Agricultural Waste–Derived Biochar for Antibiotics Removal in Aqueous Environment: A Comprehensive Outlook
Abstract
Increased antibiotic consumption and inadequate management procedures expose the environment to antibiotics, leading to significant antibiotic pollution and emerging antibiotic-resistant microbes. Biochar form agricultural waste can efficiently remove antibiotics from aquatic environments and is a renewable and cost-effective adsorbent. Biochar-producing methods from different agricultural residues, the physicochemical characteristics, and the adsorption effectiveness of biochar are discussed. Surface modification of biochar provides a variety of new interactions between the biochar and antibiotics. The key elements influencing antibiotic adsorption on biochar, including surface chemistry, pyrolysis conditions, and environmental variables, were discussed in detail. This review focuses specifically on the functional modification of the biochar, the mechanism involved in antibiotic adsorption, and the scalability of this process. The difficulties with biochar regeneration, environmental risks, and industrial viability are also addressed. The findings highlighted that the existing adsorption approaches for antibiotic removal need adsorbent with prolonged stability, process optimization, and cost-effective regenerative techniques. This review gives a broad overview of antibiotic removal using biochar and aids the decision-makers in developing environmentally friendly water treatment systems.
1. Introduction
The increasing global demand for antimicrobial agents has led to significant growth in antibiotic consumption. According to Xu et al., an estimated 200,235 metric tons of antimicrobial agents are required to combat bacterial infections and support growth prevention. Between 2000 and 2015, the worldwide consumption of antibiotics surged by 65%, and projections indicate that by 2030, the daily defined doses of pharmaceuticals will nearly double from the current 42 billion [1, 2]. The pharmaceutical industry, which serves as the backbone of modern healthcare by developing medications to treat infections and improve overall well-being, has contributed to this rapid rise in antibiotic usage. However, its expansion has also led to persistent environmental challenges, particularly pharmaceutical pollution in aquatic ecosystems [3]. This increasing presence of pharmaceutical contaminants in the environment emphasizes the urgent need for improved wastewater treatment strategies and sustainable pharmaceutical practices to mitigate the risks associated with pharmaceutical pollution.
Antibiotic pollution has been eliminated by implementing conventional water treatment techniques, such as chemical oxidation and activated carbon adsorption. However, these conventional methods have some drawbacks, such as ineffectively eliminating antibiotic contamination. These include the potential for producing hazardous byproducts and the partial breakdown of antibiotic compounds, which leaves residual contamination. Additionally, the operational costs and technical complexity are frequently excessive, particularly in settings with limited resources. Conventional methods include physical treatment, such as screening and sedimentation, and secondary treatment relies on biological processes. Tertiary treatment includes filtration, chemical treatment, and advanced oxidation. These conventional methods have certain limitations when treating antibiotics, such as adequate removal, partial degradation, and high operational cost. Although conventional water treatment methods play a role in mitigating antibiotic pollution, their limitations underscore the need for more sustainable and efficient alternatives [4].
Addressing these challenges, adsorption can be used as a remediation technology that is cost-effective, user-friendly, and efficient. The advantages of using adsorption were that it provides high removal efficiency and low energy process and can be regenerated and reused, which is more sustainable and cost-effective. Due to these benefits, adsorption gained significant attention for antibiotic removal in water bodies. Biochar, a rich carbon substance produced by pyrolysis, has been used for the treatment of water to remove antibiotic contamination, which is a promising approach. In aquatic environments, biochar, produced at specific pyrolysis temperatures, exhibits exceptional adsorption properties for various contaminants, including antibiotics. The distinguished characteristics of biochar include a high specific surface area (SSA), unique microstructure, and low regeneration costs, which lead to an efficient adsorbent. Its adsorption efficiency can be further enhanced through functional modifications [5]. Due to its porous structure, extensive surface area, functional group integration, and high cation exchange capacity (CEC), biochar is recognized as a sustainable and highly effective adsorbent [6]. Additionally, agricultural waste, such as straw, is used in producing biochar, which offers a sustainable and affordable solution for environmental sustainability [7].
The rapid increase in agricultural production generates millions of tons of waste annually, contributing to environmental pollution, particularly by burning residues such as rice, wheat, corn straw, and bagasse. This practice negatively impacts water, soil, and air quality. Proper utilization of agricultural waste supports ecological restoration and helps mitigate health risks. Converting agricultural waste into biochar offers a sustainable approach to waste management and environmental rehabilitation. By employing advanced technologies and optimizing processing parameters, agricultural waste can be transformed into biochar with tailored physicochemical properties suitable for various applications [8].
Functional modification of agricultural wastes enhances their physicochemical properties, predominantly through methods that include alkalization, acidification, esterification, etherification, carbonization, magnetization, and surface modification to enhance the performance for adsorption and selectivity for eliminating antibiotics from aqueous environments [9]. This is supported by the fact that pyrolysis-impregnation biochar made from corn husk outperformed others in terms of adsorption capacity for levofloxacin (LFX) and tetracycline. This is due to the increased presence of -OH groups, specifically –OHads, as adsorption sites. Introducing surface modifications and functional groups can enhance the surface chemistry of biochar interactions with antibiotic molecules, increasing selectivity and performance during remediation [10].
This review critically evaluates how biochar performs antibiotic adsorption in aqueous solutions. The adsorption of antibiotics using different adsorbents of recent reviews is studied thoroughly for their significant limits. Metal nanoparticles and biochar used as adsorbents resulted in high adsorption capacity but lacked health-associated risks and failed to discuss large-scale feasibility [11]. Similarly, the usage of metal oxide nanoparticles and biological treatment has high adsorption capacity but has failed to address the health risks and environmental issues, along with long-term viability, generation, and stability [12, 13]. Even though previous studies have tried to look at the potential of biochar to remove pollutants, a comprehensive analysis focused on its use with antibiotic contaminants is still required. The present investigation attempts to address this gap by reviewing antibiotic removal mechanisms, large-scale feasibility, and regeneration studies with industrial applicability and highlighting research prospects and difficulties in the field. Furthermore, in support of a circular economy model of environmental rehabilitation, this review proposes using agricultural by-products as feedstock to manufacture biochar. This review also aims to provide significant insights into the usefulness of biochar as a dual-purpose technique for water remediation and agricultural waste valorization by highlighting its dual role in the removal of contaminants and the sustainable valuation of the waste.
2. Potential Agricultural Wastes
The enormous amounts of agricultural waste produced worldwide, especially in Asia, indicate the enormous numbers of crop wastes produced each year from various crop varieties. The agricultural wastes include sugarcane leaves, tops, bagasse, maize, rice, wheat straw, stalks, husk, cobs, bran, potato foliage, tops, peels, pulps, fresh vegetable leaves, stems, peels, skins, seeds, cassava peels, stalks, and bagasse. If not properly utilized, this biomass waste will become an issue and eventually become unusable garbage that can contaminate the environment. Biomass waste must first be converted into biochar because its direct usage is considered inefficient. These agricultural wastes can be used for environmental sustainability if their composition and qualities are properly understood [14].
Several biomass sources, including municipal, animal, and agricultural wastes, can be used to produce biochar. For example, rice fields generate approximately 20 million tons of rice straw as agricultural waste annually after harvest. Similarly, India produces 25.3, 120, 80, 10.5, 9.9, 6.7, 6.1, 7.6, 17.7, 5.8, and 18.2 metric tons of agricultural residues from wheat straw, sugarcane, maize, cotton, rapeseed, mustard, bajra, gram, barley, jowar, and sunflower, respectively [15]. Several biomasses are used for the production of biochar such as cotton stock, groundnut shells, paddy straw, wheat straw, coconut shell, bamboo, pigeon pea stock, corn cob, maize straw, eucalyptus, sugarcane bagasse, orange peel, palm kernel shell, and walnut shell with a yield of 46.5%, 34.1%, 49.5%, 32.9%, 28%, 32.20%, 24.5%, 26.55%, 19.65%, 21.26%, 75%, 25.54%, 43.13%, and 28.4%, respectively. Among this sugarcane, bagasse gives a maximum yield of biochar [16]. Figure 1 illustrates the various agricultural wastes that are used as a potential feedstock/precursor for biochar and their application in different sectors such as wastewater treatment, soil enrichment, and bioenergy production.
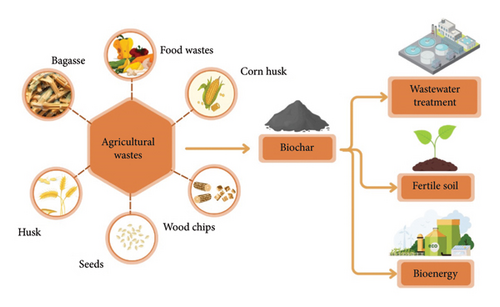
Sugarcane bagasse is one of the world’s largest agricultural residues, contributing approximately 100 million tons annually. These wastes can be used as resources for environmental sustainability, such as wastewater treatment and biofuel and biogas production [17]. Manyuchi et al. performed pyrolysis for biochar production at 600°C for 3 h [18]. The produced biochar has a particle size of 0.8–2.0 mm, a surface area of 500 m2/g, and porosity of 65%–70% with a yield of 75%. In another research, tetracycline antibiotics were effectively extracted from water using innovative magnetic biochar made from starch-rich rice waste. This biochar had a high removal rate of 96.02% and the potential to be reused. The expected expense for producing modified biochar was 5.91 USD per kg, with 1 kg of modified biochar (using 8 kg of waste rice) treating approximately 0.55 tons of tetracycline effluent [19]. Similarly, palm oil fiber biochar was used for cephalexin adsorption, yielding a maximum adsorption capacity of 57.47 mg/g, providing an economical and sustainable solution for tetracycline wastewater treatment while solving solid waste management challenges [20].
The variety and potential of biomass-based materials in water treatment applications are demonstrated by the fact that, in addition to the biomass sources previously stated, other biomasses have been transformed into biochar, which is used to remove antibiotics from water.
3. Biochar Production Using Agricultural Wastes
Biochar production using agricultural waste has gained prominence owing to its wide use, availability, and improved production methods. Biochar-producing processes include pyrolysis, gasification, torrefaction, hydrothermal carbonization, and electromodification. Pyrolysis produces high-quality biochar with large pore sizes. Biochar is made from organic waste and includes carbon. The feedstock’s composition and the pyrolysis circumstances can affect the biochar’s characteristics. Agricultural biomass is easy-to-use, affordable, sustainable, eco-friendly, and readily available material for reducing antibiotics [21, 22]. Waste generated from different agricultural activities, commonly referred to as agrarian biomass, is listed in Table 1.
| Agricultural waste | Pyrolysis conditions | Yield | Targeted antibiotics | References | ||
|---|---|---|---|---|---|---|
| Temperature (°C) | Heating rate (°C/min) | Retention/holding time (h) | ||||
| BDB, MDB, and SCGDB | 550 | 6–7 | 1.5 | 18.60%, 41.61%, and 21.23% | Ampicillin, clarithromycin, erythromycin, ofloxacin sulfamethoxazole, and tetracycline trimethoprim | [23] |
| Auricularia auricula dregs | 300, 500, and 700 | 5 | 2 | — | Tetracycline | [24] |
| Mixed food scraps | 300 | 12 | — | Tetracycline | [25] | |
| Soap nut seeds | 450 | 3 | 2 | 3.73 g | Ciprofloxacin | [26] |
| Sawdust, rice husk, and municipal organic waste | 300 and 700 | 5 | 2 | 63.33%–73.50% and 30.16%–32.95% | Moxifloxacin and ofloxacin | [27] |
| Corncob | 600 | — | 2 | — | Ciprofloxacin, ofloxacin, and delafloxacin | [28] |
| Pine bark | 400 | — | 2 | — | Tetracycline | [29] |
Despite this variability, biochar is a biomass with a stable porous structure that is an extremely effective, economical, and ecologically friendly adsorbent. Slow pyrolysis was performed on 50–60 kg of biosolids, cattle manure, and wasted spent coffee grounds in a small-scale pyrolysis capacity (12–14 kg capacity) with a supply of nitrogen at 400 mL min−1 with oxygen limitation. The feedstocks were heated to 550°C for 1.5 h at 6–7°C/min. Following pyrolysis, the resulting biochars (BDB - biosolids-derived biochar, MDB - cattle manure-derived biochar, and SCGDB - spent coffee grounds-derived biochar) were crushed, allowed to cool to room temperature, and sieved to remove particles smaller than 125 μm before being stored in polyethylene bottles. With constant nitrogen flow and a temperature range of 500°C–600°C for 1.5–2 h, uniform pyrolysis conditions produced biochars with different dry weights (41.61% for cattle dung, 18.60% for biosolids, and 21.23% for wasted coffee grounds) [23].
Similarly, the remaining material from the Auricularia auricula dregs was washed, dried for 24 h at 105°C, and then crushed into tiny bits. In a tube furnace with nitrogen flow, wastes A. auricula residues were pyrolyzed for 2 hours at 300, 500, and 700°C. The rate of heating was 5°C per minute. After the pyrolysis, the samples were allowed to cool before sieving through mesh and cleaned with deionized water to remove any remaining surface ash. They were then dried at 105°C to produce biochar. The biochars that were generated at temperatures of 300°C, 500°C, and 700°C were labeled as BC (Biochar)-300, BC-500, and BC-700, respectively. This technique made it possible to prepare three adsorbents with the same steps but at various pyrolysis temperatures [24].
Dang et al. pyrolyzed corncobs in a small kiln at Maejo University’s Bio-Energy Research Center in Chiang Mai, Thailand. 1.7 kg of dried corncobs were put into a 20-L steel container for the pyrolysis process [28]. After that, the air supply was adjusted to manually heat the kiln for approximately 2 h at approximately 600°C. After the pyrolysis process, the weight of the carbonized corncobs was determined. The carbonized corncobs were ground and passed through two mesh sieves to obtain particles with a diameter of approximately 1.5–3 mm. Both tap and deionized water were used to wash the biochar. The pH of the wash water dropped from 9.6 to 7.2 after the remaining ash was removed. The corncob char was dried in an oven at 105°C for 2 h.
In another investigation, pine bark was used as biochar. Wood fragments from pine trees, which are often cut down, were gathered, and the bark was cleaned, peeled, and dried for a full day. At 400°C, it was then carbonized for 2 h. Subsequently, the samples were sieved with varying meshes and activated in a vacuum oven at 800°C under CO2 gas flow. Finally, the biosorbent was used after being collected and ground up in mortars and laboratory containers [29].
Similarly, several studies have been carried out using agricultural waste, such as sawdust, organic waste, and rice husk, to produce biochar. To achieve a less than 10% moisture content, each biomass component was individually oven-dried at 60°C before being crushed, ground, and homogenized. Biochar was produced by the slow pyrolysis method using a muffle furnace with two varying temperatures of 300°C and 700°C. The individual samples of bio-mass were arranged alone in a covered 6 by 4 inches container and pyrolyzed for 2 h at the given temperatures under restricted air supply and at a 5°C/min raise. The generated biochar was then allowed to cool and kept in airtight receptacles. The weight loss during pyrolysis is computed to estimate the yield of biochar [30]. The thorough investigation of different feedstocks and pyrolysis conditions produced a range of biochar with unique features, dry weights, and adsorption capacities, highlighting a versatile and adjustable method of producing biochar for possible environmental uses.
4. Functional Modification of Biochar
Modification of biochar improves its physicochemical characteristics, leading to greater adsorption efficiency. The methods with most common biochar modification are classified into physical, chemical, and biological modifications [9]. The modification of biochar is performed by chemical processes such as acids, bases, oxidants, or functional compounds, which introduce specific functional groups into biochar for the enhancement of its surface characteristics. This enhancement aims to increase the surface area by enhancing the adsorbing capacity of the adsorbent, improving its interaction with pollutants. The functional modification of biochar impacts its adsorption capabilities by producing significant surface modifications. Oxygen-containing functional groups such as –COOH and –OH have undergone acid treatments, which improve hydrophilic and electrostatic interactions with antibiotics. Similarly, alkaline (KOH and NaOH) and oxidizing agents (H2O2 and KMnO4) improve porosity and negative surface charge, introducing specific adsorption sites that favor the adsorption process more selectively. Functionalized biochars have increased adsorptive qualities that, for example, are useful in water treatment applications where contaminants and pollutants need removal [31, 32]. The functional modification of biochar is illustrated in Table 2.
| Agricultural waste | Modifications | Enhanced characteristics | Targeted antibiotics | Removal capacity (mg/g) | References |
|---|---|---|---|---|---|
| New pomelo peel | KOH at 600°C | Enhanced pore filling and surface area | Tetracycline, oxytetracycline, and chlortetracycline | 476.2, 407.5, and 555.6 | [33] |
| Flueggea suffruticosa | ZnCl2 and HCl ultrasonic wash | Surface area −1556 m2/g and pore volume −0.871 cc/g | Tetracycline, oxytetracycline, and chlortetracycline | 188.7, 129.9, and 200 | [34] |
| Corncob xylose residue | NaOH at 400°C - 1 h and KOH at 850°C -1 h | Amino functional groups | Sulfamethoxazole | 1429 | [35] |
| Palm oil fiber |
|
Largest surface area and carboxylic acid groups | Cephalexin | 57.5 | [20] |
| Pomegranate peels | 85 wt% H3PO4 | Greater porosity and surface area | Cephalexin | 87.2 | [36] |
| Anthriscus sylvestris | NaOH | Oxygen-containing functional groups -C=O, C -O, and -OH | Diclofenac and cephalexin | 392.9 and 724.5 | [37] |
| Rice husk | FeCl3 | Greater porosity and surface area | Sulfamethazine(SMT), sulfamerazine (SMR), sulfadiazine (SDZ), and sulfamethoxazole (SMX) | 0.132, 0.130, 0.228, and 0.163 | [38] |
| Walnut shells | HNO3 | Abundant carbon and oxygen-contained functional groups | Sulfadiazine, sulfamethazine, and sulfachloropyridazine | 32, 46, and 40 | [39] |
Chen et al. produced two types of Fe-modified biochar utilizing the pyrolysis-impregnation and impregnation-pyrolysis [33]. Using the impregnation-pyrolysis technique, 40.5 g of FeCl·6HO was dissolved in 500 mL of distilled water, 8.377 g of biomass was added, the pH was adjusted to 10, and the mixture was shaken for two hours at 30°C. The mixture was dried and then pyrolyzed for 1 hour at 300°C in a nitrogen environment. In contrast to unmodified biochar, which had an adsorption capacity of 80 mg/g for tetracycline, this Fe-modified biochar had an adsorption capacity of 150 mg/g. This enhanced adsorption capacity due to the introduction of iron functional groups enables the interaction of tetracycline with the modified biochar. The enhancement is attributed to increased surface area and the introduction of Fe functional groups, which facilitate complexation with tetracycline molecules.
Similarly, residues from Flueggea suffruticosa were converted into biochar by activating with ZnCl2. 10 g of powdered biomass, 40 mL of deionized water, and 15 g of ZnCl2 were combined, stirred for an hour, dried, and then carbonized for 90 min at 500°C. Compared to raw biochar, which had an adsorption capacity of 100 mg/g for ciprofloxacin, the resultant ZnCl2-activated biochar had an adsorption capacity of 200 mg/g. The formation of a porous structure and the addition of functional groups that promote π–π interactions and hydrogen bonding with ciprofloxacin molecules are probably the causes of this enhancement [34].
In a recent study, biochar was modified with alkaline treatment, resulting in a larger surface area (130.520 m2/g) and pore volume (0.128 cm3/g), and explored for the adsorption of different types of antibiotics from livestock wastewater. The modified biochar showed enhanced adsorption capacity, which was mainly determined by chemisorption and moderately affected by solution pH variations (3–10). Computational analysis indicated that the -OH groups on the biochar surface served as the dominant active sites for antibiotics adsorption due to strong adsorption energies between antibiotics and -OH groups. In a recent study, nanoparticles were found to be crucial for the adsorption process [40]. However, functionalized biochars are stable for the long term. Thus, they can be used to investigate novel techniques, such as bio-inspired methodologies, to treat environmental issues and for practical applications. Research on functionalized biochar is still in its infancy, but it has enormous potential to improve agricultural practices and solve modern environmental problems.
5. Sources and Adverse Effects of Antibiotics in Aqueous Environment
Antibiotics can be categorized according to their chemical structure, broad spectrum of activity, and mechanisms of action. They may originate from synthetic, semisynthetic, or natural sources. More than 250 registered and widely used antibiotics fall under groups such as β-lactams, macrolides, fluoroquinolones, tetracyclines, and sulfonamides [41]. Antibiotics’ pervasiveness in aquatic environments has sparked worries due to their extensive usage in human health, veterinary medicine, and agriculture. Antibiotics can eventually find their way into water sources through a number of routes, including farm runoff, treated wastewater discharge, and inappropriate medicine disposal. This issue has led to surface water, groundwater, and drinking water supply contamination. Reducing possible risks to human health and the environment requires increasing knowledge of the origins and detrimental effects of antibiotics in aquatic settings [42, 43].
In addition, municipal water is contaminated with high amounts of antibiotics. Drinking water contains most of the antibiotics dissolved in it. It was discovered that there were traces of ciprofloxacin, norfloxacin, enrofloxacin, lomefloxacin, macrolides, clarithromycin, and erythromycin in tap water [44]. According to a study, azithromycin levels in freshwater from France and Lebanon ranged from 3.75–17 ng/L and 10.8–377.7 ng/L, respectively. The ranges for erythromycin and fluoxetine concentrations were 121 and 268 ng/L and 12.6 and 28.4 ng/L, respectively. Concentrations of Sotalol were said to range between 44 ng/L in France and 2.1–5 ng/L in Lebanon [45].
The study was validated by detecting various antibiotics in the surface water of Portugal at different frequencies. Abacavir was found most frequently in surface water (69%), followed by ciprofloxacin and clarithromycin (46%). The three drugs with the lowest detection frequencies were sulfamethazine, norfloxacin, and azithromycin (8%). The water samples collected from the Yamuna River of India contained antibiotics. Of all the antibiotics present, the highest concentration was ofloxacin (145.3794 ng/mL), followed by erythromycin at 2.171 ng/mL and amoxicillin at 3.033 ng/mL [46].
Another investigation found SMX and trimethoprim in River Swarna and River Netravathi waters. Due to low consumption and high population density, chloramphenicol and ciprofloxacin were isolated in 83% of the samples in R. Swarna. Interestingly, netilmicin showed seasonal variations and was not detected during the postmonsoon season. The increased amount near Mangalore city may be the result of sewage discharge, as SMX and trimethoprim are widely distributed in R. Netravathi. Most samples have the presence of chloramphenicol, netilmicin, and ciprofloxacin. The comparison investigation highlights the contrasts between the two rivers, with R. Netravathi figuring a higher prevalence of SMX and a 100% presence of chloramphenicol [47]. Pharmacological components in groundwater resources range from mg/L to μg/L. Antibiotics; psychiatric drugs such as carbamazepine (9.3–92.4 ng/L); stimulant drugs like caffeine (55 ng/L); antidepressants; antihypertensives; hormonal contraceptives; and analgesics and antipyretics such as Ibuprofen (57.9–104 ng/L), sulfamethoxazole (28.7–124.5 ng/L), and sulfamethazine (29.2–83.9 ng/L) have been detected [48, 49]. It is detrimental to living things when antibiotics are present in the environment. For example, tetracyclines, sulfonamides, and macrolides affect algae growth, photosynthesis, and defense mechanisms against oxidative stress.
In addition, it shows the possible effects on fish, demonstrating that even at levels of environmental exposure, antibiotics can impact fish survival, change the functioning of genes linked to immune responses and antioxidant activity, hinder fish hatching, prevent fish from hatching, and harm adult fish’s hearts and metabolism [49]. Bombaywala et al. demonstrated the importance of antibiotic resistance genes (ARGs), changing as a result of continued antibiotic use in both people and animals. Antibiotics administered to animals can affect the formation of ARGs in humans, and ARG reservoirs in human commensals can spread to possible pathogens traveling through the gastrointestinal tract [50].
Genetic and genomic research highlights the need for efficient waste management and treatment techniques, emphasizing the crucial role of antibiotic resistance in organisms. Research has demonstrated that present wastewater treatment facilities are not fully equipped to remove pharmaceutical chemicals, emphasizing the need for novel treatment approaches that prioritize antibiotic removal.
6. Antibiotics Removal Using Biochar
Biochar produced from biomass such as agricultural waste is a viable method for eliminating water impurities and a useful way to mitigate the harmful environmental effects of burning crop residue. For both organic and inorganic pollutants, it is an effective and sustainable adsorbent [51]. This was confirmed by research into using biochar derived from cotton gin waste (CG) and Guayule bagasse as adsorbents to remove erythromycin, docusate, and sulfapyridine (SPY) from aqueous solutions. As the biochar pyrolysis temperature was raised from 350°C to 700°C, the SSA, pH, and surface hydrophobicity increased. The biochar’s SSA and functional groups, as well as the duration of contact and pH of the solution, influenced the erythromycin, docusate, and SPY adsorption capabilities. Unexpectedly, as the pH rose from 7 to 10, the adsorption of SPY increased from 40% to 70%. Major pollutants from water can be removed using high surface area biochars that possess favorable physicochemical properties that facilitate considerable interactions with pharmaceuticals’ polar and nonpolar functionalities [52, 53].
Nguyen et al. experimented in 2021 to extract tetracycline using alkaline-modified biochar (NaOH-SCG), which is produced by pyrolyzing waste coffee grounds. Its better adsorption capacity of 113.6 mg g−1 was made possible by its ordered mesoporous structure and large SSA (116.591 m2/g), which was 2.9 times more than that of nonmodified SCG [54]. The sorption of sulfonamide antibiotics is improved by magnetite-functionalized biochar (MBC) when copyrolyzed with Fe3+ because of the influence of the hydrophobic characteristics, substituents, and dissociation constants of sulfonamide antibiotics. Mechanistic insights indicate that hexavalent zinc (SaAs) sorption capacities are arranged in the following order: SMX > SDZ > SMR > SMT, with π-bond, assisted H-bonding, and the carbonaceous matrix of MBC playing critical roles. This study highlights the contributions of oxygen-containing groups and structural changes in MBC to antibiotic sorption, which provides crucial knowledge for the development of specific biochar composites for field disposal [38].
Furthermore, tetracycline antibiotics can be effectively and economically removed from aqueous solutions using pine bark-derived biochar, which shows a maximum removal efficiency of 89.5% within 15 min of acidic pH 5.1 with 1 g of antibiotic. Structural analysis shows that tetracycline is effectively removed from aqueous solutions with a maximum adsorption capacity of 58.47 mg/g when three functional groups (hydroxyl, carbonyl, and amine), pores with a surface area of 683.33 m2/g, total volume of pore 0.418 cm3/g, and average adsorbent diameter of 2.45 nm are present. The process conforms to pseudo-second-order kinetics and the Langmuir isotherm, which makes the adsorption response effective [27].
Table 3 presents further detailed information on the process parameters for antibiotic adsorption. Biochar is a useful and affordable method for removing antibiotics. To the fullest extent possible, biochar must be used to reduce antibiotic contamination in a range of environmental contexts. Currently, research is concentrated on streamlining the procedures used in its synthesis, investigating new feedstocks, and resolving obstacles.
| Source of biochar | Targeted antibiotics | Optimal removal conditions | Isotherm and kinetic model | Mechanism | Removal capacity (mg/g) | References |
|---|---|---|---|---|---|---|
| Sunflower seed husk biochar | Tetracycline, ciprofloxacin, Ibuprofen IBP, and SMX | — | — | Chemisorption, external diffusion, and intraparticle diffusion | 429.3, 361.6, 251.3, and 251.1 | [55] |
| Vinasse wastes | Pefloxacin (PEF) and ciprofloxacin | pH - 5, T - 25°C, and RT - 72 h | Freundlich isotherm and pseudo-second order | Pore-filling effect, π-π electron donor-acceptor (EDA), π-π stacking interaction, hydrogen bonding, and hydrophobicity. | 270 and 135 | [56] |
| Reed-based biochar | Tetracycline | — | Freundlich isotherm and pseudo-second order | Van der Waals forces, π–π conjugation, and electrostatic interaction | 173.6 | [57] |
| Pomelo peel | Levofloxacin | pH −5 and T - 25°C | Freundlich isotherm and pseudo-second order | Electrostatic interactions, complexation of functional groups, hydrogen bonding, and donor-acceptor interactions between π-π electrons | 115 | [58] |
| Spent coffee ground | Tetracycline | pH −7.0, T - 25°C, and RT - 24 h | Langmuir | π-π electron donor-acceptor interactions and pore filling | 113.6 | [54] |
| Cotton gin waste and guayule bagasse | Sulfapyridine, docusate, and erythromycin | pH - 10 and RT - 24 h | Langmuir and PSO | Hydrogen bonding, hydrophobic partitioning, and EDA interactions | 1.2, 19.7, and 17.1 | [52] |
| Pomelo peels | Paracetamol | pH - 7.0, T - 25°C, and contact time - 48 h | Langmuir and PSO | Electrostatic attraction, van der Waals force, hydrogen bonding formation, pore filling, and π-π interaction | 147 | [56] |
| Peanut shells | Naproxen | pH - 7.0 and T - 30°C | Langmuir and PSO | Pore filling, π-π interaction, and van der Waals force | 324 | [59, 60] |
| Pepper stem | Ibuprofen | — | Langmuir | Pore filling, π-π interaction, and hydrogen bonding | 569.6 | [61] |
7. Affecting Factors of Antibiotic Adsorption Using Biochar
Numerous factors affect the adsorption of antibiotics using biochar, including pyrolysis temperature, biochar preparation, nature of the feedstock, and functional modifications. The temperature at which the pyrolysis process occurs, feedstock and other pyrolysis-related parameters directly impact how effectively biochar absorbs contaminants. Biochar’s properties, such as its chemistry, polarity, surface area, pore size, and aromaticity, are significantly impacted by this temperature. The type of biomass used affects the various surface characteristics of the produced biochars [46, 62]. Figure 2 shows the three main parameters that affect the adsorption process: operating conditions, environmental factors, and adsorbent properties. The operating conditions include contact time, the amount of contaminants present, and the dosage of biochar. Environmental factors include pH and temperature. Similarly, adsorbent properties include the pore size, the functional group, and the surface area.
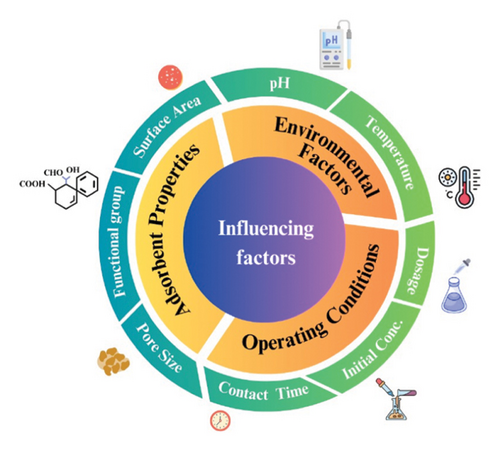
Temperature significantly influences the physicochemical qualities and features of biochar during pyrolysis and the makeup of the biowaste, as determined by the type of biochar obtained [63, 64]. The physicochemical properties of the resulting biochar, and thus its adsorption capability, are significantly influenced by the temperature during pyrolysis and the nature of the biomass feedstock. Higher pyrolysis temperatures typically increase the SSA and porosity of biochar, increasing its ability to adsorb contaminants. Chars produced at higher peak pyrolysis temperatures (450°C), for instance, have been shown to extract up to 88% of the antibiotic clarithromycin from wastewater [65].
Another important factor which affects the adsorption process was the feedstock. The surface functional groups on the biochar, which are crucial for interactions with antibiotic compounds, are also impacted by the makeup of the feedstock. Surface modification may increase the surface area and pore size for better adsorption capacity. Addition of metal oxides to the biochar results in increased adsorption capacity compared to acid or alkali modifications. However, the metal ion leakage results in environmental problems while exposed to the surroundings [66]. The optimal pH level varies for different antibiotics and biochar materials. However, because the pH-dependent heterogeneity of antibiotics affects how they interact with adsorbents, the mixture’s pH is critical to the adsorption of antibiotics by biochar. The amount and type of ions present affect the ability of biochar materials to adsorb antibiotics. Ionic strength additionally serves as a significant factor that affects the adsorption process through both a “squeeze-out” and a “salting-out” effect [67]. Considering these variables enables the enhancement of biochar-based methods for the elimination of antibiotics. The design and implementation of biochar applications for environmental remediation should consider these issues. Site-specific parameters may be required for optimized approaches for efficient antibiotic adsorption using biochar.
8. Adsorption Mechanism of Antibiotics on Biochar
The isotherms provide important details about the nature of the interactions between the adsorbent and the adsorbate as well as the adsorption capacity. The Freundlich and Langmuir models are two of the most often used isotherms among these models. According to the Langmuir isotherm, adsorption takes place as a monolayer on a homogenous adsorbent surface until all accessible sites are occupied, with no interaction between adsorbed molecules. On the other hand, the Freundlich isotherm allows for interactions between adsorbed molecules and multilayer adsorption, which accounts for a heterogeneous surface [69]. Adsorption kinetic models explain how molecules of the adsorbate attach to an adsorbent’s surface over time. These models describe the dynamics, mechanics, and reaction rate of the adsorption process. The pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion models are examples of common kinetic models.
The rate-limiting phase in the pseudo-first-order and pseudo-second-order models is surface reactions. According to the pseudo-first-order model, the difference between the equilibrium concentration (qe) and the concentration at a given time (q) determines the adsorption rate (dq/dt). The adsorption rate, on the other hand, is proportional to the square of this difference, according to the pseudo-second-order model. The Elovich model makes the assumption that there are interactions between the adsorbate molecules and that the adsorption rate changes with time. The intraparticle diffusion model, on the other hand, concentrates on the rate of diffusion inside the particles. Diffusion in the particles is the process that limits the rate of this process [70, 71].
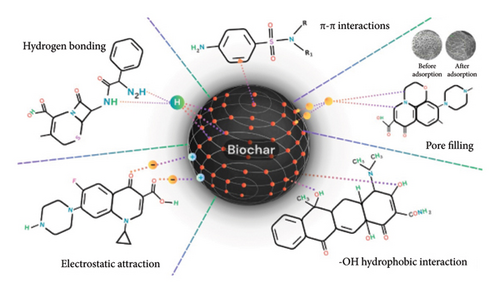
In this study, tetracycline antibiotic was adsorbed by biochar, which is a complex process that involves multiple interactions. These interactions include the formation of π-π conjugate effects between aromatic rings and the formation of H-bond among the functional groups of biochar and tetracycline, with enhanced adsorption affinity on alkaline-modified biochar (NaOH-SCG), which is attributed to a larger pore volume, mesoporous dominance, and improved surface properties. Additionally, the effect of electrostatic repulsion depends on the solution’s pH, highlighting the significant role that physicochemical properties play in tetracycline adsorption [54].
SPY’s removal from solution over a period of time using six different biochar shows a range of adsorption. Cotton gin waste CG700, which has a higher surface area and is thought to be involved in mechanisms such as hydrophobic interactions, negative charge-assisted H-bonding, and π–π EDA interactions, is responsible for the highest rate of removal (70%). The differences in SPY adsorption between CG and guayule bagasse biochar can be attributed to distinct physicochemical characteristics, highlighting the impact of biochar properties on antibiotic adsorption behavior [52]. In another study, pore filling was found to play an important role in the mechanism of naproxen adsorption onto carbonaceous porous substances, notably biochar. This mechanism is further strengthened by micropore volume dominance and the ensuing pore-blocking phenomena.
Furthermore, besides the fact that adsorption efficiency depends on oxygen-containing functional groups, as shown by some experimental and advanced techniques such as Raman spectroscopy, the π−π interactions have been shown to be a sine qua noninteraction between the aromatic ring of the biochar and π electron with the antibiotic [60, 71]. The use of bamboo-based magnetic biochar had an effect on the removal of LFX. The associated mechanisms were surface area dominance, chemisorption, contributions from carbon matrix edges, π-π interactions, liquid film diffusion, multilayer heterogeneous processes, and involvement of different types of functional groups on the surface, including C-C, O-C=O, and M-O. Also, H-bonding and increased connections via π-π EDA interactions are taken into account [58]. The adsorption performance of bio-nanocomposites for ciprofloxacin and sparfloxacin is essentially dependent on the structural morphology and surface characteristics, namely the surface area, pore volume, the nature of active regions, as well as surface chemical groups. While physical phenomena are favored, the porous structure encourages π-π EDA interactions.
In bio-nanocomposites, the graphene surface behaves in terms of electron exchange, the π-electron donor. In contrast, ciprofloxacin/sparfloxacin with large amounts of electron-withdrawing fluorine groups acts as a π-electron acceptor. So, as shown by XPS and Raman spectroscopy, the adsorption capacity would increase. This pH-dependent mechanism also involves hydrophobic forces, hydrogen bond interactions, and electrostatic interactions. Furthermore, polar aromatic pollutants (PRCs) adsorption methods onto biochar involve a range of activities, such as hydrogen bond, n-π interaction, π-π interaction, electrostatic attraction, and pore filling. The specifics of the experiment, the qualities of the adsorbent, and features of the adsorbate determine the relative significance of these processes. More specifically, the π-π interaction—supported by several experimental techniques—becomes a significant factor. This is also supported by the interactions of hydrogen bonding, pore filling, and other factors, the significance of which is determined by the unique characteristics of the spherical and nonspherical types of biochar. The work emphasizes how these mechanisms interact intricately to shape PRC’s adsorption behavior and how the characteristics of biochar affect how much each activity contributes [59, 72].
Numerous factors, including hydrophobic forces, surface chemistry, electrostatic interactions, and the structural characteristics of both the antibiotic and the biochar, influence the intricate and varied process of antibiotic adsorption on biochar. Optimizing the use of biochar as an antimicrobial adsorbent for water treatment applications requires an understanding of these complex processes.
9. Regeneration and Industrial Applications of Biochar
The regeneration of biochar is a multifaceted process involving desorption mechanisms influenced by various factors, including the properties of the adsorbate, the intrinsic characteristics of the biochar, and the specific conditions during desorption. According to recent research, biochar may be efficiently regenerated using a variety of techniques, including chemical treatment, thermal treatment, and biological processes, ensuring repeated usage without sustaining a significant decrease in adsorption capacity. This recyclability eliminates waste production and lowers operating expenses, which are consistent with the circular economy. However, the kind of impurities adsorbed and the characteristics of the biochar might affect how effective regeneration techniques are. Thus, a further study is needed to optimize these procedures for particular uses. Furthermore, regeneration may restore biochar’s functioning, but repeated cycles may cause structural changes or slow deterioration, impairing the material’s long-term performance [73].
Similarly, employing large-scale biochar usage for removing antibiotics from water impacts several aspects, such as production costs, feedstock availability, and the effectiveness of biochar modification processes. The high energy need of the pyrolysis process is one of the main obstacles; its substantial energy consumption accounts for around 36% of the entire manufacturing cost. Optimizing pyrolysis temperatures and investigating energy-efficient manufacturing techniques are crucial for improving economic viability. Furthermore, because it directly affects both environmental and financial results, choosing sustainable and plentiful feedstocks is essential for the large-scale synthesis of biochar. Furthermore, although biochar has demonstrated potential in the adsorption of antibiotics, surface modifications can increase its adsorption capability even further. However, these changes might result in additional costs and other environmental issues, such as secondary contamination from the chemicals used to make the changes [74].
Therefore, for biochar to be used practically in industrial wastewater treatment, evaluating the advantages of improved adsorption against the expenses and environmental effects of modification is essential. To fully exploit the promise of biochar as an economical and sustainable option for antibiotic elimination on an industrial scale, these issues must be resolved via technological breakthroughs and strategic planning.
10. Current Challenges and Future Perspectives
Antibiotic adsorption on biochar results in secondary waste since the contaminants accumulate on the biochar’s surface and may be disposed of into the environment. In order to this, concerns regarding the proper disposal or regeneration of biochar will be required. Effective regeneration techniques or alternative disposal methods are considered to be major obstacles. In large-scale treatment, the high surface area and low bulk density of biochar make it difficult to separate from treated water, resulting in problems during separation procedures. In order to avoid difficult recovery procedures, magnetic modification of biochar has been proposed; however, this approach may result in additional expenses and potential environmental problems due to metal leaching. Modifications such as molecular imprinting, iron doping, or heteroatom doping are frequently used to increase the adsorption potential of biochar. Despite their effectiveness, these techniques can be costly and complex, making them difficult to scale and practically applicable [75].
The sustainability and cost-effectiveness can be increased by proposing new methods for the regeneration of used biochar with low-energy and reuse of the leftover biochar for different purposes, such as the production of biofuel and soil enrichment to enrich the productivity [76, 77]. By carefully regulating the feedstock content and pyrolysis conditions, biochar’s physicochemical characteristics can be strategically adjusted to maximize its native adsorption capabilities and reduce the need for expensive postmodification. Optimizing the design of efficient biochar requires elucidating the connection between production variables and adsorption performance. Combining biochar adsorption with other treatment technologies, such as advanced oxidation processes, or with other therapies may have synergistic effects that lead to a more thorough elimination of antibiotics and their byproducts. The drawbacks of individual treatments might be addressed by integrated techniques, which would also increase overall effectiveness. To ensure the ecological safety of biochar-based remediation technology, thorough assessments of the long-term environmental effects of biochar use, such as leaching of adsorbed antibiotics and microbiome effects on soil and water biota, must be carried out [78].
11. Conclusion
One promising and long-term strategy for reducing water contamination is the use of biochar to remove antibiotics by recycling agricultural waste. The most crucial elements influencing antibiotic adsorption onto biochar, including feedstock, surface modification, pyrolysis conditions, and environmental parameters, have been highlighted in the review. Despite the high adsorption efficiency of biochar, problems with a large-scale application, cost-effective regeneration, and postadsorption disposal have to be focused. However, in order to reach its full potential, future research must focus on optimizing biochar manufacturing techniques to improve adsorption capacity, enhance the sustainability of biochar and long-term usage, and enable large-scale applicability for the real world. Even though biochar shows promise in treating antibiotic contamination, interdisciplinary collaboration, policy frameworks, and innovations are necessary to bridge the gap between research and reality. For biochar to be marketed as a workable, long-term solution for cleaning water of antibiotics, future procedures must concentrate on sustainable production, efficient regeneration, and multipurpose uses. In conclusion, biochar presents a promising way to reduce antibiotic contamination in water, but its successful and long-term application depends on addressing these issues through targeted research and technical developments.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Varalakshmi Varatharajan: investigation and writing – original draft. Karthik V.: conceptualization, supervision, validation, and writing – review and editing. Selvakumar Periyasamy: conceptualization, data curation, validation, and writing – review and editing.
Funding
No funding was received for this research.
Acknowledgments
The authors would like to express their gratitude to P.S.R Engineering College, Sivakasi, Tamilnadu, India, Government College Technology, Coimbatore, India, and Adama Science and Technology University, Adama, Ethiopia, for their assistance in completing this review.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




