Nitrogen and Metal Ions Accumulation in Two Sensitive Himalayan Lichens From Western Nepal: A Reference for Ecosystem Health Monitoring
Abstract
In this study, thalli nitrogen (N) and metal ions contents in the two fruticose lichens (Ramalina intermedia and Usnea cornuta) were quantified, which were collected from a pristine Himalayan forest of Shey Phoksundo National Park of western Nepal. Besides, the probable impacts of N and metal ions in physicochemical responses such as electrical conductivity, chlorophyll contents, and chlorophyll degradation were studied. Our initial hypothesis was there are considerable amounts of thallus N and metal ions in lichens from the study area, and it has substantial impacts on physicochemical responses. In comparison, the thalli N concentration was observed to be the greatest in R. intermedia thalli (0.24%–1.15%). However, comparable amounts of metal ions in both lichen species were observed. Regression analysis revealed the least or no impacts of N and metal ions in physicochemical responses. The principal component analysis suggests the contribution of different environmental variable and their correlation with lichen’s variables. This study provides evidence of the least concentration of N and metal ions and only minor impacts on the physicochemical integrity of the two sensitive lichens in our study area. This study is likely to contribute as field-based reference information for further studies on the species-specific N and metal ions accumulation in lichens from different Himalayan forest systems to monitor ecosystem health.
1. Introduction
The ability of ecosystems to function depends on a finely balanced availability and regulation of major nutrients including nitrogen (N) [1]. The N is essential in sustaining life; however, excess reactive nitrogen (Nr) in the atmosphere, which is formed through various sources ranging from intensive agriculture to fossil fuel burning, is now a serious threat to the world’s biodiversity [2]. The N deposition in the atmosphere has grown nearly threefold since 1995, and it is expected to quadruple by 2050 as the world’s population grows [3]. As a result, concentrations of ammonium-N and oxides of N are increasing globally, even in remote ecosystems [4]. Given that many ecosystems are structured by N-deficient conditions, even small increases in Nr deposition can induce the dual impacts of eutrophication and acidification, with far-reaching negative consequences for biodiversity and ecosystem services [5, 6].
Lichens have been used as a highly sensitive bioindicator for excess atmospheric N [7]. The point at which lichens start to suffer negative impacts has been used to establish ammonia (NH3) critical levels and N critical loads for ecosystems [8], as well as in monitoring the wider effects of N pollution [9]. While atmospheric N has stabilized or declined in temperate Europe and North America [10], excessive N is increasing in the tropics, and the highest values for atmospheric NH3 are now found in South Asia [11, 12]. Recent desk-based estimates—based on the transfer of lichen critical levels and loads—suggest that ca. 80%–95% of Himalayan forests may have surpassed thresholds for N pollution [13]. In order to monitor this rapidly changing environmental situation, it is urgent to establish a field-based reference against which future change can be assessed. Moreover, such references—if they can be established—can help contextualize earlier studies concentrated in Europe and North America, which have often been conducted in landscapes that had been recurrently affected by N pollution over long periods of time, thereby skewing the baseline from which N impacts are inferred. In addition to N, different elements are essential or otherwise affect lichen metabolic activities, and they can be trapped and remain within lichen thalli for long periods [14]. Metal ions present in lichen thalli can be beneficial in maintaining physiological health as they support the production of antioxidants for free radical scavenging [15]. However, metal ions such as copper, cobalt, iron, nickel, manganese, molybdenum, and mercury also cause toxicity if they are present in excessive concentrations [14, 16]. Thus, deposition sources and their impacts on lichen communities are relevant for wider bioindication studies.
Among the various forms of lichens around the globe, fruticose lichens are the most sensitive to pollution and have the least tolerance [17]. The richness of fruticose lichens among high altitudinal gradients of Nepal is noticeably high, compared to other life forms [18]. In this study, we focused on lichen epiphytes, growing in the Himalayan forest of Nepal and probably unaffected by pollution to construct a field-based reference for the thallus concentration of N and metal ions. The thallus N and metal ions concentration in two sensitive fruticose lichens of two different families: Ramalina intermedia (Delise ex Nyl.) Nyl. (1873) of Ramalinaceae and Usnea cornuta Körb. (1965) of Parmeliaceae, distributed in the subalpine forest of Shey Phoksundo National Park of western Nepal was quantified for the first time. Our initial hypothesis was there are considerable amounts of thallus N and metal ions in lichens from the study area, and it has substantial impacts on physicochemical responses. Our second hypothesis was there is comparable thallus N and metal ions concentration, and physicochemical responses in examined lichens. The outputs of this study can be used as a reference baseline for impact monitoring of the increasing atmospheric pollution due to N and metal ions in the Himalayan forest ecosystems.
2. Materials and Methods
2.1. Study Area
The sampling site (Figure 1) was a forest dominated by Pinus wallichiana A. B. Jacks. in a subalpine climatic zone of Shey Phoksundo National Park of Dolpa district of western Nepal. Based on model data, background atmospheric total N deposition is in the range of 3.197–15.033 kg N/(ha yr), and atmospheric NH3 concentration is in the range of 0.53–1.61 μg/m3. This makes it possible to sample from locations with N deposition below the critical load of 8.26 kg N/(ha yr) and NH3 below the critical level of 1.44 μg/m3, established as thresholds based on a recent meta-analysis [13], providing a reference baseline for N deposition. The probable sources of N deposition and accumulation in the study area are local agricultural practices, household emissions, and emissions from frequent forest fires. The annual average temperature of the region is about 12°C with annual precipitation ranging from 500 to 1200 mm.
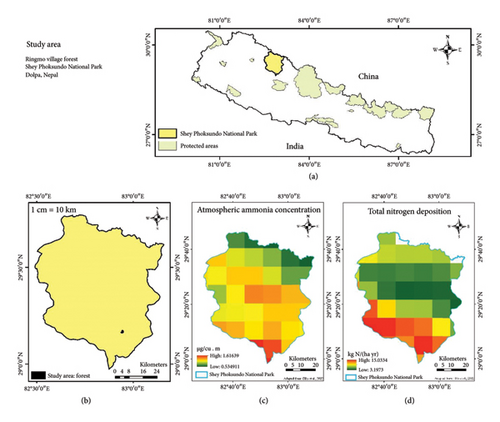
2.2. Samples Collection and Processing
Lichen samples, bark substratum, and soil were collected in September 2021. The three replicates of each lichen sample were collected from 16 P. wallichiana trees (Table 1), which comprise two duplicated trees at the same elevation, ranging from 3635 to 3885 m above sea level (m a.s.l.). The minimum distance between trees was 50 m, and the distance between duplicated trees was 100 m. Approximately 50 g of thallus material of two lichen species (R. intermedia and U. cornuta) per tree were collected by random stratified sampling to select trees (eight transects at equal intervals of 50-m vertical distance) and lichens (from multiple randomly positioned 10 × 10 cm quadrats). Approximately 10-g bark samples were collected from each tree using the same approach. For the soil, three replicates of soil samples were collected from a depth of approximately 15 cm, at positions surrounding each tree. The collected samples were brought to Kathmandu University for further laboratory analyses and kept at 4°C until use. The lichens were identified by the National Herbarium and Plant Laboratories, Godawari, Lalitpur, Nepal, with reference letter number 079/80-246. The impurities in the outer surface of lichens were removed before the analysis.
| Trees | Coordinates | Elevation (m a.s.l.) | DBH (cm) | Crown cover (%) | Tree height (m) | Tree slope (°) |
|---|---|---|---|---|---|---|
| T1a | 29°10′24.14″N 82°56′25.96″E | 3652 | 29 | 83 | 14 | 23 |
| T1b | 29°10′21.29″N 82°56′23.69″E | 25 | 68 | 12 | 22 | |
| T2a | 29°10′20.86″N 82°56′28.51″E | 3682 | 19 | 59 | 9 | 32 |
| T2b | 29°10′17.76″N 82°56′28.34″E | 20 | 55 | 8 | 29 | |
| T3a | 29°10′16.17″N 82°56′32.11″E | 3713 | 40 | 47 | 10.72 | 10 |
| T3b | 29°10′12.66″N 82°56′31.45″E | 38 | 42 | 11 | 12 | |
| T4a | 29°10′14.28″N 82°56′34.10″E | 3744 | 38 | 60 | 12 | 15 |
| T4b | 29°10′10.71″N 82°56′33.00″E | 35 | 62 | 11 | 17 | |
| T5a | 29°10′12.43″N 82°56′36.16″E | 3775 | 23 | 58 | 10 | 29 |
| T5b | 29°10′9.31″N 82°56′34.83″E | 26 | 58 | 9 | 32 | |
| T6a | 29°10′10.83″N 82°56′37.73″E | 3806 | 29 | 57 | 8.74 | 47 |
| T6b | 29°10′8.06″N 82°56′36.41″E | 26 | 55 | 8 | 45 | |
| T7a | 29°10′9.34″N 82°56′39.65″E | 3838 | 37.70 | 65 | 11 | 23 |
| T7b | 29°10′6.36″N 82°56′38.31″E | 36.10 | 65 | 10.50 | 22 | |
| T8a | 29°10′7.85″N 82°56′41.89″E | 3869 | 26 | 46 | 9 | 42 |
| T8b | 29°10′5.11″N 82°56′40.35″E | 26 | 43 | 9.70 | 43 |
2.3. Lichen Abundance and Bark Roughness
The abundance categories of the lichens were quantified based on quadrat sampling. The number of lichen thalli was counted within each quadrat. With a modification to the Braun-Blanquet density score, high abundance (a) was applied if more than five lichen thalli were present in a single quadrat, and moderate abundance (b) was applied for the presence of three to five lichen thalli in the quadrat, while fewer than three thalli accounted for low abundance (c) [19]. Moreover, the roughness categories of bark were high (a), moderate (b), and smooth (c), quantified based on smoothness and depth of crevices adopting the methods of previous investigators [20].
2.4. Total Kjeldahl N in Lichens, Bark, and Soil
The total Kjeldahl nitrogen (TKN) in lichens and bark samples was determined using the macro-Kjeldahl (Vapodest, VAP50S) through the boric acid titrimetric method [21], for which 0.5 g of air-dried lichen thalli and 1 g of bark samples were subjected for analyses. The total available N in the soil was determined using the Kjeldahl method [22] for which 5 g of soil samples were subjected to analyses. The results were expressed as the percentage of N per gram of lichen, bark, and soil.
2.5. Metal Ions in Lichens, Bark, and Soil
The tri-acid digestion method with slight modification was used for the extraction and determination of metal ions in lichen, bark, and soil samples [23]. Briefly, 1 g of dry lichen, bark, and soil were digested with 25 mL of the tri-acid mixture (concentrated HNO3, concentrated HClO4, and concentrated H2SO4; 10:4:1 v/v) at 80°C. The digested samples were diluted to 50 mL with deionized water. The solutions were filtered through a Whatman filter (0.45 micron) and processed for the quantification of sodium (Na+), magnesium (Mg2+), potassium (K+), calcium (Ca2+), chromium (Cr4+), manganese (Mn2+), iron (Fe3+), nickel (Ni2+), cobalt (Co2+), copper (Cu2+), zinc (Zn2+), lead (Pb2+), and cadmium (Cd2+) using a direct air-acetylene method in atomic absorption spectrophotometer (SavantAA GBC).
2.6. Physicochemical Parameters in Soil, Bark, and Lichens
For the determination of electrical conductivity (EC) in lichens, 2 g of lichen samples were soaked in 20-mL ultrapure water and left for 1 h before measurement. Similarly, for the determination of EC and pH in bark, 2 g of bark samples were soaked in 20-mL ultrapure water and left overnight before measurement. Moreover, for the determination of soil pH, 10 g of soil was dissolved in 10-mL ultrapure water and left for 1 h before measurement. For the determination of EC in soil, 5 gm of soil was dissolved in ultrapure water and made it slurry. The mixture was then filtered through the Whatman filter paper before measuring EC. The pH and EC were measured using a standard pH meter (pH 50 VioLab, XS, Italy) and a standard EC meter (COND 7, Vio Set, Italy), respectively.
2.7. Chlorophyll Content and Degradation in Lichens
2.8. Source Ascertaining for Metal Ions
2.9. Statistical Analyses
All measurements were carried out at least in triplicate, and results are presented in mean ± standard deviation (mean ± SD). A significant difference in the mean values of different variables between the lichen species was tested using an independent sample t-test, and a significant difference in the mean value of different variables within the same lichen species but from different tree species was tested using one-way ANOVA, post hoc Tukey, multiple comparison test with a logarithm transformation of data to achieve normal distribution, and results considered significant for p < 0.05. Moreover, Pearson’s correlation between the lichen’s variables and principal component analysis (PCA) was performed in R (version 4.2). A regression analysis of all the variables of the two lichen samples was performed to compare species-specific bioindication potential.
3. Results and Discussion
3.1. Forest Environment and Lichen Abundance
Our study reports the reference for the N and metal ions in two lichens in a pristine region of the Himalayan forest. This is relevant to future trend analysis considering the increase in N pollution in South Asia and the potential of this to affect Himalayan forest ecosystems. To standardize for tree species, we selected P. wallichiana trees which are highly abundant and distributed in the alpine climatic zone of western Nepal. This was based on the evenness of tree species in the study area. The moderate-to-high abundance of both lichens was observed in the study area along with moderate-to-rough bark roughness categories (Table 2). In addition to selective two lichens, several other lichens such as Hypotrachyna spp., Lecidea spp., Coccocarpia spp., Pertusaria spp., Punctelia spp., Lecanora spp., Bacidia spp., Coenogonium spp., Usnea spp., Ramalina spp., Peltigera spp., Hypogymnia spp., Xanthoria spp., Heterodermia spp., Cladonia spp., Leptogium spp., Flavoparmelia spp., Physcia spp., Physconia spp., Bacidia sp., and Dolichousnea sp. were abundant in the study area.
| Trees | Lichens abundance | Bark roughness | ||
|---|---|---|---|---|
| R. intermedia | U. cornuta | R. intermedia | U. cornuta | |
| T1 | b | b | c | c |
| T2 | b | b | b | b |
| T3 | a | b | a | a |
| T4 | b | a | b | b |
| T5 | b | b | b | b |
| T6 | a | b | b | b |
| T7 | b | b | b | b |
| T8 | b | a | c | c |
- Note: Abundance: a, high; b, moderate; and c, low and roughness: a, smooth; b, moderate; and c, rough.
3.2. Thalli N Content
Although N is a limiting agent in most of the ecosystem, the excess N will help deteriorate the ecosystem’s health by causing eutrophication, acidification, and alternation in nutrient cycling [28]. Lichens may primarily fulfill N requirements in the form of ammonium, nitrate, organic N, and, in the case of cyano-lichens, gaseous N [29]. The Nr species (mainly in the form of gaseous NH3 and oxides of N) have negative impacts on terrestrial as well as aquatic biodiversity [30]. Atmospheric NH3 has accumulated to high levels in the Indo-Gangetic Plain (IGP), contributing to extensive atmospheric pollution [31]. In this study, we report thallus N concentrations of 0.24%–1.15% in R. intermedia and 0.22%–0.62% in U. cornuta as TKN. Although there was no significant difference in the mean N concentration between the two lichens, there was a significant difference among different trees (p < 0.05)—indicating the effects of the local environment under baseline conditions (Table 3 and Supporting Information File S1). The N concentration in the U. cornuta is comparatively low as indicated in a previous finding from the American region for other Usnea spp [32]. Further, in comparison, N deposition in Usnea and Ramalina species from some parts of China was remarkably higher than our findings [33]. Although several studies focusing on computational modeling [34] and the impacts of ammonium-N on the functional traits of some lichen species for critical level analysis are well understood in other parts of the world [7, 35–38], the studies get the least attention in the South Asian region. Our results report a considerably low concentration of thalli N concentration in both lichen species. Additionally, based on the fact that accumulation of N in lichens thalli can alter the physicochemical and biological responses in lichens [39], we further use thallus N concentration in U. cornuta and R. intermedia as a proxy for establishing the relations with physicochemical responses such as chlorophyll contents, chlorophyll degradation, and cell membrane integrity.
| Parameters | Variables | Lichens | |||
|---|---|---|---|---|---|
| R. intermedia | U. cornuta | ||||
| Range | Mean ± SD | Range | Mean ± SD | ||
| N | TKN (%) | 0.24–1.15 | 0.5 ± 0.86 | 0.22–0.62 | 0.41 ± 0.09 |
| Metals (ppm) | Na | 22.74–348.80 | 162.85 ± 118.86 | 6.25–326.88 | 128.40 ± 106.38 |
| Mg | 267.69–294.41 | 285.75 ± 7.86 | 246.48–299.05 | 283.28 ± 12.48 | |
| K | 137.71–329.64 | 256.65 ± 48.86 | 164.02–350.24 | 258.43 ± 54.81 | |
| Ca | 3.41–37.75 | 17.40 ± 10.86 | 7.70–38.73 | 17.11 ± 7.53 | |
| Cr | ND | ND | ND | ND | |
| Mn | 6.06–19.21 | 12.60 ± 3.86 | 5.18–23.82 | 11.12 ± 4.58 | |
| Fe | 169.86–853.74 | 384.55 ± 166.86 ∗ | 85.74–421.52 | 200.89 ± 73.99 ∗ | |
| Ni | 0.43–6.06 | 2.45 ± 1.86 | 0.21–8.89 | 2.71 ± 2.41 | |
| Co | ND | ND | ND | ND | |
| Cu | 0.40–3.46 | 1.73 ± 0.86 | 0.40–4.28 | 1.50 ± 0.82 | |
| Zn | 2.37–10.60 | 6.71 ± 2.86 | 2.06–9.61 | 4.55 ± 1.91 | |
| Pb | 0.26–42.10 | 17.37 ± 10.86 | 1.05–28.60 | 10.68 ± 9.60 | |
| Cd | 0.11–0.65 | 0.46 ± 0.86 | 0.11–0.63 | 0.45 ± 0.14 | |
| Physicochemical responses | EC (μS/cm) | 21.75–73.26 | 41.57 ± 11.86 | 27.16–82.45 | 46.08 ± 11.59 |
| Chlorophyll-a (mg/kg) | 4.48–21.97 | 10.39 ± 3.86 ∗ | 0.49–13.75 | 7.73 ± 3.28 ∗ | |
| Chlorophyll-b (mg/kg) | 1.05–7.57 | 3.64 ± 1.86 | 0.42–8.44 | 2.73 ± 1.73 | |
| Total chlorophyll (mg/kg) | 7.82–26.40 | 14.04 ± 4.86 ∗ | 3.33–18.66 | 10.46 ± 4.03 ∗ | |
| Chlorophyll degradation | 0.69–1.07 | 0.93 ± 0.86 ∗ | 0.49–0.96 | 0.74 ± 0.14 ∗ | |
- Note: All experiments were carried out in six replicates, and results are presented as mean ± standard deviation (mean ± SD) of 8 trees (n = 8).
- Abbreviation: ND, not detectable.
- ∗Significant difference at p < 0.05, independent sample t-test with log transformation of variables.
3.3. Metal Ions in Lichen Thalli
Metals in lichens are generally from atmospheric deposition. Moreover, the transformation and deposition of metals in lichens are sometimes substrate-dependent [40]. At an optimum level, the metals are crucial for the proper functioning of the physicochemical and biological processes of lichens as well as other biotic systems [41]. In contrast, the hyperaccumulation of metals can have environmental and biological ramifications [42]. Our study revealed a comparative tendency for metal ions accumulation in both R. intermedia and U. cornuta. The highest concentration was observed for Fe, Mg, K, and Na, and the least was observed for Cu, Pb, and Ni followed by Cd in both lichen species. Moreover, CO and Cr were not detectable in both the lichen species. No significant difference between the mean concentration of metal ions in R. intermedia and U. cornuta was observed except for Fe. However, a significant difference in mean values of metal ion concentration of the same lichen species was observed between sampling trees (p < 0.05) (Table 3 and Supporting Information File S1). These pilot results provide evidence for the accumulative properties of our examined lichen species toward different metal ions. Further, the prevalence of Ni in both lichen species suggests household emissions and forest fires near the study area [43]. Our study also revealed a comparatively low concentration of metal ions deposition in lichen thalli, which is consistent with the baseline status—suggesting the lack of direct source and long-range transport to the Himalayan forest. For example, a study in Shennongjia National Nature Reserve in China revealed a similar concentration of metal ions in Usnea aciculifera and Usnea luridorufa [44]. This indicates the least concentration of metal ions in our studied lichens. However, the influences of climatic settings and other environmental factors should be taken into account when comparing results from different parts of the world. Additionally, the concentration of metal ions in lichen’s thalli was further used as a proxy for considering alternation in physicochemical responses.
3.4. Physicochemical Responses in Lichens
The EC as a measure of cell membrane integrity indicates the membrane damage and leaching of ions from cells and is a marker for environmental stresses in lichens [45]. The loss of important ions in the lichen thallus is therefore a frequent indicator of the toxicity of N and other pollutants [46]. Generally, higher EC in lichens thalli represents the stressed environmental condition that enhanced the ions’ leaching. Our study revealed the EC range from 21.70 to 73.26 µS/cm in R. intermedia and 27.16 to 82.45 µS/cm in U. cornuta. The EC was significantly different between the lichen samples of different trees (p < 0.05). However, no significant difference between the mean concentration of EC between R. intermedia and U. cornuta was observed (Table 3 and Supporting Information File S2). Several previous studies from different parts of the world revealed comparatively high EC in Ramalina lacera [47] and Usnea amblyoclada [48]. However, there are no recent trends in determining the EC of lichens in natural ecosystems, and there are no such reports for EC in lichens from this region. Our results can be used as a reference for lichens of the Himalayas for future monitoring and trend analysis against environmental stresses, especially atmospheric N pollution which might be important in both forest ecosystem and climate studies. Further, our study revealed no significant impacts of N and metal ions on EC in lichens indicating the low or no impacts of pollution in lichens (Supporting Information Files S3 and S4).
The amount of chlorophyll in the lichen thallus has been correlated with the degree of environmental stresses; with less chlorophyll under stressful conditions [49]. Moreover, the deterioration of photosynthetic pigments is also used as a lichen viability indicator. Our study revealed noteworthy chlorophyll-a, chlorophyll-b, and total chlorophyll content in lichens, accompanying some degree of chlorophyll degradation. Among the two lichens, R. intermedia had the highest chlorophyll-a (10.39 ± 3.86 mg/kg), chlorophyll-b (3.64 ± 1.86 mg/kg), total chlorophyll content (14.04 ± 4.86 mg/kg), and chlorophyll degradation (0.93 ± 0.86). A significant difference in mean concentrations of chlorophyll-a, total chlorophyll, and chlorophyll degradation was observed between R. intermedia and U. cornuta (p < 0.05). Also, a significant difference in mean concentrations of chlorophyll pigments was found between the same lichen species of different trees (p < 0.05) (Table 3 and Supporting Information File S2) suggesting the possible variation due to local environmental setup. Several studies have reported and suggested higher total chlorophyll contents in lichens from less polluted areas [49–51]. Moreover, a study suggests, under field conditions, chlorophyll degradation in lichens from contaminated locations is associated with the accumulation of metals in the thallus [52]. Our results revealed the upkeep of chlorophyll content in lichen thalli suggesting little or no impacts of pollutants in the studied area.
3.5. Correlation Matrix and Principal Components (PCs)
Rather than acting alone, the accumulation of N along with metal ions has a crucial additive effect in shaping the functionality and physicochemical responses in lichens [14]. This is generally expected that the hyperaccumulation of metal ions enhances the toxicity and physicochemical alteration in lichens which can be further triggered by N and NH3 deposition [40, 46]. Assessing relationships between N and metal ions concentrations in lichens, and their relationship with changes in physicochemical and biochemical responses, is thus crucial. In the context of R. intermedia, Pearson’s correlation revealed a negative correlation of thallus N content with chlorophyll degradation and chlorophyll content. However, EC showed a strong positive correlation with chlorophyll degradation. Moreover, Na, Fe, and Pb showed a positive correlation with chlorophyll degradation, and Ni and Zn showed a strong negative correlation with the same. Further, Na showed a positive correlation with EC, and Cd showed a strong negative correlation with the same. Among others, Ni and K showed a strong negative correlation with chlorophyll-a content (Supporting Information File S5). However, in U. cornuta, no correlation between thallus N and physicochemical responses was observed. Still, Cu showed a negative correlation with chlorophyll content, whereas Cd showed a strong negative correlation with EC and Mg showed a strong positive correlation with the same. Moreover, Fe and Ni showed a positive correlation with chlorophyll degradation (Supporting Information File S6). In addition to this, the intercorrelation among different metal ions was observed. A previous study reported the enhanced EC and degradation of photosynthetic pigments caused by ion leaching (Na and Ca) [45], which might be the case in our findings as well.
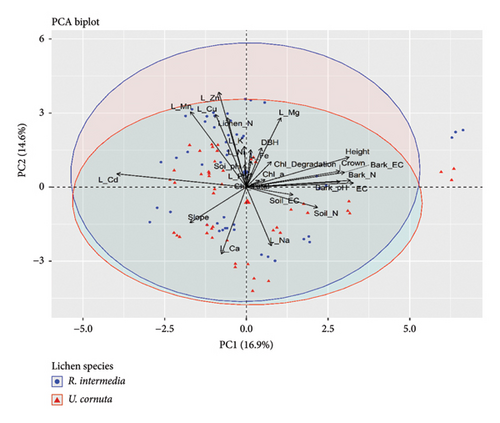
Further, the PCA revealed the five PCs loading in the lichens with eigenvalue greater than 1 with Kaiser normalization which explains 71% of components loadings. Among these PCs, PC1 explains the 22% loadings, and PC2 explains the 21% loading contributed mainly by N, trace elements, and chlorophyll contents. In both species, N content in thalli negatively impacted EC, chlorophyll contents, and degradation but to the least extent. In addition, the EC of lichens was negatively correlated with Zn, Cu, K, and Ni content. Similarly, EC, Na, and Ca of lichens showed a negative correlation with chlorophyll content and degradation (Figure 2). Among the two lichen species, R. intermedia showed a somewhat higher accumulation of N and metal ions and their impacts on physicochemical responses; however, U. cornuta remains unaffected.
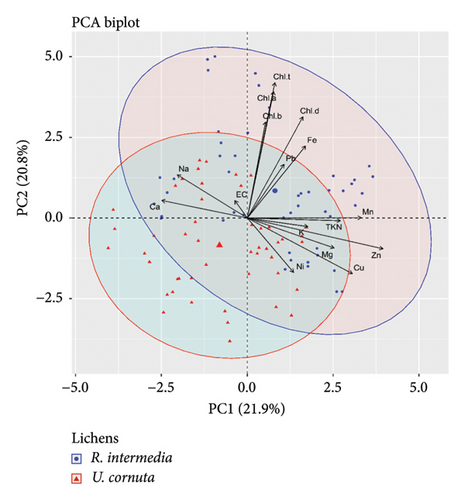
3.6. Soil and Bark Characteristics
Soil and bark (substrata) play a vital role in shaping the properties of lichens including their accumulation of atmospheric pollutants [45, 53]. To characterize the overall health of the forest, we analyzed soil and bark physicochemical parameters that might have impacts on lichen diversity and health. In our study, the total available N in soil was found in the range of 0.22%–0.62%, and a slightly basic pH was observed (7.93 ± 0.19) along with moderate EC (46.08 ± 11.59 µS/cm). The neutral or slightly basic pH in the pine forest might be due to the contribution of the buffering capacity of ash formed during forest fires. Moreover, moderate concentrations of major cations and micronutrients were observed. Among others, the highest concentrations of K (258.43 ± 54.81 ppm), Mg (283.28 ± 12.48 ppm), and Fe (200.89 ± 73.99 ppm) were observed (Supporting Information File S7). Further, in the context of bark, TKN ranged from 0.04% to 0.49% along with slightly acidic pH (4.22 ± 0.36) and moderate EC (189.19 ± 80.91 µS/cm). Moreover, the remarkably high concentration of major cations, especially Ca (17,953.46 ± 1720.99 ppm), was observed which has a geogenic origin in the study area (see Section 3.8). Similarly, for trace elements, the concentration of Fe (364.62 ± 282.87 ppm) accounted highest (Supporting Information File S8).
The PCA in soil variables revealed the seven PCs that explain 77% of component loading. Among these PCs, PC1 explains the 19% component loadings, and PC2 explains the 16% component loading with the major contribution of N, EC, major cations, and trace elements (Cr, Ni, and Pb). The soil pH and Zn concentration negatively influence N, EC, and major cations, while major cations positively influence N and EC. Trace elements were correlated with each other and loaded in PC2. The overall results suggested the least impact of Cd along with pH and the highest impact of Na, K, Mg, Ni, Cr, and Pb along with EC in the soil environment (Figure 3). In the context of bark, PCA revealed the six PCs that explain 79% of component loading. Among these PCs, PC1 explains the 26% component loadings, and PC2 explains the 17% component loading with the major contribution of N, pH, EC, and major ions. Most of the metal ions were correlated negatively with pH, N, and EC. However, Cu and Zn correlated positively with these variables. These results signify the notable impact of pH and N in metal ions deposition in the bark itself (Figure 4).
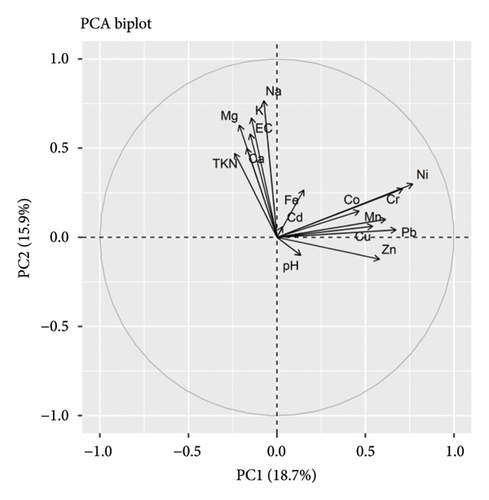
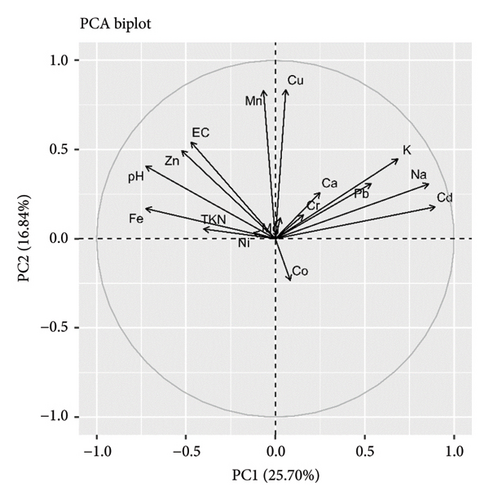
3.7. Tree Environment and Its Relationships With Lichens
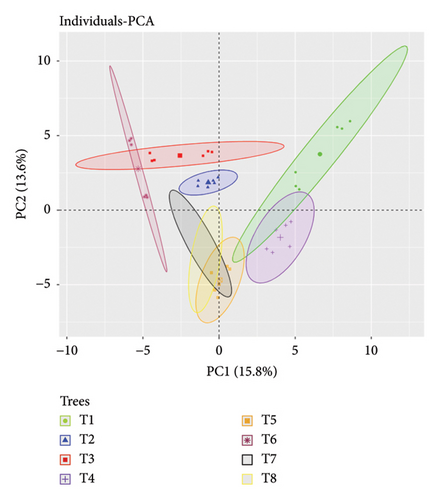
Epiphytic lichens and their distribution are affected by the different environmental factors and tree characteristics in any landscape. Key factors at a tree scale include type of species, height, canopy cover, branch size, age, nutrient availability, and microclimate [54, 55]. Thus, the environmental and substrate-related variables might have a separate or combined impact on the presence of lichens, their development, their absorption of elements, and their physiological functioning [56]. As a result, it could be more challenging to identify the direct effects of increased N deposition on the lichen. The overall PCA demonstrated the impacts of different environmental variables and tree characteristics in lichen variables. PCA of inclusion of all environmental variables, bark, soil, and lichens variables revealed the eight PCs among which PC1 contributes 17% explanation and PC2 explains 15% of component loading with a major contribution of bark EC, bark pH, bark N, tree height, crown cover, slope, and metal ions in lichens. Among the different environmental variables, the slope of the landscape played a vital role in shaping N and metal ions deposition and physicochemical responses in both lichens. With increasing slopes, a negative correlation was observed in N content, chlorophyll contents, and most of the metal ions. Slope also interferes with the tree height, DBH, crown cover, EC of bark, and N in the bark. However, these variables enhance the chlorophyll contents, chlorophyll degradation, and EC of lichens (Figure 5). The overall results revealed the least impact of environmental variables on lichens’ physicochemical responses. Our study revealed crown cover as the most important factor affecting a lichen’s physicochemical responses. The acidic bark in studied trees might make a suitable habitat for both acidophytic lichens (both U. cornuta and R. intermedia) used for the study. A previous study suggests intermediate canopy cover and low-intermediate light intensity for a high abundance of Usnea longissima [57]. Moreover, the PCA among all the variables of eight sampled trees revealed the tree-wise variation in the physical environment and their impacts on N deposition, metal ions deposition, and physicochemical responses in lichens (Figure 6). This indicates the impact of the individual tree characteristics and microclimatic settings influencing the thalli N and metal ions contents in lichens and their relationship with physicochemical responses.
3.8. Sources of Metal Ions Accumulation in Lichens
The transformation and deposition of metal ions from different geogenic and anthropogenic sources and long-range transport mechanisms are crucial in understanding the extent of pollution whether it is local, regional, or long-range transport. Further, under favorable conditions, metal ions can be transferred from soil and bark to lichen and vice versa [40, 58]. Higher concentrations of toxic elements in the substrata could impede the metabolism and decrease biological accumulation in the lichens. However, extracellular passive binding of metals and particulate trapping would still occur [14]. The results on EF and TF in examined lichens are presented in Table 4. In R. intermedia, except for Na, Mg, Cd, Ni, and Fe, EF was less than 1.5. Similarly, in U. cornuta, the highest EF value was found for Ni followed by Na. A significant difference in the mean EF value of the examined lichens was found only for Fe (p < 0.05). Further, the TF analysis was performed to check the accumulation of metal ions from bark to lichens. Our results revealed the range of TF in lichens for the baseline site. In brief, R. intermedia possesses the highest TF for Fe, whereas Ca showed the lowest. Further, the TF of metals from the bark in U. cornuta revealed the highest values for Mg, while Ca had the lowest values. A significant difference in mean TF between the species was observed only for Fe (p < 0.05). Overall, the EF provides evidence of no long-range transport of metal ions into the forest ecosystem of Shey Phoksundo National Park, and deposition was mainly geogenic and anthropogenic. Moreover, the comparatively low TF in both lichen species suggests no probable accumulation of metal ions from the substrate to lichens.
| Metals | Enrichment factor | Transfer factor | ||
|---|---|---|---|---|
| R. intermedia | U. cornuta | R. intermedia | U. cornuta | |
| Na | 2.97 ± 2.17 | 2.70 ± 1.77 | 0.72 ± 0.69 | 0.68 ± 0.63 |
| Mg | 1.14 ± 0.14 | 1.13 ± 0.14 | 0.92 ± 0.06 | 0.91 ± 0.08 |
| K | 0.15 ± 0.02 | 0.15 ± 0.03 | 0.55 ± 0.11 | 0.60 ± 0.16 |
| Ca | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Mn | 0.88 ± 0.27 | 0.77 ± 0.27 | 0.71 ± 0.54 | 0.41 ± 0.21 |
| Fe | 1.09 ± 0.38 ∗ | 0.58 ± 0.13 ∗ | 1.45 ± 0.81 ∗ | 0.72 ± 0.19 ∗ |
| Ni | 2.38 ± 1.46 | 2.99 ± 2.04 | 0.14 ± 0.06 | 0.16 ± 0.14 |
| Cu | 0.58 ± 0.28 | 0.49 ± 0.19 | 0.43 ± 0.38 | 0.37 ± 0.15 |
| Zn | 0.15 ± 0.06 | 0.10 ± 0.02 | 0.31 ± 0.25 | 0.13 ± 0.06 |
| Pb | 0.97 ± 0.43 | 0.61 ± 0.30 | 1.18 ± 0.72 | 0.66 ± 0.40 |
| Cd | 1.93 ± 0.63 | 1.88 ± 0.67 | 0.65 ± 0.31 | 0.55 ± 0.20 |
- Note: All experiments were carried out in six replicates, and results are presented as mean ± standard deviation (mean ± SD) of 8 trees (n = 8).
- ∗Significant difference at p < 0.05, independent sample t-test with log transformation of variables.
3.9. Species-Specific Response
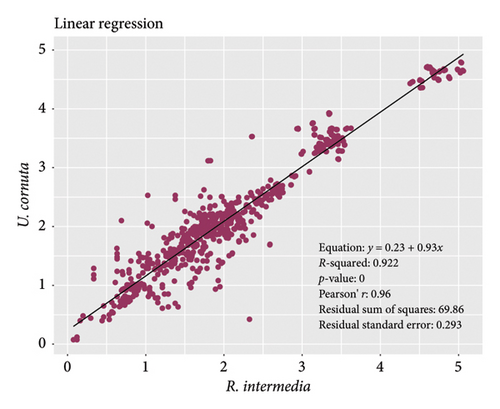
The selection of appropriate lichen species for biomonitoring is a subject of interest in both lichenological and pollution research [59]. The response abilities of different lichens differ depending on their traits, thallus chemistry, photobiont-mycobiont symbiosis, and the surrounding environmental conditions [60]. The regression analysis between the total variables of R. intermedia and U. cornuta showed a significant correlation (R = 0.96) and regression (R2 = 0.92; p < 0.001) (Figure 7) that indicates the comparative trends of both lichens toward N and metal ions accumulation and their relations to physicochemical responses—suggesting the similar potential.
4. Conclusions
This study provides a field-based reference for N and metal ions concentration in lichen’s thalli from the pristine Himalayan forest of western Nepal to which no alternation in physicochemical responses occurs—which supports the null hypothesis. Further, comparatively similar potential of both species indicated by comparable N and metal ions concentration and physicochemical responses supports our second hypothesis. This study might be useful for the field-based reference for future studies based on the N critical loads in examining the ecosystem health of Himalayan forests using lichen epiphytes as a bioindicative tool. Thus, it is recommended to expand further studies among different eco-regions, forest types, and lichen species in the pollution gradient of the Himalayan landscapes that might provide holistic pictures of the impacts of N and metal ions deposition on the forest ecosystem and lichen diversity before it exceeded the critical loads—which helps to urge the sustainable N management and necessary policy formation and implementation for the biodiversity conservation.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.P.P.: conceptualization, fieldwork, laboratory work, data analysis and verification, manuscript drafting and revision, and project coordination. H.B.: fieldwork, laboratory work, data analysis and visualization, manuscript drafting, and revision. B.L.: laboratory works, data analysis, and revision of the manuscript. N.D.: field and laboratory works. B.P.P.: project coordination and revision of the manuscript. S.S.: project administration. S.P.P. and H.B. contributed equally to this work.
Funding
This work was funded by the United Kingdom Research Innovation through Global Challenges Research Fund (UKRI-GCRF) for South Asian Nitrogen Hub (SANH) with grant ID NE/S009019/1.
Acknowledgments
The authors are extremely grateful to Dr. Christopher J. Ellis from Royal Botanic Garden Edinburgh for his suggestions and Mr. Prayon Joshi from Aquatic Ecology Centre, Kathmandu University, Nepal, for providing a base map of the study area. The authors are also grateful to the Department of National Park and Wildlife Conservation, Kathmandu, Nepal, for the research permit to Shey Phoksundo National Park. The authors are also thankful to the National Herbarium and Plant Laboratory, Lalitpur, Nepal, for lichen identification and authorization. The authors also like to thank the Aquatic Ecology Centre, Kathmandu University, Kavre, Nepal, for its laboratory facilities.
Supporting Information
Supporting Information File S1: Tree-wise variation in N and metal ions in lichens. All experiments are carried out in six replicates, and results are presented as mean ± standard deviation (mean ± SD) of 8 trees (n = 8). Different letters in the superscript of the same column for a lichen species indicate the significant difference in mean values. One-way ANOVA, post hoc Tukey, and multiple comparison test (p < 0.05).
Supporting Information File S2: Tree-wise variation in physicochemical responses in lichens. All experiments are carried out in six replicates, and results are presented as mean ± standard deviation (mean ± SD) of 8 trees (n = 8). Different letters in the superscript of the same column for a lichen species indicate the significant difference in mean values. One-way ANOVA, post hoc Tukey, and multiple comparison test (p < 0.05).
Supporting Information File S3: Regression coefficient for R. intermedia.
Supporting Information File S4: Regression coefficient for U. cornuta.
Supporting Information File S5: Correlation matrix for R. intermedia.
Supporting Information File S6: Correlation matrix for U. cornuta.
Supporting Information File S7: Physicochemical characteristics of the soil. All experiments were carried out in six replicates, and results are presented as mean ± standard deviation (mean ± SD) of 8 trees (n = 8).
Supporting Information File S8: Physicochemical characteristics of bark. All experiments were carried out in six replicates, and results are presented as mean ± standard deviation (mean ± SD) of 8 trees (n = 8).
Open Research
Data Availability Statement
Data will be made available by corresponding authors upon request.




