Sitosterol acetate | C31H52O2 |
| 9 | 29.92 | 12,563 | 468.80 | Lup-20(29)-en-3-ol, acetate (3α) | C32H52O2 |
| 10 | 31.24 | 210,905 | 304.57 | 4-Pyrimidenecarboxylic acid,2,6-bis[(tert-butyldimethylsilyl)oxy]-,tert-butyldimethylsilylester | C14H32O3Si2 |
| 11 | 31.61 | 120,237 | 889.85 | Tetracosamethyl-cyclododecasiloxane | C24H72O12Si12 |
| Peak number | Retention time | Peak value | Molecular weight | Name of the compound | Molecular formula |
|---|---|---|---|---|---|
| 1 | 4.47 | 135,655 | 164.20 | 3-Allyl-6-methoxyphenol | C10H12O2 |
| 2 | 5.69 | 1735 | 196.37 | 1-Tetradecene | C14H28 |
| 3 | 6.90 | 22,616 | 196.00 | Tetradecane | C14H30 |
| 4 | 7.35 | 38,708 | 591.20 | 3-Butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C19H54O7Si7 |
| 5 | 7.63 | 22,635 | 212.41 | Pentadecane | C15H32 |
| 6 | 13.22 | 9771 | 270.45 | Hexadecanoic acid, methyl ester | C17H34O2 |
| 7 | 16.26 | 18,782 | 282.50 | Cis-veccenic acid | C18H34O2 |
| 8 | 16.52 | 7273 | 264.35 | 2,2-Difluoroheptacosanoic acid | C14H26F2O2 |
| 9 | 23.20 | 102,989 | 214.42 | 1-Cyclohexyldimethylsilyloxybutane | C12H26OSi |
| 10 | 24.99 | 156,509 | 290.50 | 5-(7a-isopropenyl-4,5-dimethyl-octadihydroinden-4-yl)-3-methyl-pen-2-enal | C20H34O |
| 11 | 27.76 | 5713 | 764.94 | Digitoxin | C41H64O13 |
| 12 | 28.93 | 18,730 | 444.73 | Tricyclo[20.8.0.0(7.16)]triacontane-1(22),7(16)-diepoxy- | C30H52O2 |
| 13 | 29.87 | 65,832 | 430.70 | Cholestan-3-one, cyclic 1,2-ethanediyl acetal, (5α)- | C29H50O2 |
| 14 | 30.91 | 42,692 | 355.00 | 2,7-Diphenyl-1,6-dioxopyridazino[4,5′,2’]pyrrolo[4′,5’]pyridazine | C20H13N5O2 |
| 15 | 31.24 | 6904 | 537.00 | 1-Heptatriacotanol | C37H76O |
| 16 | 32.40 | 18,922 | 270.45 | i-Propyl 9-tetradecenoate | C17H34O2 |
| 17 | 33.10 | 23,506 | 584.83 | Octatriacontyl pentafluoropropionate | C33H61F5O2 |
Digitoxin and diepoxy have been established in the literature as treatment options for cardiac arrhythmias and heart failures, and they have antimicrobial and cytotoxic properties [22]. The authors of reference [23] in their research findings have established the antimicrobial properties of 2,2-difluoroheptacosanoic acid [23]. The current study also established the presence of propionic acid and t-butyl ester which have been determined to possess several biological activities, including antityrosinase, antioxidant, and antibacterial qualities [24]. Hexadecenoic acid as profiled in Tables 3 and 4 has shown to have antibacterial activities against a range of pathogenic bacteria, including Salmonella typhi, Escherichia coli, and Staphylococcus aureus [25].
Phenol and 4-heptyl as identified in the aqueous crude extracts of Terminalia laxiflora root and Mitragyna inermis stem bark (Tables 3 and 4) possess anti-inflammatory, antioxidant, and anticancer effects in previous researches [26]. The authors in reference [21] in a similar research profiled the biological activities of tetracosamethyl-cyclododecasiloxane, 3-allyl-6-methoxyphenol [21], 10-hexadecenoic acid, methyl ester, and cis-veccenic acid (Z) (Tables 3 and 4) Studies have demonstrated the antitumor, antibacterial, anti-inflammatory, anticancer, and antioxidant properties [25]. The authors in reference [27] in their research findings reported the anti-inflammatory activities of lup-20(29)-en-3-ol, acetate (3α). The findings in the current study are all plants’ secondary metabolites. Treatment and cure for a variety of disorders may be aided by the use of extracts from the stem bark of Mitragyna inermis and Terminalia laxiflora root [28].
3.2. Conclusion
In the current study, FTIR and GC–MS analyses of the aqueous crude extracts of Mitragyna inermis stem bark and Terminalia laxiflora root revealed the presence of common bonds stretching from O-H, N-H, C-H, C=C to C-X, corresponding to the presence of alkaloids, flavonoids, terpene, saponins, and phenolic compounds, which can be isolated and further screened for various biological activities depending on their therapeutic uses. The structural analysis of the aqueous crude extracts of the plants under study revealed the presence of important biologically active compounds. The claimed efficacy of these plants in the current study by traditional folks might be due to the presence of alkaloids, tannins, saponins, flavonoids, and phenols. Further studies are required to isolate the most active compounds from the aqueous crude extracts of Mitragyna inermis stem bark and Terminalia laxiflora root.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
The original concept was initiated by Samson Abah Abagale and Isaac Sackey. Laboratory analysis was conducted by Sylvenus Aguree. The manuscript was drafted by Sylvenus Aguree. The manuscript was edited and approved by all authors.
Funding
This research did not receive any funding or grant from any organization or co-operate body and or not-for-profit sectors.
Acknowledgments
The authors acknowledge the contributions and support of the staff of Professor Marion Martinson Biotechnology laboratory, Brandenburg University of Technology, Cottbus, Germany, for their tremendous inputs into this study. We (authors) also appreciate the suggestions of the faculty members of C. K. Tedam University of Technology and Applied Sciences, School of Chemical and Biochemical Sciences, Navrongo, Ghana.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




