Green Synthesis and Biological Study of Novel Cyclopenta[b]pyridines: Multicomponent Reactions of Meldrum’s Acid
Abstract
A novel multicomponent method was utilized to attain high yields in the synthesis of new cyclopenta[b]pyridine derivatives. The procedure utilized vinilydene Meldrum’s acid, ethyl 2-amino-4-dioxo-4-arylbutanoates, activated acetylenic compounds, and primary amines. The operation was performed at ambient temperature and in the presence of water. This technique has numerous advantages, such as elevated product yields, rapid reaction times, and uncomplicated product separation methods. The antioxidant activity of the newly synthesized compounds is ascribed to their NH group, which has undergone two testing procedures. The antibacterial efficacy of the synthesized cyclopenta[b]pyridines was evaluated by a disc diffusion method, utilizing two strains of Gram-negative bacteria. These compounds were also discovered to inhibit the growth of Gram-positive bacteria.
1. Introduction
Multicomponent reactions (MCRs) are sequential processes that utilize commonly available or commercially obtainable starting components to produce a specific chemical compound. These reactions have been deliberately utilized in various synthetic processes, while traditional approaches typically need many steps and protracted procedures [1, 2]. The target compounds can be produced in a singular reaction with markedly reduced procedural steps. Consequently, MCRs have attracted significant interest in medicinal and combinatorial chemistry for the synthesis of heterocyclic scaffolds [3, 4]. MCR methodologies produce excellent efficiency, economic viability, decreased reaction duration, environmental sustainability, and function as an effective means for producing a varied array of novel chemical compounds [5–12]. Researchers have explored sustainable ways for synthesizing heterocyclic compounds to improve energy efficiency, reduce waste, and augment reaction yields [13, 14]. Creating highly efficient processes that function at ambient temperature and employ alternate energy sources has emerged as an attractive possibility. MCRs represent a significant improvement in synthetic organic chemistry. They entail the utilization of over three reactants in a singular reaction, all amalgamated within one vessel [15–19]. Consequently, the MCR approach has gained significant recognition and application in pharmaceutical chemistry and drug development, especially in the management of stereoisomers [20]. The efficacy of MCRs is significantly dependent on the intercoordination among the reactants, solvent, and catalyst within the reaction sequence [21, 22]. Consequently, MCRs are esteemed for their role in the generation of distinctive organic compounds and the synthesis of captivating heterocyclic structures utilizing various molecular entities as precursors [23, 24]. The characteristics of heterocycles are affected by the inclusion of heteroatoms and the strain inside the ring structure [25]. Heterocyclic structures are ubiquitous and essential components in nearly all physiologically and biologically active organic compounds [26, 27]. A considerable quantity of heterocyclic compounds functions as precursors in the synthesis of various pharmaceuticals exhibiting antimalarial [28], antiulcer [29], diuretic [30], anthelmintic [31], antidepressant [32], anticancer [33], antineoplastic, and antipsychotic [34–36] properties. Heterocycles may exhibit either electrophilic or nucleophilic characteristics, contingent upon the reaction. Nitrogen-based compounds are the most common subunits in several pharmaceuticals and agricultural goods among heterocycles, due to their remarkable features [37–39]. Cyclopenta[b]pyridine derivatives are constituents of alkaloids and exhibit a wide array of pharmacological actions [40]. Among these compounds, hypoglycemic derivatives [41], calcium channel antagonists [42], fluorescence probes [43], and FGFR1 protein kinase inhibitors [44] were identified. The principal methods for synthesizing cyclopenta[b]pyridines mostly include the integration of the pyridine ring into substituted cyclopentanone or its enamine [45, 46]. The production of these compounds can be accomplished by converting linear molecules [47] or via the intramolecular recyclization of substituted pyrimidine [48]. The synthesized compounds may exhibit biological features, including antimicrobial effects [49] and oxidation prevention or inhibition [50]. Organic molecules are believed to possess a more straightforward chemical structure due to their capacity to function as antioxidants [51]. The harmful effects of free radicals can be alleviated with the assistance of DPPH-free radicals [52]. Moreover, substances possessing antioxidant properties can inhibit the advancement of some disorders [53–56]. The current research focusses on investigating the potential antibacterial activities of the synthesized compounds [57]. Antibiotic-resistant microorganisms are detrimental and can induce many symptoms associated with infectious diseases.
Organic molecules are presumed to possess a simplified molecular structure due to their antioxidative capacity [58]. The detrimental effects of free radicals can be mitigated with the assistance of DPPH-free radicals. Moreover, numerous diseases may be mitigated by compounds possessing antioxidant properties. This study is presently investigating the possible antibacterial effects of the synthesized compounds. Antibiotic-resistant bacteria are detrimental and can lead to various diseases associated with infectious conditions. It is essential to determine the most effective technique to stop or conclude this process due to many causes. An environmentally sustainable approach was utilized to synthesize cyclopenta[b]pyridines 5.
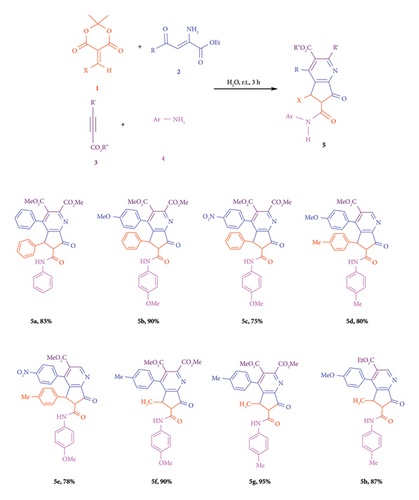
This involved using vinilydene Meldrum’s acid 1, ethyl 2-amino-4-dioxo-4-arylbutanoates 2, activated acetylenic compounds 3, and primary amines 4 as MCRs in an aqueous solution at room temperature (Scheme 1) (see Supporting Information for further details, Figures S1–S33). This signaled the initiation of the advancement of inventive methodologies for the creation of novel heterocyclic compounds [59, 60].
2. Results and Discussion
This study effectively synthesized cyclopenta[b]pyridines 5 through four-component reactions that included vinylidene Meldrum’s acid 1, ethyl 2-amino-4-dioxo-4-arylbutanoates 2, activated acetylenic compounds 3, and primary amines 4. The reactions occurred in aqueous solutions at room temperature (Scheme 1). The presence of a solvent is essential for reactions to proceed and attain high product yields. Table 1 indicates that water is the preferred solvent for sample processing. Water serves as an optimal solvent for this reaction because to its inert characteristics, which reduce its environmental impact. In the “on-water” reactions, water is not used as a solvent but floats reactants on the water-emulsion surfaces. On-water reactions are a group of organic reactions that take place as an emulsion in water. This effect has been known in 2005 by researchers in the group of K. Barry Sharpless published a systematic study into this phenomenon. In contrast to in-water reactions, lipophilic substrates aggregate to form an aqueous suspension (on-water). When surfactants form the self-assembled aggregates to accommodate lipophilic reactants, reactions inside the aggregates can be distinguished from others. In these reactions, the hydrogen bonding maybe contributed in the performing reaction in water. Increasing the number of H-bonds by increasing the interfacial surface area should further enhance the reaction rate. Indeed, the same authors demonstrated, via optical measurements, the correlation between stirring speed and interfacial area of the reaction between cyclopentadiene and methyl acrylate in water. They demonstrated that the higher these values, the higher the rates of conversion.
| Entry | Solvent | Time (h) | Yield%a |
|---|---|---|---|
| 1 | EtOH | 15 | 68 |
| 2 | CH2Cl2 | 8 | 78 |
| 3 | CHCl3 | 5 | 75 |
| 4 | H2O | 3 | 83 |
| 5 | Solvent free | 8 | — |
| 6 | DMF | 12 | 25 |
| 7 | Toluene | 12 | 70 |
- Note: The bold entry is the best solvent for these reactions.
- aIsolated yields.
The current research effectively synthesized cyclopenta[b]pyridines 5 through four-component reactions that included vinilydene Meldrum’s acid 1, ethyl 2-amino-4-dioxo-4-arylbutanoates 2, activated acetylenic compounds 3, and primary amines 4. The reactions occurred in aqueous solutions at ambient temperature (Scheme 1). The presence of a solvent is essential for reactions to proceed and attain high product yields. Table 1 indicates that water is the preferred solvent for sample processing. Water serves as an optimal solvent for this reaction because to its inert characteristics, which reduce its environmental impact. Water dissolves reactants located on the surface of the liquid emulsion, rather than being affected by its property of being in water. Water enables numerous biological functions. Barry Sharpless and his colleagues performed an investigation and a thorough analysis of this issue. Similar solvent reactions occur in both nonaqueous and aqueous environments. Owing to their organic makeup, none of the reactants demonstrate solubility in water. The configuration of the synthesized cyclopenta[b]pyridines 5 can be determined via product purification and analytical methods including 1H NMR, 13C NMR, IR, and mass spectrometry (see Supporting Information, Figures S1–S33).
The presence of carbonyl groups in the molecular structure of compound 5a was confirmed using infrared spectral analysis. The mechanism underlying these reactions is elucidated in Scheme 2. However, no details or explanations are provided on the stages involved in the formation of cyclopenta[b]pyridines 5. Intermediate 6 was formed through the reaction of enaminone 2 on alkylidene Meldum’s acid 1. Intermolecular cyclization of 6 produce intermediate 7 which react with compound 3 to produce intermediate 8. Intermolecular cyclization of intermediate 8 produced 9, which by elimination of water intermediate 10 was generated. Compound 10 react with primary amines 4 and by elimination of water and CO2 produce product 5 [61].
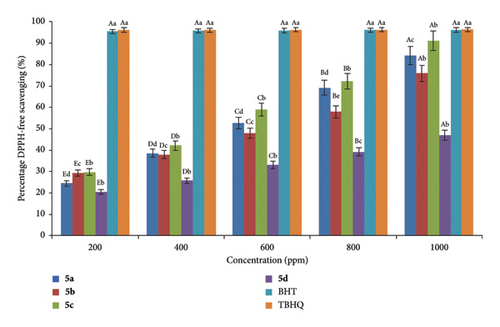
2.1. Assessment of the Antioxidant Potential of Synthesized Cyclopenta[b]pyridines via DPPH Methodology
The synthesized cyclopenta[b]pyridines may exhibit antioxidant properties owing to their significant nucleus. The DPPH radical was employed to validate the attainment of this objective. The DPPH-free radical is utilized to investigate the biological properties of food, biological structures, and synthetic cyclopenta[b]pyridines by the absorption of hydrogen or electrons. This radical is confined within the process [62, 63]. The present investigation seeks to validate the antioxidant efficacy of the synthesized cyclopenta[b]pyridines 5a–d by assessing their capacity to take hydrogen or electrons from the DPPH-free radical. Furthermore, the degree of antioxidant activity demonstrated by synthetic substances is intricately associated with the velocity at which DPPH radicals sequester electrons or hydrogen. The absorbance of DPPH diminished to 517 nm as a result of the electrons or hydrogens from cyclopenta[b]pyridines 5a–d. The researchers investigated the antioxidant properties of compounds 5a–d by employing varying amounts of butylated hydroxytoluene (BHT) and 2-tert-butylhydroquinone (TBHQ), which are commonly utilized antioxidants.
The antioxidant capabilities of synthesized compounds 5a–d were found in the following order: TBHQ ≈ BHT > 5c > 5a > 5b > 5b [64, 65] (Figure 1).
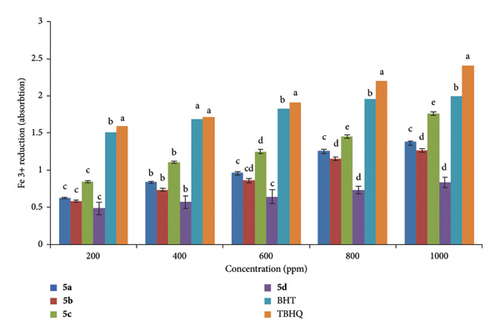
2.2. Assessment of the Antioxidant Capacity of Cyclopenta[b]pyridines Using a Fe3+ Reduction Method
Compounds 5a–d were assessed for their antioxidant activity by quantifying their ability to reduce Fe3+/ferricyanide to Fe2+/ferrous at a wavelength of 700 nm. The sequence of the synthesized chemicals is illustrated in Figure 2. Regarding its efficacy in reduction, compound 5c outperformed the other cyclopenta[b]pyridines. As a result, the effectiveness of cyclopenta[b]pyridines was reduced in the following sequence: TBHQ≈BHT > 5c > 5a > 5b > 5b.
2.3. Investigation of Antibacterial Properties of Synthesized Cyclopenta[b]pyridines
A comparison was conducted between the bactericidal properties of synthesized cyclopenta[b]pyridines and two commonly used antibiotics, streptomycin and gentamicin. Table 2 displays the findings of the experiment. The diameter of the inhibitory zone is directly influenced by two crucial factors: the presence of certain bacteria and the quantity of chromoazepines. Cyclopenta[b]pyridines 5a, 5b, 5d, 5f, and 5h exhibit high efficacy against Escherichia coli in comparison with both Gram-positive and Gram-negative bacteria due to the significant diameter of the zone of inhibition.
| Staphylococcus aureus (+) PTCC 1337 | Bacillus cereus (+) PTCC 1023 | Escherichia coli (−) PTCC1270 | Klebsiella pneumoniae (−) PTCC 1290 | |
|---|---|---|---|---|
| Compounds | ZOI(mm) | ZOI(mm) | ZOI(mm) | ZOI(mm) |
| 5a | 15 ± 0.01 | 17 ± 0.01 | 20 ± 0.01 | 18 ± 0.01 |
| 5b | 18 ± 0.01 | 19 ± 0.02 | 22 ± 0.02 | 18 ± 0.01 |
| 5c | 6 ± 0.02 | 9 ± 0.00 | 10 ± 0.03 | 10 ± 0.01 |
| 5d | 17 ± 0.00 | 18 ± 0.00 | 22 ± 0.00 | 18 ± 0.01 |
| 5e | 7 ± 0.01 | 9 ± 0.01 | 8 ± 0.01 | 7 ± 0.03 |
| 5f | 19 ± 0.00 | 22 ± 0.01 | 20 ± 0.02 | 19 ± 0.00 |
| 5g | 9 ± 0.03 | 9 ± 0.01 | 10 ± 0.01 | 8 ± 0.01 |
| 5h | 18 ± 0.01 | 21 ± 0.01 | 22 ± 0.01 | 18 ± 0.01 |
| Streptomycin | 21 ± 0.00 | 23 ± 0.00 | 23 ± 0.00 | 22 ± 0.01 |
| Gentamicin | 20 ± 0.01 | 24 ± 0.00 | 22 ± 0.01 | 20 ± 0.01 |
- Note: The bold values are the compounds with antibacterial activity.
3. Conclusion
This study investigated the ecological benefits of ethyl 2-amino-4-dioxo-4-arylbutanoates, primary amines, activated acetylenic compounds, and vinilydene Meldum’s acid in aqueous solutions at ambient temperature. The inquiry resulted in the successful synthesis of novel cyclopenta[b]pyridine derivatives, attaining high yields. Furthermore, two methodologies were utilized to assess the antioxidant efficiency of the synthesized cyclopenta[b]pyridines 5a–d. These compounds exhibit considerable activity in FRAP and DPPH radical scavenging experiments, unlike conventional antioxidants. Among them, compound 5c have the high antioxidant activity relative to other compounds. Furthermore, the antibacterial capabilities of the synthesized cyclopenta[b]pyridines 5a, 5b, 5d, 5f, and 5h were demonstrated through the application of the disc diffusion method on both Gram-positive and Gram-negative bacteria. The findings demonstrated that the synthesized cyclopenta[b]pyridines possess the capability to impede bacterial proliferation. These reactions offer the benefit of producing high-yield molecules, efficiently using a substantial number of atoms, and employing simple procedures.
3.1. Experimental Section
3.1.1. General
The research team utilized analytical quality reagents and solvents, ensuring that their physical and chemical properties were unaltered. The Shimadzu IR-460 spectrometer was utilized to record the FT-IR spectra of the organized nanocatalysts in a KBr medium. In addition, the 1H and 13C NMR spectra of the synthesized compounds were acquired using a Bruker DRX-400 AVANCE spectrometer. In order to obtain the spectrum of the compounds that were created, the spectrometer was adjusted to a frequency of 400 MHz. The solvent used was CDCl3, and the internal standard employed was TMS. The mass spectra of the synthesized compounds, which possess an ionization potential of 70 eV, were acquired using the Finnigan MAT 8430 spectrometer.
3.1.2. General Synthetic Method of the Vinilydene Meldrum’s Acid 1 [66]
Aldehyde (1.0 mmol, 1.0 equiv), Meldrum’s acid (158 mg, 1.1 mmol, 1.1 equiv), and dry benzene (distilled from Na/benzophenone, 5.0 mL, 0.2 M) were added to a glass vial equipped with a magnetic stir bar. To this was added 200 lL of a 0.5 mM solution of pyrrolidinium acetate in benzene (prepared by dropwise addition of AcOH to pyrrolidine in benzene, 0.1 mmol, 10 mol %) at room temperature. The vial was capped tightly and stirred at the appropriate temperature (heated reactions performed in preheated oil baths) for 24 h. Products can be purified either by diluting the reaction with EtOAc, washing the mixture with saturated NaHCO3 solution, drying over MgSO4, and concentrating dry or by removal of benzene by rotary evaporation and recrystallizing the resulting solid from MeOH.
3.1.3. General Synthetic Method of Cyclopenta[b]pyridines 5a–h
The vinilydene Meldrum’s acid 1 (2 mmol) and the ethyl 2-amino-4-dioxo-4-arylbutanoates 2 (2 mmol) were mixed together and stirred for 30 min at room temperature. After 30 min, activated acetylenic compound 3 (2 mmol) was added to the previous mixture. The new mixture was then stirred for 45 min. Subsequently, primary amine 4 (2 mmol) was added to the mixture and the resulting mixture was thoroughly mixed for a duration of 3 h at room temperature. The reaction was completed after 3h, which was monitored by TLC. At this stage, the solid was isolated using filtering and then dissolved in CH2Cl2 (3 mL). The resulting solution was then purified using column chromatography (5:1 hexane/EtOAc) in order to get purified cyclopentapyridines 5.
Dimethyl 7-oxo-4,5-diphenyl-6-(phenylcarbamoyl) -6,7-dihydro-5H-cyclopenta[b]pyridine-2,3-dicarboxylate (5a): yellow powder, m.p.123–125°C, yield 83%. IR (KBr) (νmax/cm−1): 3439 (NH), 1732 (C=O), 1624 (C=C), 1456 (C-O) and 1230 (C-H) cm−1. 1H NMR (500 MHz, CDCl3): δ 3.84 (3 H, s, MeO), 3.91 (3 H, s, MeO), 4.35 (1 H, d, 3J = 6.5 Hz, CH), 4.95 (1 H, d, 3J = 6.5 Hz, CH), 7.08–7.63 (15 H, m, 15 CH), 10.73 (1 H, s, NH) ppm. 13C NMR: δ 199.2 (C=O), 170.7 (C=O), 166.0 (C=O), 165.7 (C=O), 150.1 (C), 146.1 (C), 142.5, 138.8, 138.2, 136.3, 131.4, 130.7, 129.8, 129.0, 128.7, 128.4, 128.3, 127.8, 127.4, 124.5, 120.9, 60.01 (CH), 52.49 (MeO), 52.31 (MeO), 46.07 (CH) ppm. MS (EI, 70 eV): m/z (%) = 520 (M+, 10), 31 (100). Anal. Calcd for C31H24N2O6 (520.54): C, 71.53; H, 4.65; N, 5.38. Found: C, 71.68; H, 4.82; N, 5.52%.
Dimethyl 4-(4-methoxyphenyl)-6-((4-methoxyphenyl) carbamoyl)-7-oxo-5-phenyl-6,7-dihydro-5H-cyclopenta[b]pyridine-2,3-dicarboxylate (5b): yellow powder, m.p.142–144°C, yield 90%. IR (KBr) (νmax/cm−1): 3427 (NH), 1732 (C=O), 1626 (C=C), 1585 (C-O), and 1227 (C-H) cm−1. 1H NMR (500 MHz, CDCl3): δ 3.79 (3 H, s, MeO), 3.82 (3 H, s, MeO), 3.84 (3 H, s, MeO), 3.91 (3 H, s, MeO), 4.35 (1 H, d, 3J = 6.2 Hz, CH), 4.95 (1 H, d, 3J = 6.2 Hz, CH), 7.25–7.52 (13 H, m, 13 CH), 10.65 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 199.3 (C=O), 170.8 (C=O), 166.3 (C=O), 165.9 (C=O), 159.6 (C), 156.7 (C), 153.8 (C), 145.4, 143.1, 139.0, 133.9, 132.6, 132.1, 131.5, 130.6, 128.8, 128.4, 127.7, 122.5, 114.6, 114.1, 59.5 (CH), 55.3 (MeO), 55.4 (MeO), 52.5 (MeO), 52.3 (MeO), 46.1 (CH) ppm. MS (EI, 70 eV): m/z (%) = 580 (M+, 10), 31 (100). Anal. Calcd for C33H28N2O8 (580.59): C, 68.27; H, 4.86; N, 4.83. Found: C, 68.42; H, 4.98; N, 4.96%.
Dimethyl 6-((4-methoxyphenyl) carbamoyl)-4-(4-nitrophenyl)-7-oxo-5-phenyl-6,7-dihydro-5H-cyclopenta[b]pyridine-2,3-dicarboxylate (5c): yellow powder, m.p.168–170°C, yield 90%. IR (KBr) (νmax/cm−1): 3446 (NH), 1719 (C=O), 1628 (C=C), 1597 (C-O), and 1257 (C-H) cm−1. 1H NMR (500 MHz, CDCl3): δ 3.79 (3 H, s, MeO), 3.82 (3 H, s, MeO), 3.84 (3 H, s, MeO), 3.91 (3 H, s, MeO), 4.35 (1 H, d, 3J = 6.2 Hz, CH), 4.95 (1 H, d, 3J = 6.2 Hz, CH), 7.25–7.52 (13 H, m, 13 CH), 10.67 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 199.3 (C=O), 170.8 (C=O), 166.3 (C=O), 165.9 (C=O), 156.7 (C=O), 153.7 (C), 147.1 (C), 145.41, 143.51, 140.82, 139.01, 133.95, 132.01, 131.46, 130.51, 128.82, 128.38, 127.65, 124.16, 122.46, 114.60, 59.5 (CH), 55.3 (MeO), 52.5 (MeO), 52.3 (MeO), 46.1 (CH) ppm. MS (EI, 70 eV): m/z (%) = 595 (M+, 10), 31 (100). Anal. Calcd for C32H25N3O9 (595): C, 64.54; H, 4.23; N, 7.06. Found: C, 64.68; H, 4.36; N, 7.19%.
Methyl 4-(4-methoxyphenyl)-7-oxo-5-(p-tolyl)-6-(p-tolylcarbamoyl)-6,7-dihydro-5H-cyclopenta[b]pyridine-3-carboxylate (5d): yellow powder, m.p.153–155°C, yield 90%. IR (KBr) (νmax/cm−1): 3450 (N-H), 1735 (C=O), 1625 (C=C), 1508 (C-O), and 1265 (C-H) cm−1. 1H NMR (500 MHz, CDCl3): δ 3.79 (3 H, s, MeO), 3.82 (3 H, s, MeO), 3.84 (3 H, s, MeO), 3.91 (3 H, s, MeO), 4.35 (1 H, d, 3J = 6.2 Hz, CH), 4.95 (1 H, d, 3J = 6.2 Hz, CH), 7.25–7.52 (13 H, m, 13 CH), 10.65 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 199.8 (C=O), 170.8 (C=O), 166.2 (C=O), 159.6 (C), 155.8 (C), 151.4 (C), 143.2 (C), 137.86, 137.60, 136.92, 134.27, 132.81, 131.48, 130.87, 129.82, 129.46, 128.22, 127.31, 120.29, 114.31, 59.7 (CH), 55.3 (MeO), 52.4 (MeO), 46.6 (CH), 21.1 (Me), 20.7 (Me) ppm. MS (EI, 70 eV): m/z (%) = 520 (M+, 10), 31 (100). Anal. Calcd for C32H28N2O5 (520): C, 73.83; H, 5.42; N, 5.38. Found: C, 73.97; H, 5.58; N, 5.52%.
Methyl 6-((4-methoxyphenyl)carbamoyl)-4-(4-nitrophenyl)-7-oxo-5-(p-tolyl)-6,7-dihydro-5H-cyclopenta[b]pyridine-3-carboxylate (5e): yellow powder, m.p.162–164°C, yield 78%. IR (KBr) (νmax/cm−1): 3452 (NH), 1729 (C=O), 1628 (C=C), 1585 (C-O), and 1266 (C-H) cm−1. 1H NMR (500 MHz, CDCl3): δ 2.35 (3 H, s, Me), 3.78 (3 H, s, MeO), 3.87 (3 H, s, MeO), 4.40 (1 H, d, 3J = 6.2 Hz, CH), 5.08 (1 H, d, 3J = 6.2 Hz, CH), 6.86–8.22 (13 H, m, 13 CH), 8.91 (1 H, s, CH), 10.28 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 199.8 (C=O), 170.8 (C=O), 166.2 (C=O), 156.7 (c), 155.8 (C), 151.44, 147.09, 143.39, 141.82, 137.86, 136.92, 133.95, 131.63, 130.87, 129.82, 128.22, 127.59, 124.17, 122.46, 114.60, 59.7 (CH), 55.3 (MeO), 52.4 (MeO), 46.5 (CH), 21.1 (CH) ppm. MS (EI, 70 eV): m/z (%) = 551 (M+, 10), 31 (100). Anal. Calcd for C31H25N3O7 (551.56): C, 67.51; H, 4.57; N, 7.62. Found: C, 67.62; H, 4.68; N, 7.76%.
Dimethyl 6-((4-methoxyphenyl)carbamoyl)-5-methyl-7-oxo-4-(p-tolyl)-6,7-dihydro-5H-cyclopenta[b]pyridine-2,3-dicarboxylate (5f): Yellow powder, m.p.156–158°C, yield 90%. IR (KBr) (νmax/cm−1): 3448 (NH), 1716 (C=O), 1663 (C=C), 1547 (C-O), and 1228 (CH) cm−1. 1H NMR (500 MHz, CDCl3): δ 1.50 (3 H, d, 3J = 7.3 Hz, Me), 2.41 (3 H, s, Me), 3.68 (1 H, q, 3J = 7.3 Hz, CH), 3.78 (3 H, s, MeO), 3.87 (3 H, s, MeO), 3.91 (3 H, s, MeO), 4.10 (1 H, d, 3J = 6.7 Hz, CH), 6.86–7.53 (8 H, m, 8 CH), 10.30 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 206.1 (C=O), 166.2 (C=O), 165.8 (C=O), 150.1 (C), 144.7 (C), 144.18, 139.12, 134.46, 131.74, 131.20, 128.98, 128.97, 61.0 (CH), 55.3 (MeO), 52.5 (MeO), 52.3 (MeO), 44.4 (CH), 32.7 (CH), 21.2 (Me), 20.1 (Me) ppm. MS (EI, 70 eV): m/z (%) = 502 (M+, 10), 31 (100). Anal. Calcd for C28H26N2O7 (502.52): C, 66.92; H, 5.22; N, 5.57. Found: C, 67.12; H, 5.38; N, 5.69%.
Dimethyl 5-methyl-7-oxo-4-(p-tolyl)-6-(p-tolyl carbamoyl)-6,7-dihydro-5H-cyclopenta[b]pyridine-2,3-dicarboxylate (5g): yellow powder, m.p.138–140°C, yield 95%. IR (KBr) (νmax/cm−1): 3439 (NH), 1710 (C=O), 1635 (C=C), 1213 (CH) cm−1. 1H NMR (500 MHz, CDCl3): δ 1.51 (1 H, q, 3J = 6.2 Hz, Me), 2.35 (3 H, s, Me), 2.41 (3 H, s, Me), 3.65–3.74 (1 H, m, CH), 3.84 (3 H, s, MeO), 3.91 (3 H, s, MeO), 4.11 (1 H, d, 3J = 6.4 Hz, CH), 7.12–7.43 (8 H, m, 8 CH), 10.27 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 200.7 (C=O), 171.2 (C=O), 166.3 (C=O), 165.9 (C=O), 153.2 (C), 144.4 (C), 142.7 (C), 139.12, 137.58, 135.31, 134.27, 131.68, 131.38, 129.46, 129.05, 128.97, 120.30, 61.0 (CH), 52.5 (MeO), 52.3 (MeO), 37.7 (CH), 21.2 (Me), 20.7 (CH), 17.8 (CH3) ppm. MS (EI, 70 eV): m/z (%) = 486 (M+, 10), 31 (100). Anal. Calcd for C28H26N2O6 (486.52): C, 69.12; H, 5.39; N, 5.76. Found: C, 69.28; H, 5.52; N, 5.92%.
Ethyl 4-(4-methoxyphenyl)-5-methyl-7-oxo-6-(p-tolylcarbamoyl)-6,7-dihydro-5H-cyclopenta[b] pyridine-3-carboxylate (5h): yellow powder, m.p.126–128°C, yield 87%. IR (KBr) (νmax/cm−1): 3442 (NH), 1727 (C=O), 1630 (C=C), and 1113 (CH) cm−1. 1H NMR (500 MHz, CDCl3): δ 1.38 (3 H, t, 3J = 7.4 Hz, Me), 1.51 (3 H, d, 3J = 6.4 Hz, CH), 2.35 (3 H, s, Me), 3.59–3.64 (1 H, m, CH), 3.82 (3 H, s, MeO), 4.13 (1 H, d, 3J = 6.5 Hz, CH), 4.95 (2 H, q, 3J = 7.4 Hz, CH2O), 6.97–87.54 (8 H, m, 8 CH), 8.89 (1 H, s, CH), 10.27 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): δ 201.16, 171.20, 167.31, 159.62, 151.14, 150.22, 143.51, 137.58, 134.27, 132.54, 131.48, 129.46, 128.85, 126.30, 120.30, 114.31, 61.3 (CH2O), 61.1 (CH), 55.3 (MeO), 37.4 (CH), 20.7 (Me), 17.9 (Me), 14.3 (Me) ppm. MS (EI, 70 eV): m/z (%) = 458 (M+, 10), 31 (100). Anal. Calcd for C27H26N2O5 (458.51): C, 70.73; H, 5.72; N, 6.11. Found: C, 70.93; H, 5.92; N, 6.24%.
3.2. DPPH as Reagent for Evaluation of Antioxidant Properties of Cyclopenta[b]pyridines
The antioxidant properties of cyclopenta[b]pyridines 5a–d were evaluated using the DPPH assay, as outlined by Shimada et al. [67]. The antioxidant properties of the synthesized cyclopenta[b]pyridines 5a–d were tested using the Shimada technique. In order to examine this property, various concentrations (ranging from 200 to 1000 ppm) of these specific compounds were added to an equal volume of methanolic DPPH solution (1 mmol/L). The absorbance of the mixture was measured at a wavelength of 517 nm after a 30-min incubation with DPPH at room temperature. Afterwards, the combo was placed in an area with dim lighting. The antioxidant activity of synthesized cyclopenta[b] pyridines and oxathiepines 5a–d was assessed by comparing it to the results of the tests conducted by Yen and Duh [68]. In this study, a 3 mL quantity of methanol was employed as a substitute for two synthetic antioxidant standards, specifically BHT and TBHQ. DPPH can be used to measure the extent to which free radicals are inhibited.
3.3. FRAP Process for the Study of Antioxidant Properties of Cyclopenta[b]pyridines
The antioxidant properties of the synthesized cyclopenta[b]pyridines were assessed using the methodologies outlined by Yildirim et al. [69]. The cyclopenta[b]pyridines 5a–d functioned as reducing agents for iron (III) in this approach. A mixture of cyclopenta[b]pyridines (1 mL), potassium ferricyanide (2.6 mL), and phosphate buffer (2.6 mL) was stirred for 35 min at a temperature of 55°C. After adding 2.5 mL of trichloroacetic acid to the prior mixture, it was subjected to centrifugation for 10 minutes. The absorbance at a wavelength of 700 nm was determined for a combination containing 0.6 mL of FeCl3, 2.6 mL of deionized water, and 2.5 mL of supernatant. It is crucial to bear in mind that the high absorption is a consequence of significant decreases. The experiments were conducted three times in order to acquire the most favorable results.
3.4. Study of Cyclopenta[b]pyridines as Antibacterial Agents
The evaluation of bacterial samples involves the analysis of both Gram-positive and Gram-negative microorganisms, which is a specific field of study. The Persian Cultural Collection (PTCC) in Tehran, Iran has offered these specific types that are used in the process of distributing discs. To get the required bacteria for this process, the two bacterial species were cultivated for a duration of 16–24 h at a temperature of 37°C, using the same McFarland Standard no. 0.5. The two standards, gentamicin and streptomycin, were utilized to eliminate two distinct classifications of bacteria. The bacteria (1.5 × 108 CFU/mL) were inoculated onto Mueller–Hinton agar using a sterile swab and McFarland standard 0.5 for culture. Afterwards, a comparison was performed between this and the inhibitory zone to confirm the antibacterial effectiveness of the specified derivatives of these substances. Gentamicin and streptomycin were utilized to compare, measure, and arrange the plates.
Disclosure
The 1H, 13C NMR, FT-IR, and mass spectra of synthesized compounds 5a–5h are available in the Supporting Information of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed in all sections of this manuscript but in detail, the role of each author was listed as follows.
Faezeh Shafaei: investigation and writing the original draft.
Hadi Jouladehroodbar: investigation; formal analysis; and writing the original draft.
Fariba Zamani Hargalani: software; investigation; and formal analysis.
Zinatossadat Hossaini: writing the original draft and reviewing and editing.
Funding
No funding was received for this study.
Acknowledgments
The author sincerely would like to acknowledge the Islamic Azad University for spiritual support.
Supporting Information
Figure S1: 1H NMR spectrum of compound 5a, Figure S2: 13C NMR spectrum of compound 5a, Figure S3: Mass spectrum of compound 5a, Figure S4: FT-IR spectrum of compound 5a, Figure S5: 1H NMR spectrum of compound 5b, Figure S6: 13C NMR spectrum of compound 5b, Figure S7: Mass spectrum of compound 5b, Figure S8: FT-IR spectrum compound 5b, Figure S9: 1H NMR spectrum of compound 5c, Figure S10: 13C NMR spectrum of compound 5c, Figure S11: Mass spectrum of compound 5c, Figure S12: FT-IR spectrum of compound 5c, Figure S13: 1H NMR spectrum of compound 5d, Figure S14: 13C NMR spectrum of compound 5d, Figure S15: Mass spectrum of compound 5d, Figure S16: FT-IR spectrum of compound 5d, Figure S17: 1H NMR spectrum of compound 5e, Figure S18: 13C NMR spectrum of compound 5e, Figure S19: Mass spectrum of compound 5e, Figure S20: FT-IR spectrum of compound 5e, Figure S21: 1H NMR spectrum of compound 5f, Figure S22: 13C NMR spectrum of compound 5f, Figure S23: Mass spectrum of compound 5f, Figure S25: FT-IR spectrum of compound 5f, Figure S26: 1H NMR spectrum of compound 5g, Figure S27: 13C NMR spectrum of compound 5g, Figure S28: Mass spectrum of compound 5g, Figure S29: FT-IR spectrum of compound 5g, Figure S30: 1H NMR spectrum of compound 5h, Figure S31: 13C NMR spectrum of compound 5h, Figure S32: Mass spectrum of compound 5h, and Figure S33: FT-IR spectrum of compound 5h.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




