Influence of Pectin/Xanthan Gum Mixtures on the Physical and Oxidative Stability of Rice Bran Protein Hydrolyzate-Stabilized Fish Oil-in-Water Emulsions
Abstract
The effects of pectin and xanthan gum (XG) on the physical and oxidation stability of fish oil-in-water emulsions stabilized by rice bran protein hydrolyzate (RBPH) under different pH conditions (pH = 7 or 3.5) were investigated. At both pH 7 or pH 3.5, the addition of pectin and XG improved the physical stability of the emulsions due to electrostatic repulsion and steric hindrance. In contrast, when pH 7, the effect of polysaccharides on the oxidative stability of the emulsion: pectin was increased while XG was decreased. All properties improved at pH 3.5. In general, the oxidation stability of the emulsion at pH 7 is higher than pH 3.5, which is due to the high solubility of iron under acidic conditions. These effects are due to differences in the ability of the two polysaccharides to bind pro-oxidant iron ions, which affects emulsion aggregation. This research provides a theoretical basis for delivery systems with plant-based emulsifiers.
1. Introduction
Long-chain ω-3 polyunsaturated fatty acids (PUFAs) are essential nutrients for the human body and must be intaked through dietary [1]. However, ω-3 PUFAs have many characteristics, such as natural oxidation, low solubility, unpleasant odor, and variable bioavailability, which significantly limit their application [2]. Accordingly, it is a considerable challenge to develop new product opportunities and technologies to expand the range of fish oil applications in the food industry.
Although oil-in-water emulsions have been widely used in ω-3 PUFA delivery systems [3], they still have many limitations, such as their poor lipid oxidation stability. Lipid oxidation is an adverse reaction that leads to food deterioration and nutrient loss. Moreover, the polyunsaturated lipids formed by lipid oxidation often cause some potentially toxic reactions [4]. The addition of antioxidants is undoubtedly one of the simplest and most effective. Among antioxidants, plant-based ingredients are gradually replacing synthetic or animal-based emulsifiers as consumers seek more environmental friendliness [5]. Given that some traditional surfactants are harmful to human health and pollute the environment [6], natural plant-based emulsifier show higher stability and lower toxicity [7]. Our past studies have shown that the rice bran protein hydrolyzates (RBPHs) with a degree of hydrolysis of 3% could effectively stabilize the emulsion as an emulsifier [8]. Rice bran is an important byproduct of rice production, and it has been used as fodder or has simply been discarded in the past. Using rice bran as an emulsifier will broaden its range of applications and increase its economic value.
However, protein-stabilized emulsions tend to be unstable due to environmental effects. We previously found that adding pectin or xanthan gum (XG) significantly improves the physical stability of RBPH-stabilized emulsions, even around the isoelectric point [8]. However, the extent to which polysaccharides improve the oxidation stability of emulsions depends on the type of emulsifier and the surrounding environment [9]. Polysaccharides can directly or indirectly promote pro-oxidation or oxidation in emulsion systems. Therefore, it is necessary to investigate the oxidation stability of emulsions with different polysaccharides under different environmental conditions. Furthermore, a better understanding of the protein hydrolyzate–polysaccharide complex emulsion system is crucial for further development.
2. Materials and Methods
2.1. Materials
Rice bran protein (72.03 wt%, dry basis) was prepared in our laboratory. Fish oil was purchased from Piping Rock Health Products Ltd. (Ronkonkoma, USA). XG with an average molecular weight of 6000 kDa was purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Pectin extracted from citrus fruit (DE > 50%) with an average molecular weight of 200 kDa was provided by Sigma-Aldrich Chemical Co. (St. Louis, MO). Other chemicals were provided by Tianjin Tianli Chemical Reagent Co. (Tianjin, China).
2.2. Preparation of RBPH
RBPH was obtained following the method described earlier [8]. The enzymatic hydrolysis time was 4.40 min, and the mixture was heated at 90°C for 10 min to inactivate the enzyme and then cooled to room temperature in an ice bath quickly. The supernatant was centrifuged at 6000 g for 15 min at 4°C after adjusting the pH to 7 with 1 M NaOH or 1 M HCl and then lyophilized for later use.
Phosphate buffer (5 mM, pH 7) was used to dilute RBPH to make a 3 wt% protein solution and to dissolve polysaccharide solutions (1 wt%). The protein and polysaccharide solution was stored in a refrigerator at 4°C overnight to ensure that the protein was fully hydrated after stirring for 2 h. The pH of the solution was readjusted to 7 if necessary.
2.3. Emulsion Preparation
The primary emulsion was prepared by mixing 10 wt% fish oil and 90 wt% RBPH suspension (3%, pH 7.0) at 10,000 rpm for 2 min with a T18 basic high-speed blender (IKA, Staufen, Germany). Then, an EP FPG12805 high-pressure homogenizer equipped with a cooling device (STANSTED FLUID POWER LTD., Essex, United Kingdom) was used to emulsify primary emulsions at 80 MPa with 3 cycles. The secondary emulsions was a mixture of primary emulsion, polysaccharide solution, and buffer solution (10 mmol/L, pH 7.0 phosphate buffer solution) in different proportions, which is composed of 1 wt% fish oil, 0.3 wt% RBPH, and 0.2 wt% polysaccharide, pH 7. After stirring for 30 min, sodium azide (0.005 wt%) was added as a microbial inhibitor. The pH of the emulsion was adjusted to 3.5 or 7 with 0.5 M HCl and NaOH and then stirred for 1 h.
2.4. Particle Size and Surface Charge (ζ-Potential) Measurements
The particle size and ζ-potential of the emulsion were measured by a Mastersizer 2000 (Malvern Instruments, Worcestershire, UK) and a ZS-90 Zetasizer Nano ZS-90 (Zetasizer Nano ZS-90, Malvern Instruments, Worcestershire, UK), respectively. To avoid multiple scattering effects, all emulsions were diluted 100-fold and measured; the parameters were recorded and analyzed.
2.5. Confocal Laser Scanning Microscopy (CLSM) Analysis
The microstructure of the emulsion were observed by a CLSM (Leica TCS SP2, Leica Microsystems, Wetzlar, Germany). The oil and protein in the emulsion (1 mL) were stained with Nile red solution (40 μL, 0.01 wt% in isopropanol) and Nile blue solution (40 μL, 1 wt% in deionized water), respectively. The stained emulsion sample (10 μL) was placed on the slide for visualization after 30 min. The excitation and emission wavelengths were 488 nm and 633 nm, respectively.
2.6. Oxidation Measurements
All the samples were incubated for 29 days at 37°C away from light, and the samples were taken out periodically to determine their corresponding indicators.
2.6.1. Hydroperoxides
Hydroperoxides were analyzed according to Celus et al. [10]. In brief, 0.3 mL of emulsion and 1.5 mL of iso-octane/2-propanol (3:1 v/v) were added to a centrifuge tube and centrifuged (Sigma 3-18KS centrifuge, Germany) at 1000 g and 20°C for 2 min after vortexing three times for 10 s. Then, 0.2 mL of the supernatant was transferred to the test tube, and 2.8 mL of methanol/2-propanol (2:1 v/v) solution, 15 μL of 3.94 M ammonium thiocyanate solution, and 15 μL of 0.0072 M Fe2+ solution were successively added and mixed. The absorbance of the mixture was measured at 510 nm after 20 min of storage without light. The peroxide concentration in the emulsion was calculated by dividing the Fe3+ concentration by 55.845 (the atomic weight of Fe3+), and the Fe3+ concentration was determined by reference to the standard curve of FeCl3 solution.
2.6.2. TBARS
The formation of TBARS was measured according to Xu et al. [11] with slight modifications. In brief, 1 mL of emulsion and 2 mL of thiobarbituric acid (TBA) solution were added to a capped test tube. After boiling for 15 min, cool quickly to room temperature in 10 min. Centrifuge 1000 g for 15 min. The absorbance of the supernatant was measured at 532 nm. The concentration of secondary oxidation products was obtained by a 1,1,3,3-tetraethoxypropane (TEP) standard curve.
2.7. Statistical Analyses
The data were analyzed by one-way analysis of variance (ANOVA) using Duncan’s multiple range test of SPSS 19.0 statistical analysis software (SPSS Inc., Chicago, USA). There was statistical significance when p < 0.05. All analyses were performed on three samples, each of which was repeated at least three times. Results are presented as the measured mean ± standard deviation.
3. Results and Discussion
3.1. Physical Stability
3.1.1. Similar Charges on Droplets and Polysaccharides (pH 7.0)
Both RBPH-coated oil droplets and polysaccharides carry negative charges at pH 7.0 [9], which should cause electrostatic repulsion between them.
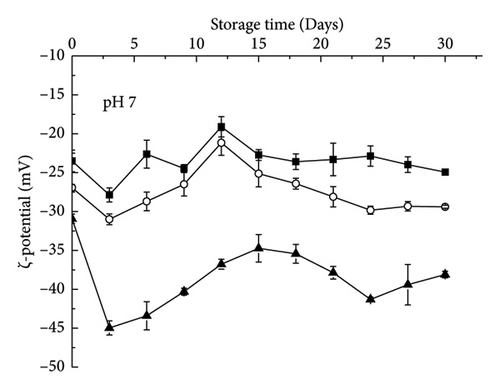
The emulsion containing XG remained relatively stable without significant phase separation (Figure 1). The control emulsion also showed this stable state when the storage time was less than 21 days (Figure 1). In addition, no large aggregation was observed in the 29-day microscopic images (Figure 2(a)). The mean particle diameter (d3,2) remained at approximately 0.96 ± 0.28 μm (control emulsion) and 1.55 ± 0.36 μm (containing XG) (Figure 3(a)). This system was stable because it was maintained by electrostatic repulsion between RBPH-coated droplets or RBPH-coated droplets and polysaccharide molecules. In particular, the emulsion with XG always had a high charge, from approximately −30 mV to −40 mV (Figure 4(a)). This result may be related to changes in interface composition during storage. One possible reason for this observation is that the anionic reaction products formed during lipid oxidation accumulated on the surface of the oil droplets, thereby increasing their negative charge. An increase in electrostatic repulsion also reduced the accumulation of oil droplets [12].
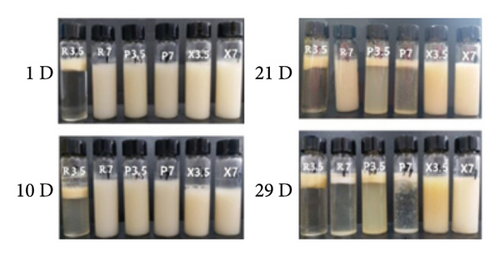
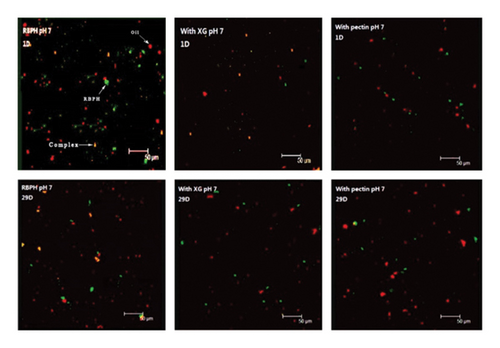
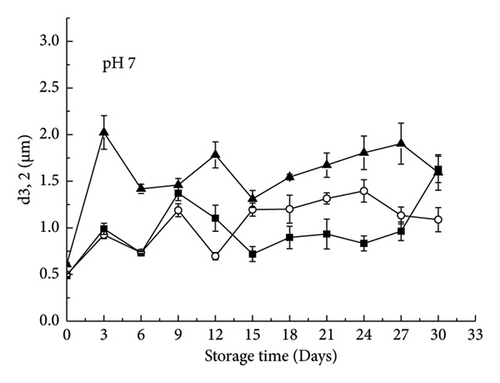

Pectin-containing emulsions also showed considerable aggregation and creaming stability at the beginning of the storage period. The d3,2 value remained at 1.03 ± 0.2 μm (Figure 3(a)), and rapid phase separation was not observed (Figure 1). No substantial amount of aggregation was observed in microscopic images (Figure 2(a)). The initial particle size (1.03 ± 0.21 μm) of pectin-coated emulsions was more extensive than that of the control emulsions (0.96 ± 0.28 μm), probably due to the presence of a layer of pectin molecules on the surface of the oil droplets. The pectin layer can be adsorbed because the anion group carries binds to the cationic region on the surface of the RBPH-coated droplet. This is similar to the reported protein–polysaccharide system above the isoelectric point of proteins [8]. The excellent aggregation stability of pectin-stabilized emulsions may also be due to the synergistic reaction of electrostatic and steric repulsion between the RBPH-coated droplets and the pectin-coated droplet [13].
3.1.2. Opposite Charges on Droplets and Polysaccharides (pH 3.5)
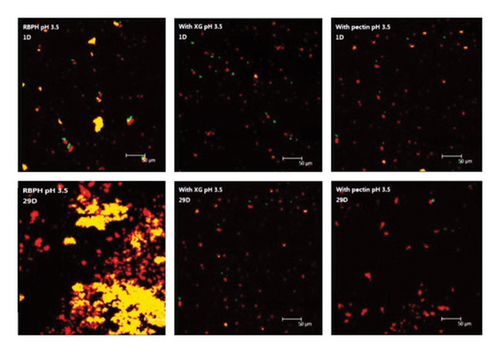
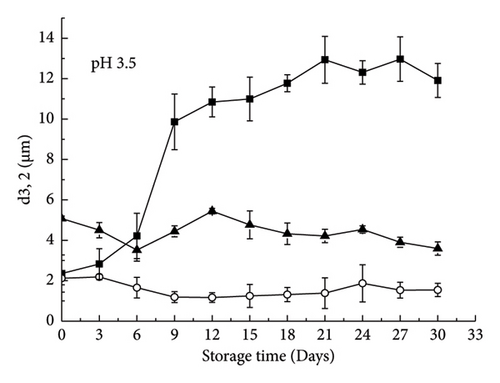
At pH 3.5, the control emulsion was very unstable due to droplet aggregation and creaming: After 29 days of storage, the d3,2 value increased from 2.35 μm to 11.91 μm (Figure 3(b)). Rapid phase separation occurred after 24 h (Figure 1). Large droplets and flocs were clearly observed under a microscope (Figure 2). The reason for these droplets and flocs was that the pH of the solution was close to the isoelectric point of the adsorbed protein. At the same time, the electric repulsion between oil droplets was not sufficient to overcome van der Waals forces and hydrophobic interactions and thus led to droplet aggregation. The particle size of the control emulsion remained stable for the first 6 days and then increased rapidly (Figure 3(b)).
At pH 3.5, the RBPH-coated oil droplets had a positive charge, while the polysaccharide molecules had a negative charge. The addition of polysaccharides has been shown to promote a strong electrostatic attraction between these molecules [9]. Compared with the control emulsion, the emulsion containing anionic polysaccharide had a smaller particle size (Figure 3(b)), better emulsion stability (Figure 1), and less aggregation (Figures 1 and 2(b)). It can be seen that the aggregation and creaming stability of the emulsion will be increased after the addition of polysaccharide. The results show that the electrostatic repulsion and steric hindrance between the anionic polysaccharides adsorbed on the surface of cationic oil droplets can be increased, and their aggregation stability can be greatly improved [13]. Therefore, this mechanism reasonably explains the phenomenon: the physical stability of the emulsion added with XG and pectin was increased.
XG molecules adhere more strongly to the surface of the oil droplets and generate greater electrostatic repulsion. In contrast, pectin has weak adhesion to the surface of the droplet. When some pectin molecules are separated from one droplet and adsorb to the surface of another droplet, bridging flocculation occurs [14]. In addition, the electrostatic repulsion between the droplets coated with pectin was weaker than that of the droplets coated with XG, which was also the reason for the greater droplet aggregation. The ζ-potential also reflected that the droplets coated with XG carried a significantly higher charge than those coated with pectin (Figure 4(b)). It is interesting that at pH 3.5, the ζ-potential coated by an anionic polysaccharide did not change over time, suggesting that their interface composition remained relatively constant.
3.2. Chemical Stability
3.2.1. Similar Charges on Droplets and Polysaccharides (pH 7.0)
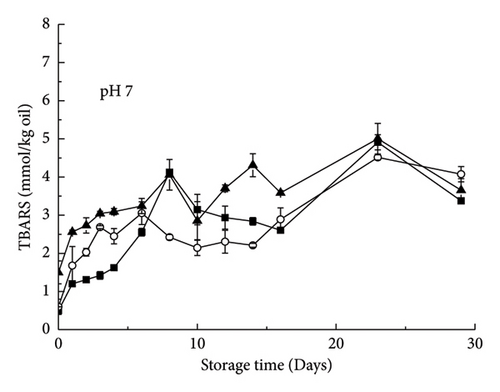
During preservation at neutral pH (pH 7.0), although the primary oxidation products of the emulsion at the initial stage increased, lipid oxidation occurred, but the addition of polysaccharides had a certain delay effect on the increase in peroxidation value. During the initial 16 days of storage, lipid hydroperoxides and TBARS formed very similarly in all emulsion systems (Figures 5(a) and 5(b)) and increased slowly. However, during longer storage periods, the degree of lipid oxidation in the samples showed significant differences, and the general trend was XG-containing emulsion > control emulsion > pectin-containing emulsion. The rapid formation of TBARS occurs after the formation of lipid hydroperoxides because of the decomposition of primary oxidation products before the formation of secondary oxidation products [15]. The results showed that under these conditions, XG played a small role in promoting oxidation, while pectin played a small role in antioxidation. One possible reason is that the negatively charged polysaccharides in the aqueous phase can bind to positively charged transition metals (e.g., Fe2+), preventing them from binding to the ω-3 PUFAs contained in the droplets [10]. However, a similar phenomenon was observed in emulsions stabilized with whey protein: the rate of lipid oxidation in emulsions containing XG was significantly higher than that in control emulsions [10]. One possible reason for this observation is that XG increased the water solubility of metals at this pH. Metal ions are poorly water soluble at a neutral pH of 7.0, which may reduce their oxidation-promoting activity [16]. Therefore, if the highly anionic XG molecule increases the solubility of iron ions, it can increase the oxidation capacity of lipids. The addition of high anionic phospholipids to a neutral aqueous solution containing iron has a similar effect [17].

3.2.2. Opposite Charges on Droplets and Polysaccharides (pH 3.5)
As shown in Figure 6, the curves of the control emulsion oxidation products with RBPH stabilization were all above the curves of emulsions containing a polysaccharide (pectin, XG), which demonstrated the inhibition of polysaccharide addition on oxidation at pH 3.5. The lipid hydroperoxide and TBARS contents increased initially but decreased with prolonged storage time (Figure 6). The possible reasons for this effect were the initial formation of these reaction products and their degradation as lipid oxidation progressed. lipid oxidation increased in the order of XG-containing emulsion < pectin-containing emulsion < control emulsion. This pattern was consistent with previous reports that the addition of XG or pectin under acidic conditions can reduce lipid oxidation in emulsions [18, 19]. At pH 3.5, both XG and pectin can inhibit lipid oxidation to a certain extent, but XG has a stronger inhibitory capacity than pectin.
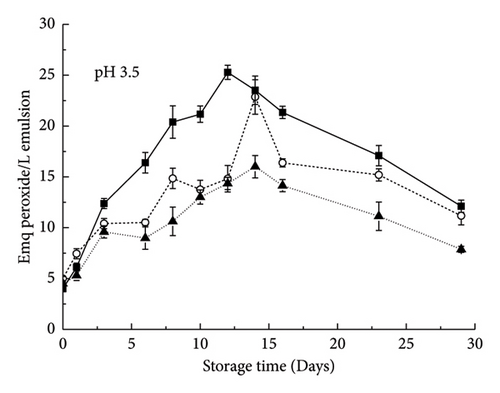
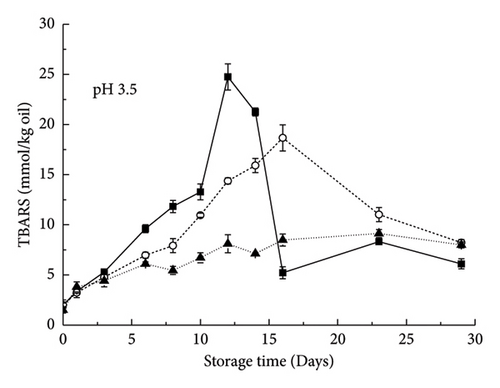
Previous studies have shown that iron is more soluble under acidic conditions than under neutral conditions [14]. Therefore, under these conditions, anionic polysaccharides have a strong binding ability with cationic transition metal ions, which may reduce their ability to interact with oil droplets rich in omega-3 unsaturated fatty acids. An important reason for the antioxidant activity of anionic polysaccharides is that the negative electric groups carried by them can chelate with cationic metals [20]. Under acidic conditions, because XG has a more negative charge than pectin [9], it has a stronger ability to bind transition metals. XG can inhibit lipid oxidation through physical and chemical effects. XG can chelate metal ions via the negatively charged pyruvate moiety and quench peroxides in the oil by passivation of Fe2+ ions. This is why it is a more potent oxidant than pectin under these conditions. Under acidic conditions, the control emulsions were extremely unstable and tended to aggregate compared with those containing polysaccharides (Figure 1). Lipid oxidation may occur more rapidly when the oil droplets are relatively close together because free radicals produced in one oil droplet may be more easily transported to adjacent oil droplets. This explains why emulsions containing XG have greater antioxidant capacity than emulsions containing pectin. Similar conclusions also appeared in the study of the hydrolyzate of rice gluten [11], suggesting that the main role of rice bran protein may be gluten.
4. Conclusions
The effects of the addition of anionic polysaccharides on the physical and oxidative stability of RBPH-coated fish oil emulsions were investigated under different environmental conditions (neutral and acidic pH). Under neutral conditions (pH = 7), RBPH and anionic polysaccharides had similar charges. The aggregation, creaming, and oxidation stability of emulsions containing pectin were all improved. In contrast, the physical stability of the emulsion containing XG was not markedly different from that of the control emulsion, and the oxidation stability of XG played a role in promoting the impact. Under acidic conditions (pH = 3.5), RBPH and anionic polysaccharides had opposite charges. The emulsions containing pectin and XG showed excellent physical and oxidation stability, and the effect of adding XG was better than that of adding pectin. In general, because iron is more water soluble under acidic conditions than under neutral conditions, the oxidation rate of lipids at pH 7 was significantly lower than that at pH 3.5. The effect of polysaccharides on the oxygenation stability of the emulsion was because polysaccharides can bind to iron, which competitively reduced the potential chance of their binding to lipids. However, the interaction mechanism between polysaccharide and emulsion components needs further study. Fourier infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and biolayer interference (Biofilm) are used as the main means to study the interaction mechanism of emulsion components, which will provide help for further study of the interaction mechanism. This study provides a necessary theoretical basis for the design of unsaturated lipid transport systems. Such delivery systems can be used to deliver lipophilic vitamins, carotenoids, phenolic bioactives, minerals, flavor compounds, probiotics, and unsaturated fatty acids. In particular, emulsion gels, which are rich in unsaturated fatty acids, have proved to be good fat substitutes.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by Science and Technology Department of Heilongjiang Province, the Natural Science Foundation of Heilongjiang Province (grant number: SS2021H003).
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.




