3-Methyl-4-Nitrobenzoate Derivates as Antifungal Drug Candidates: Biological and In Silico Evaluation
Abstract
The Candida genus includes many opportunistic pathogens of great clinical importance, being commonly involved in several superficial and systemic human fungal infections. In this study, eleven 3-methyl-4-nitrobenzoate derivates were prepared and evaluated against four Candida strains (Candida albicans ATCC-90028, C. glabrata 90,030, C. krusei 34,125, and C. guilliermondii 207). The relationship between the chemical structure of the prepared compounds and their antifungal activity was also investigated. The chemical structure of the obtained products was confirmed using Infrared, 1H and APT-13C nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry. The antifungal assay results showed that the screened compounds exhibited different levels of activity depending on the structural variation and tested strain. Among the 11 nitrobenzoate analogs, the compounds methyl 3-methyl-4-nitrobenzoate (1) and pentyl 3-methyl-4-nitrobenzoate (6) presented great antifungal activity against C. guilliermondii 207, with MIC values of 39 and 31 µM, respectively. Modeling studies of 6, the most bioactive compound against C. guilliermondii, demonstrated that it interacted with TPMK protein, which has been proposed as a molecular target in studies of potential antifungal agents. Finally, the preliminary SAR study of this series furnished information on possibly structural features that may affect the antifungal activity, like the importance of alkyl side chains for bioactivity.
1. Introduction
The increasing incidence of fungal infections caused by Candida species is a cause for concern among health authorities. Therefore, efforts should be made to research new drugs capable of reducing morbidity and mortality, especially in immunocompromised patients [1, 2]. Candida albicans rank as a leading cause of healthcare-associated bloodstream infections caused by the pathogenic species of the Candida genus. Candida glabrata, a non-albicans species, is the main cause of invasive candidiasis [1, 3, 4].
The higher involvement of resistant C. glabrata and C. krusei has promoted an increase in candidemia mortality rates [5]. Difficulties and failures when treating disseminated Candida spp. infections are mainly related to the few antifungal drugs available and the increasing appearance of fungal strains that are resistant to these antifungal agents [6]. Given this problem, much research is looking for molecules that are bioactive against human pathogenic fungi [7, 8]. Figure 1 presents the three major groups of antifungal drugs in clinical use, azoles, polyenes, and echinocandins. Despite the high efficacy of drugs from these chemical classes, in addition to other drugs traditionally used in antifungal therapy, resistant strains in the hospital environment represent a new challenge for the development of an effective clinical approach. Therefore, the investigation of new antifungal chemical classes is a priority to increase the options of antimicrobial agents for use in patient therapy [6].
The 3-methyl-4-nitrobenzoic acid is a di-substituted benzoic acid derivative obtained through synthesis, and used in studies as a precursor to obtain bioactive molecules [9]. The nitro group (NO2) is shown to contribute to a wide range of biological activities including anti-inflammatory, antioxidant [10], antitumour [11, 12], leishmanicidal [13], antibacterial [14, 15], and antifungal activities [16, 17]. Regarding antimicrobial activity, the NO2 can generate free radicals in the cells of microorganisms and promote oxidative stress with damage to the cell membrane, proteins, and nucleic acids [18–21].
In the study of Shahid et al. [22], one synthetic nitro compound (1-methyl-4-nitro-1H-imidazole) showed antifungal potential against several Candida species. Another study, performed by Nascimento et al. [23], showed the antimicrobial activity of derivatives of the o-nitro-cinnamic acid esterified with alkyl and benzyl carbon chains against four Candida species: C. albicans, C. tropicalis, C. parapsilosis, and C. krusei. The bioactivity of these molecules was attributed to side chain modulation and a possible influence of the NO2, which potentiated antimicrobial activity in the derivatives.
Given the bioactivity of nitro compounds, the purpose of this research was to evaluate the antifungal potential of 11 compounds derived from 3-methyl-4-nitrobenzoic acid against four Candida strains (C. albicans ATCC-90028, C. glabrata ATCC-90030, C. krusei ATCC-34125 and C. guilliermondii 207), establish the structural chemical characteristics identify that influence biological activity, and carry out a perform molecular docking for the molecule that presents the best antifungal capacity.
2. Materials and Methods
According to Figure 2, this work was divided into three parts: synthesis of nitrobenzoate derivatives, antifungal assay, and in silico study.

2.1. Chemistry
2.1.1. Materials
The reactions were carried out with high-purity reagents and solvents and monitored by TLC Analytical Thin Layer Chromatography (TLC) using silica gel 60 as the stationary phase and mixtures of ethyl acetate and hexane as mobile phase. The structural identification was made by Infrared (IR) spectroscopy, 1H and APT-13C Nuclear Magnetic Resonance (NMR) analysis, Mass Spectrometry (MS), melting point (m.p.), and comparison with the literature data.
The 1H and APT-13C NMR were obtained using two spectrometers: AscenedTM-Bruker operating at 400 MHz for 1H NMR and 100 MHz for APT-13C NMR, and Varian-NMR-System operating at 500 MHz (1H NMR) and 125 MHz (APT-13C NMR). The IR spectra were obtained in an FTIR spectrophotometer model IRPrestige-21, from the manufacturer Shimadzu, using KBr tablets. The frequency was measured in cm−1. The m.p. were performed using the heating plate of a GEHAKA digital device, model PF 1500, operating at 110V with temperatures ranging from 0°C to 350°C. High-Resolution Electrospray Ionization Mass Spectrum (HREIMS) was obtained using a QTOF Impact HD (Bruker Daltonics GmbH, Alemanha) mass spectrophotometer equipped with a high-performance solid-state laser (λ = 355 nm) and reflector. The spectra of the spectroscopic analyses are in the Supporting Information (Spectrum S1–S33).
2.1.2. General Procedure for the Preparation of Analogues 1–5
The 3-methyl-4-nitrobenzoic acid (100 mg, 0.552 mmol) was dissolved in the alcohol (20 mL), 0.1 mL of H2SO4 was then added and refluxed for 6–24 h. The alcohol was removed under reduced pressure and the residue was transferred to a separating funnel containing water (10 mL) and ethyl acetate (10 mL). The derivative ester was extracted using ethyl acetate (2 × 10 mL). The organic phase was neutralized with NaHCO3 5% and dried over Na2SO4. The ethyl acetate was then evaporated resulting in a pure product [24, 25].
2.1.3. General Procedure for the Preparation of Analogues 6–9
The 3-methyl-4-nitrobenzoic acid (100 mg, 0.552 mmol) and halide (0.552 mmol) were added in a flask containing acetone. After adding triethylamine (0.1 mL, 0.552 mmol), the mixture was refluxed for 48–72 h. The solvent was evaporated, and the product was extracted in a separation funnel with dichloromethane or chloroform (3 × 10 mL) and water. The organic phase was dried over Na2SO4, and the solvent was then evaporated [26, 27].
2.1.4. General Procedure for the Preparation of Analogue 10
3-Methyl-4-nitrobenzoic acid (100 mg, 0.552 mmol) and benzyl alcohol (0.552 mmol) in tetrahydrofuran (2 mL) were cooled and stirred for 30 minutes. Then, added 140 mg (0.552 mmol) of triphenylphosphine and 0.11 mL (0.552 mmol) of diethyl azodicarboxylate, and the reaction was stirred at room temperature for 48 h. The solvent was removed, and the product was extracted with H2O and dichloromethane (3 × 10 mL). The organic phase was neutralized with NaHCO3 5% (10 mL), dried over Na2SO4, and the solvent evaporated [28].
2.1.5. General Procedure for the Preparation of Analogue 11
The 3-methyl-4-nitrobenzoic acid (100 mg, 0.552 mmol), (+)-borneol (1.104 mmol, 170 mg), and 4-dimethylamino pyridine (0.055 mmol, 6.74 mg) were stirred in dichloromethane (4 mL). After the addition of N,N′-dicyclohexylcarbodiimide (0.6072 mmol, 125 mg) solubilized in dichloromethane, the reaction was stirred for 48 h at room temperature. Afterward, the reaction content was filtered three times. The solvent was then evaporated, and the product was extracted using H2O and dichloromethane (3 × 10 mL). The organic phase was neutralized with 10 mL of HCl 0.5 N and NaHCO3 5%, and dried over Na2SO4 [29].
The products 6–11 were purified in a chromatographic column over silica gel using a hexane-ethyl acetate eluent mixture.
2.1.6. Spectral Data
- a.
Methyl 3-methyl-4-nitrobenzoate (1): yellow crystals, yield 83.3% (95.8 mg), m.p.: 80°C–81°C (literature: 80°C–81°C [30]). IR (KBr, cm−1): 3043 (C-H sp2), 2958 (C-H sp3), 1730 (C=O), 1616 and 1583 (aromatic C=C bending), 1517 and 1342 (NO2 stretching), 1280 and 1197 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.01–7.97 (unresolved, 1H), 7.96–7.93 (unresolved, 2H), 3.95 (s, 3H, H-1′), 2.60 (s, 3H, H-8). APT-13C NMR (100 MHz, CDCl3): δC ppm 165.43 (C-7), 151.97 (C-4), 134.10 (C-2), 133.87 (C-1), 133.57 (C-3), 128.15 (C-6), 124.67 (C-5), 52.82 (C-1′), 20.15 (C-8) [31].
- b.
Ethyl 3-methyl-4-nitrobenzoate (2): brown solid, yield 49.3% (57 mg), m.p: 51°C–52°C (literature: 53°C–54°C [32]). IR (KBr, cm−1): 3047 (C-H sp2), 2985 (C-H sp3), 1726 (C=O), 1612 e 1587 (aromatic C=C bending), 1523 and 1363 (NO2 stretching); 1284 and 1199 (C-O stretching). 1H NMR (500 MHz, CDCl3): δH ppm 8.02–7.97 (unresolved, 2H, H-2, H-6), 7.96 (d, J = 8.4, 1H, H-5), 4.42 (q, J = 5.7 Hz, 2H, H-1′), 2.62 (s, 3H, H-8), 1.42 (t, J = 5.7 Hz, 3H, H-2′). APT-13C NMR (125 MHz, CDCl3): δC ppm 164.95 (C-7), 151.84 (C-4), 134.17 (C-1), 134.06 (C-2), 133.55 (C-3), 128.09 (C-6), 124.63 (C-5), 61.94 (C-1′), 20.28 (C-8), 14.37 (C-2′) [32].
- c.
Propyl 3-methyl-4-nitrobenzoate (3): brown solid, yield 79.8% (98 mg), m.p.: 37°C–38°C. IR (KBr, cm−1): 3047 (C-H sp2), 2966 (C-H sp3), 1722 (C=O), 1614 and 1583 (aromatic C=C bending), 1521 and 1346 (NO2 stretching), 1284 and 1193 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.05–8.03 (unresolved, 1H), 8.00–7.97 (unresolved, 2H), 4.31 (t, J = 6.6 Hz; 2H, H-1′), 2.62 (s, 3H, H-8), 1.81 (sext, J = 7.4 Hz; 2H, H-2′), 1.03 (t, J = 7.4 Hz, 2H, H-3′). APT-13C NMR (100 MHz, CDCl3): δC ppm 165.01 (C-7), 151.86 (C-4), 134.19 (C-1), 134.05 (C-2), 133.56 (C-3), 128.14 (C-6), 124.70 (C-5), 67.45 (C-1′), 22.12 (C-2′), 20.19 (C-8), 10.58 (C-3′) [33–35].
- d.
Isopropyl 3-methyl-4-nitrobenzoate (4): yellow solid, yield 75.5% (93 mg), m.p.: 58°C–59°C. IR (KBr, cm−1): 3045 (C-H sp2), 2990 (C-H sp3), 1718 (C=O), 1614 and 1583 (aromatic C=C bending), 1519 and 1342 (NO2 stretching), 1284 and 1193 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.00–7.99 (unresolved, 1H), 7.96–7.93 (unresolved, 2H), 5.26 (sept, J = 6.3 Hz, 1H, H-1′), 2.62 (s, 3H, H-8), 1.38 (d, J = 6.3 Hz, 6H, H-2′, H-2″). APT-13C NMR (100 MHz, CDCl3): δC ppm 164.46 (C-7), 151.84 (C-4), 134.64 (C-1), 133.99 (C-2), 133.49 (C-3), 128.15 (C-6), 124.63 (C-5), 69.63 (C-1′), 22.12 (C-2′, C-2″), 20.19 (C-8) [36].
- e.
2-Methoxyethyl 3-methyl-4-nitrobenzoate (5): green solid, yield 48.5% (64 mg), m.p.: 36°C–37°C. IR (KBr, cm−1): 3045 (C-H sp2), 2931 (C-H sp3), 1722 (C=O), 1614 and 1583 (aromatic C=C bending), 1519 and 1340 (NO2 stretching), 1282 and 1193 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.05–8.02 (unresolved, 1H), 8.01–7.99 (unresolved, 1H), 7.95 (d, J = 8.2, 1H, H-5), 4.48 (t, J = 4.6 Hz, 2H, H-1′), 3.72 (t, J = 4.6 Hz, 2H, H-2′), 3.40 (s, 3H, H-3′), 2.59 (s, 3H, H-8). APT-13C NMR (100 MHz, CDCl3): δC ppm 164.92 (C-7), 151.98 (C-4), 134.14 (C-2), 133.75 (C-1), 133.51 (C-3), 128.27 (C-6), 124.60 (C-5), 70.42 (C-1′), 64.80 (C-2′), 59.12 (C-3′), 20.09 (C-8). Positive mode HRESIMS m/z 240.0866 (M+H)+ (calc. for C11H14NO5, 240.0872).
- f.
Pentyl 3-methyl-4-nitrobenzoate (6): green oil, yield 30% (42 mg). IR (KBr, cm−1): 2958 (C-H sp2), 2931 (C-H sp3), 1726 (C=O), 1610 and 1589 (aromatic C=C bending), 1533 and 1354 (NO2 stretching), 1286 and 1195 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.03–8.01 (unresolved, 1H), 7.99–7.96 (unresolved, 1H), 7.93 (d, J = 8.4, 1H, H-5), 4.34 (t, J = 6.7 Hz, 2H, H-1′), 2.61 (s, 3H, H-8), 1.77 (quint, J = 6.8 Hz, 2H, H-2′), 1.42–1.37 (m, 4H, H-3′, H-4′), 0.93 (t, J = 7.1 Hz, 3H, H-5′). APT-13C NMR (100 MHz, CDCl3): δC ppm 165.02 (C-7), 151.91 (C-4), 134.24 (C-1), 134.03 (C-2), 133.54 (C-3), 128.11 (C-6), 124.65 (C-5), 66.07 (C-1′), 28.43 (C-2′), 28.23 (C-3′), 22.44 (C-4′), 20.19 (C-8), 14.07 (C-5′) [37].
- g.
Diphenylmethyl 3-methyl-4-nitrobenzoate (7): white amorphous solid, yield 12.7% (24.3 mg), m.p.: 100°C–101°C. IR (KBr, cm−1): 3032 (C-H sp2), 2924 (C-H sp3), 1726 (C=O), 1614 and 1583 (aromatic C=C bending), 1519 and 1346 (NO2 stretching), 1280 and 1192 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.11–8.09 (unresolved, 2H), 7.99 (d, J = 8.2, 1H, H-5), 7.47–7.32 (m, 10H, H-2′ to H-6′ and H-2″ to H-6″), 7.17 (s, 1H, H-7′), 2.64 (s, 3H, H-8). APT-13C NMR (100 MHz, CDCl3): δC ppm 163.97 (C-7), 152.07 (C-4), 139.73 (C-1′, C-1″), 134.18 (C-2), 133.88 (C-1), 133.62 (C-3), 128.77 (C-2′, C-6′, C-2″, C-6″), 128.33 (C-4′, C-4″), 128.30 (C-6), 127.23 (C-3′, C-5′, C-3″, C-5″), 124.70 (C-5), 78.43 (C-7′), 20.13 (C-8) [38].
- h.
4-Chloro-benzyl 3-methyl-4-nitrobenzoate (8): white solid, yield 52% (87.8 mg), m.p. 96°C–97°C. IR (KBr, cm−1): 2933 (C-H sp2), 1722 (C=O), 1614 and 1581 (aromatic C=C bending), 1521 and 1344 (NO2 stretching), 1276 and 1192 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.02–7.98 (unresolved, 2H), 7.96 (d, J = 8.4, 1H, H-5), 7.40–3.35 (m, 4H, H-2′, H-3′, H-5′, H-6′), 5.34 (s, 2H, H-7′), 2.61 (s, 3H, H-8). APT-13C NMR (100 MHz, CDCl3): δC ppm 164.71 (C-7), 152.10 (C-4), 134.69 (C-1′), 134.17 (C-2), 133.96 (C-1), 133.65 (C-4′), 133.62 (C-3), 129.96 (C-2′, C-6′) 129.06 (C-3′, C-5′), 128.27 (C-6), 124.71 (C-5), 66.79 (C-7′), 20.15 (C-8) [39].
- i.
2-Methyl-naphthalenyl 3-methyl-4-nitrobenzoate (9): white solid, yield 34.1% (60.4 mg), m.p.: 101°C–102°C. IR (KBr, cm−1): 3057 (C-H sp2), 2935 (C-H sp3), 1720 (C=O), 1612 and 1583 (aromatic C=C bending), 1519 and 1344 (NO2 stretching), 1278 and 1193 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.06–8.04 (unresolved, 1H), 8.03–8.01 (unresolved, 1H), 7.95 (d, J = 8.4, 1H, H-5), 7.91–7.85 (m, 4H, H-1′, H-4′, H-8′, H-5′), 7.57–7.49 (m, 3H, H-3′, H-6′, H-7′), 5.55 (s, 2H, H-9′), 2.61 (s, 3H, H-8). APT-13C NMR (100 MHz, CDCl3): δC ppm 164.87 (C-7), 152.06 (C-4), 134.21 (C-2), 133.85 (C-2′), 133.63 (C-1′), 133.39 (C-8′a), 133.30 (C-4′a), 132.85 (C-3), 128.74 (C-4′), 128.32 (C-8′), 128.15 (C-5′), 127.93 (C-1′), 127.89 (C-7′), 126.66 (C-6), 126.62 (C-6′), 126.07 (C-3′), 124.71 (C-5), 67.84 (C-9′), 20.17 (C-8) [40].
- j.
Benzyl 3-methyl-4-nitrobenzoate (10): white solid, yield 43.2% (62.2 mg), m. p.: 35°C–36°C. IR (KBr, cm−1): 3037 (C-H sp2), 2968 (C-H sp3), 1718 (C=O), 1612 and 1585 (aromatic C=C bending), 1527 and 1358 (NO2 stretching), 1280 and 1192 (C-O stretching). 1H NMR (500 MHz, CDCl3): δH ppm 8.05–8.04 (unresolved, 1H), 8.03–8.01 (unresolved, 1H), 7.95 (d, J = 8.4, 1H, H-5), 7.43–7.37 (m, 5H, H-2′, H-3′, H-4′, H-5′, H-6′), 5.39 (s, 2H, H-7′), 2.61 (s, 3H, H-8). APT-13C NMR (125 MHz, CDCl3): δC ppm 164.81 (C-7), 152.08 (C-4), 135.51 (C-1′), 134.18 (C-2), 133.87 (C-1), 133.59 (C-3) 128.85 (C-3′, C-5′), 128.72 (C-6), 128.54 (C-2′, C-6′), 128.29 (C-4′), 124.67 (C-5), 67.64 (C-7′), 20.14 (C-8) [41].
- k.
Bornyl 3-methyl-4-nitrobenzoate (11): white solid, yield: 28% (49 mg), m.p.: 160°C–161°C. IR (KBr, cm−1): 2960 (C-H sp3), 1726 (C=O), 1612 and 1589 (aromatic C=C bending), 1529 and 1352 (NO2 stretching), 1286 and 1195 (C-O stretching). 1H NMR (400 MHz, CDCl3): δH ppm 8.01–8.00 (unresolved, 1H), 7.98–9.95 (unresolved, 2H), 5.14 (ddd, J = 9.9, 3.4, 2.2 Hz, 1H, H-2′), 2.63 (s, 3H, H-8), 2.53–2.44 (m, 1H, H-3′), 2.11–2.04 (m, 1H, H-6′), 1.86–1.74 (m, 1H, H-5′), 1.76 (t, J = 4.5 Hz, 1H, H-4′), 1.47–1.39 (m, 1H, H-6′), 1.35–1.28 (m, 1H, H-5′), 1.12 (dd, J = 13.9, 3.4 Hz, 1H, H-3′), 0.97 (s, 3H, H-8′), 0.92 (s, 3H, H-9′), 0.91 (s, 3H, H-10′). APT-13C NMR (100 MHz, CDCl3): δC ppm 165.22 (C-7), 151.91 (C-4), 134.63 (C-1), 133.99 (C-2), 133.59 (C-3), 128.08 (C-6), 124.71 (C-5), 81.75 (C-2′), 49.31 (C-1′), 48.12 (C-7′), 45.08 (C-4′), 36.98 (C-3′), 28.22 (C-5′), 27.55 (C-6′), 20.29 (C-8), 19.85 (C-9′), 19.04 (C-8′), 13.77 (C-10′) [42].
2.2. Antifungal Activity
2.2.1. Microorganisms
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were determined using reference strains of Candida sp. obtained from both American Type Culture Collection (ATCC, Rockville, MD, EUA) (Candida albicans ATCC 90028, C. glabrata ATCC 90030, and C. krusei ATCC 34125) and Instituto Zimotécnico (ESALQ/USP, Campinas, São Paulo, Brazil) (C. guilliermondii 207).
2.2.2. Determination of the MIC and MFC
The microdilution method using 96 U-bottom microtiter plates was performed to define the MIC of the compounds in accordance with that described by the Clinical and Laboratory Standards Institute (CLSI) [43]. It used a fungal inoculum of 2.5 × 103 CFC/mL. The products were tested at concentrations ranging from 1000–7.8 µg/mL. The nitrobenzoate derivatives were solubilized in 5% dimethyl sulfoxide (DMSO) and distilled water. The plates were incubated for 24 h at a temperature of 35 ± 2°C. Then, to confirm the cell viability, 50 μL of 1% 2,3,5-triphenyl tetrazolium chloride (a colorimetric oxide-reduction indicator for fungi) was added to each well and the plates were incubated at 35 ± 2°C for 24 h again [44]. Nystatin (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control [45]. The sterility of culture medium, yeast viability, and 5% DMSO solution were also checked. All assays were performed in triplicate in three independent experiments, and the data were analyzed using descriptive statistics, with the results expressed as the mode of MIC values for each compound.
MFC was determined by subculturing an aliquot (50 μL) from each incubated well with a concentration higher than the MIC on Sabouraud Dextrose Agar plates (KASVI, Curitiba, Brazil). The plates were incubated at 35°C for 48 h. The MFC was defined as the lowest concentration that visually inhibited fungal growth in a solid medium. The MFC/MIC ratio was then calculated to determine whether the substances were fungistatic (MFC/MIC ≥ 4) or fungicidal (MFC/MIC < 4) [46].
2.3. Modeling Studies
2.3.1. Molecular Targets
Potential molecular targets of compound 6 in C. guilliermondii were identified following the homology-based methodology previously reported [47]. First, the potential targets of 6 were identified with the Similarity Ensemble Approach (SEA) web server [48]. Then, the SEA-provided proteins were used as queries in a Blast [49] search against the C. guilliermondii (tax id: 4929) proteome using the NCBI Blast implementation [50]. Any protein in the fungus having at least 40% of identity to the targets predicted by the SEA method and covered in a minimum of 75% of their sequences by the Blast alignment were selected as possible targets of compound 6 in C. guilliermondii.
2.3.2. Molecular Docking
Molecular docking proceeded following the consensus approach employed in previous publications [47]. The structural models for the potential receptors of compound 6 in C. guilliermondii were obtained with the Swiss Model web server [51]. Diverse homology models were built for each protein and the QMEANDisCo global score was employed as the criterion for selecting the best homology models. For compound 6, one three-dimensional conformer was generated, and partial atomic charges of type am1-bcc were added to the compound model with OpenEye’s Omega [52, 53] and Molcharge [54], respectively.
The Gold software [55] was employed for docking calculations using the ChemPLP scoring function for primary docking. For defining the ligand binding site, the ligands present in the homology models’ templates were used as references. Any relevant cofactor was considered during molecular docking calculations. A total of 30 different docking solutions were explored for each receptor. These docking poses were re-scored with the ChemScore, GoldScore, and ASP scoring functions. The scoring values of each scoring function were scaled to Z-scores at a target level. The consensus score of each pose was calculated as the average Z-score across the four scoring functions. All the predicted receptor-compound 6 complexes with Z-score larger than 1 were selected for additional calculations.
2.3.3. Molecular Dynamics (MD) and Free Energies of Binding
Amber 22 [56] was employed for MD simulations following the previously reported procedure [57]. All complexes were prepared using the same protocol. Proteins were parameterized with the ff19SB force field, the compound with the gaff2 one, and parameters for cofactors were retrieved from the repository maintained by the Bryce Group at The University of Manchester (https://amber.manchester.ac.uk/index.html).
Each system was enclosed in a truncated octahedron box that extended at least 10 Å away from any solute atom. This box was solvated with OPC water molecules and excess formal charge was neutralized by adding Na+ and Cl− counterions at a concentration of 0.15 M following the approach described in [58]. Two energy minimizations were performed, with the first one including restraints to the solute. For the second energy minimization stage, all restraints were released.
The energy-minimized systems were gradually heated from 0 to 300 K during 20 ps. An equilibration step that lasted 100 ps in the NPT ensemble followed heating. The equilibrated systems were the starting coordinates for five short (4 ns) production runs using the same parameters employed for equilibration. Atomic velocities were randomly initialized before starting each production run to obtain a better exploration of the conformational space of the complexes. This setup was conducted to a total production time of 20 ns per complex. Free energies of binding were predicted with the MM-PBSA method as implemented in Amber 22. For this, 20 MD snapshots equally spaced in simulation time were extracted from the five production runs. Snapshots were extracted from the 1 ns–4 ns time interval. This led to selecting 100 MD snapshots for estimating the free energies of binding. The ionic strength for free energy calculations was set to 0.15 M.
3. Results and Discussion
3.1. Chemistry
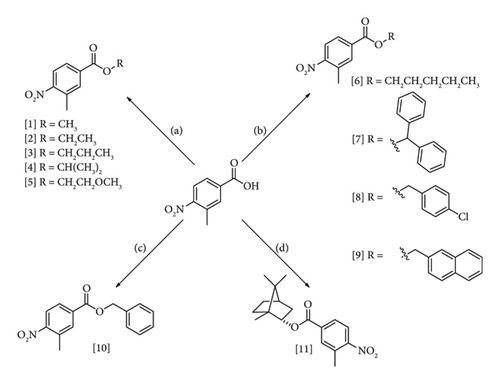
The compounds derived from 3-methyl-4-nitrobenzoic acid were prepared using four reactions: Fischer esterification (1–5), alkyl and benzyl halide esterification (6–9), Mitsunobu reaction (10), and Steglich reaction (11) (Figure 3). The chemical methods were selected according to the feasibility of product synthesis and reports in the scientific literature. The reaction conditions were established according to the best yields obtained and previous published studies.
Structure confirmation of the analogues was performed using m.p., IR spectroscopy, APT-13C and 1H NMR, and high-resolution MS (HRMS). In the IR spectra, we can observe a strong absorption between 1730 and 1718 cm−1 due to carbonyl (C=O), two esters stretching C-O absorptions between 1286 and 1280 cm−1 and 1197-1192 cm−1, asymmetric stretching between 1550 and 1490 cm−1, and symmetrical stretching between 1355 and 1315 cm−1 for the NO2 group [38–41]. In the 1H NMR spectra, the signals for aromatic hydrogens (H-2, H-5, and H-6) are between 8.06 and 7.94 ppm, and a singlet (s) between 2.61 and 2.63 ppm, typical of methyl hydrogens attached to aromatic ring [38–41]. In the 13C NMR spectra, was observed a greater carbonyl carbon displacement (between 166 and 164 ppm) and carbon directly linked to the NO2 group (between 153 and 151 ppm).
Compound 5 was the only synthesized for the first time, being obtained as a green solid with m.p. = 36°C–37°C. The HRESIMS spectrum of this compound in positive mode exhibited a pseudomolecular ion peak at m/z 240.0866 (M+H)+ (calc. for C11H14NO5, 240.0872), compatible with a 3-methyl-4-nitrobenzoate derivative with molecular formula C11H13NO5, corresponding to six degrees of unsaturation.
3.2. Evaluation of Antifungal Activity
This study was conducted to analyze the antifungal potential of 11 structurally correlated 3-methyl-4-nitrobenzoate derivatives against four Candida strains: C. albicans (ATCC 90028), C. glabrata (ATCC 90030), C. krusei (ATCC 34125), and C. guilliermondii (207).
The antifungal assays were performed by the microdilution method in 96-well microplates to measure the MIC value, expressed in both μg/mL and μM. MIC value represents the lowest concentration of the testing compounds able to inhibit microbial growth, and the MIC was only determined for microorganisms that showed susceptibility to the compounds. All assays were performed in triplicate; the nystatin antifungal agent (100 IU/mL) was utilized as positive control, and 5% DMSO solution as negative control. The MIC values were used to define structural chemical characteristics that influence bioactivity.
Alves et al. [59] established a standardized classification scheme for the antifungal potency of compounds screened against Candida species. According to this categorization [59], the antifungal bioactivity of the synthetic molecules can be classified into the following categories: (a) very strong bioactivity (MIC < 3.515 µg/mL), (b) strong bioactivity (MIC between 3.516 and 25 µg/mL), (c) moderate bioactivity (MIC between 26 and 100 µg/mL), (d) weak bioactivity (MIC from 101 to 500 µg/mL), (e) very weak bioactivity (MIC in the range of 501–2000 µg/mL), and (f) no bioactivity (MIC > 2000 μg/mL).
Table 1 summarizes the antifungal activity of the 11 assayed compounds (MIC values in μg/mL and μM). In general, among the evaluated nitrobenzoate derivatives, compound 6 (pentyl 3-methyl-4-nitrobenzoate) was found to exhibit the best antifungal activity, with MIC values of 1000; 1000 and 7.8 µg/mL against C. albicans ATCC 90028, C. krusei ATCC 34125, and C. guilliermondii 207, respectively; followed by methyl 3-methyl-4-nitrobenzoate (1) (MIC = 1000 and 7.8 µg/mL for C. albicans ATCC 90028, and C. guilliermondii 207, respectively). C. glabrata 90,030 was the less susceptible species to nitrobenzoate derivatives.
| Compounds | Candida albicans 90028 | Candida glabrata 90030 | Candida krusei 34125 | Candida guilliermondii 207 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | MIC (μM) | |
| 1 | 1000 | 5120 | + | + | + | + | 7.8 | 39 |
| 2 | 1000 | 4780 | + | + | 1000 | 4780 | 125 | 59.7 |
| 3 | 1000 | 4470 | 1000 | 4470 | + | + | 250 | 1110 |
| 4 | 1000 | 4470 | 1000 | 4470 | + | + | 1000 | 4470 |
| 5 | 1000 | 4180 | 1000 | 4180 | 1000 | 4180 | 1000 | 4180 |
| 6 | 1000 | 3970 | + | + | 1000 | 3970 | 7.8 | 31 |
| 7 | 1000 | 2870 | + | + | + | + | + | + |
| 8 | 1000 | 3270 | + | + | 1000 | 3270 | + | + |
| 9 | 1000 | 3110 | + | + | + | + | + | + |
| 10 | 1000 | 3680 | + | + | + | + | 500 | 1840 |
| 11 | + | + | + | + | 1000 | 3150 | 500 | 1570 |
| Nystatin | 3.75 | 4 | 3.75 | 4 | 3.75 | 4 | 3.75 | 4 |
| Control medium | − | − | − | − | − | − | − | − |
| 5% DMSO solution | + | + | + | + | + | + | + | + |
| Fungal growth control | + | + | + | + | + | + | + | + |
- Note: +: indicates growth of the microorganism; −: indicates no growth of the microorganism.
MFC and the MFC/MIC ratio were also obtained, which is important to define whether the compounds exhibit fungicidal or fungistatic activity. When the MFC/MIC ratio of a compound is < 4, this molecule is considered to have fungicidal effects. If the MFC/MIC ratio is ≥ 4, the compound is considered fungistatic against the pathogen [46]. According to Table 2, with the exception of compound 7, all compounds showed MFC/MIC ratio was < 4 which suggests that they exhibited fungicidal property against the tested Candida species. Fungistatic activity (at MFC/MIC ≥ 4) was not found.
| Compounds | Candida albicans 90028 | Candida glabrata 90030 | Candida krusei 34125 | Candida guilliermondii 207 | ||||
|---|---|---|---|---|---|---|---|---|
| MFC | MFC/MIC | MFC | MFC/MIC | MFC | MFC/MIC | MFC | MFC/MIC | |
| 1 | 5120 | 1 | — | — | — | — | 78 | 2 |
| 2 | 9560 | 2 | — | — | 4780 | 1 | 59.7 | 1 |
| 3 | 4470 | 1 | 4470 | 1 | — | — | 1110 | 1 |
| 4 | 8940 | 2 | 4470 | 1 | — | — | 4470 | 1 |
| 5 | 4180 | 1 | 4180 | 1 | 4180 | 1 | 4180 | 1 |
| 6 | 3970 | 1 | — | — | 3970 | 1 | 31 | 1 |
| 7 | — | — | — | — | — | — | — | — |
| 8 | — | — | — | — | 3270 | 1 | — | — |
| 9 | 3110 | 1 | — | — | — | — | — | — |
| 10 | 7360 | 2 | — | — | — | — | — | — |
| 11 | — | — | — | — | 3150 | 1 | 1570 | 1 |
- Abbreviations: MFC, minimal fungicidal concentration; MIC, minimum inhibitory concentration.
In the SAR study, most of the compounds exhibited weak bioactivity (MIC = 1000 μg/mL) against the C. albicans (ATCC 90028) strain. An increase in lateral aliphatic chains did not contribute to the enhancement of bioactivity of 1 to 6 against this strain, as well as the introduction of side chains containing aromatic rings (compounds 7–10). The nitrobenzoate derivative 11 (bornyl 3-methyl-4-nitrobenzoate) was the only inactive against C. albicans, being thus possible that the volume and spatial disposition of the substituents in bornyl group contributed to its inactivity against the fungus. Compounds 1-6 and 9-10 presented fungicidal capacity (MFC/MIC = 1 or 2).
In the tests performed against C. glabrata (ATCC 90030), compounds 1, 2, and 6-11 displayed no bioactivity. Only 3, 4, and 5 (at the highest tested concentration) were bioactive, presenting a weak antifungal effect (MIC = 1000 μg/mL) and a fungicidal action against this pathogen. The branching of the side chain in 4 (R = CH(CH3)2; MIC = 1000 μg/mL = 4470 μM; MFC = 4470 μM; MFC/MIC = 1) and 5 (R = CH2CH2OCH3; MIC = 1000 μg/mL = 4180 μM; MFC = 4180 μM; MFC/MIC = 1) did not influence or optimize the antifungal activity when comparing them with their analog 3 (R = CH2CH2CH3; MIC = 1,000 μg/mL = 4470 μM; MFC = 4,470 µM; MFC/MIC = 1).
C. krusei (ATCC 34125) species presented susceptibility to derivatives 2 (MIC = 1,000 μg/mL = 4780 μM), 5 (MIC = 1000 μg/mL = 4180 μM), 6 (MIC = 1000 μg/mL = 3970 μM), 8 (MIC = 1000 μg/mL = 3270 μM), and 11 (MIC = 1000 μg/mL = 3150 μM). These compounds had weak bioactivity against this strain and presented fungicidal capacity (MFC/MIC = 1). The influence of alkyl side chains for the bioactivity of 2, 5, and 6 was observed when compared with 1. On the contrary, the insertion of benzyl groups resulted in inactivity for derivatives 7, 9, and 10. Possibly, the chloride substituent (p-Cl) in the aromatic ring of the side chain of 8 resulted in bioactivity against C. krusei strain (ATCC 34125) when compared to 10, which was inactive. In the study performed by Araújo Neto et al. [60], the presence of electronegative atoms in the p-phenyl position contributed positively to the fungicidal activity of 5-nitro-thiophenes-thiosemicarbazones derivatives against Candida species. Therefore, this data is in agreement with the finding in the present study. Further, the introduction of the terpenic substructure in 11 (MIC = MFC = 3150 μM) did not enhance antifungal capacity when comparing it to 2.
The antifungal results of the compounds against C. guilliermondii 207 were the most significant, with emphasis on derivatives 1 (MIC = 39 μM; MFC = 78 μM; MFC/MIC = 2) and 6 (MIC = 31 μM; MFC = 31 μM; MFC/MIC = 1), that presented strong bioactivity [59]. In an earlier study, Lima et al. [61] also evaluated the antifungal effect of methyl 3-methyl-4-nitrobenzoate (1) against C. albicans ATCC 76645 strain, yielding a MIC of 256 μg/mL (1310 μM). This data corroborates the importance of the structural characteristics of this compound for the bioactivity against Candida species. Compared to compounds 1, 2, 3, and 4, we can observe that the antifungal activity was reduced with the increase of the aliphatic chain. Side chain modification to ethyl (2), propyl (3), and isopropyl (4) contributed to the reduction in the biological capacity of compound 1 (methyl) against C. guilliermondii 207.
However, increasing the aliphatic carbon chain to a pentyl (6; MIC = 31 μM) resulted in an enhancement of bioactivity, as observed for compound 1 (MIC = 39 μM) against C. guilliermondii 207, indicating the importance of aliphatic carbon chains for bioactivity against this species. Other studies also suggest the influence of the size of the carbon chain on studied biological activity [62–64]. The increase in the alkyl carbonic side chain promotes an increase in hydrophobicity of the bioactive molecules, which will modify the penetration of the compounds in cell membranes and their possible interaction with macromolecules, which may or may not contribute to better bioactivity [65]. The progressive increase of the alkyl carbonic chain may positively influence the antifungal activity of bioactive structures to a certain extent [63], with the need to carry out future studies to define the ideal extension of the linear alkyl chain that will provide better bioactivity against Candida species.
With relation to compounds with bulky groups in their structures, only 10 (MIC = 1840 μM) and 11 (MIC = MFC = 1570 μM) were active against C. guilliermondii 207, presenting weak bioactivity. Compound 11 also showed fungicidal capacity (MFC/MIC = 1), unlike 10. The antifungal capacity of monoterpenes, like borneol, was earlier observed against different pathogens, including Aspergillus and Candida species [66–68]. Finally, compound 11 had a better antifungal property when compared to 7, 8, and 9; however, it did not present better bioactivity than derivatives 1 and 6. This emphasizes the importance of aliphatic carbon chains for bioactivity against C. guilliermondii 207 [69].
Previously reported studies demonstrate the antifungal action of ester derivatives with similar structures or containing substructures of the products investigated [70–72]. The presence of a spacer between the aromatic ring and the ester group has not been shown to be relevant for the biological activity [70]. Terpenic derivatives have also shown antifungal action [71]. Meanwhile, the use of formulations containing aromatic esters may also be an interesting strategy aiming at optimizing the antimicrobial action of the bioactive compound [72–74].
3.3. Modeling Studies
Considering that compound 6 was active against three of the four tested strains and showed very strong activity against C. guilliermondii 207 (MIC = MFC = 31 μM), it was submitted to modeling studies. The modeling strategy employed in this research aimed at predicting the most possible targets of compound 6 in C. guilliermondii. For this, possible targets of the chemical were first identified and ligand-receptor binding hypotheses were generated with molecular docking calculations. Since different proteins were evaluated, the criterion for prioritizing molecular targets of compound 6 was the MD-derived free energies of binding. In this way, we avoid the biases associated with comparing docking scores across different molecular targets.
The potential molecular targets identified using the target fishing approach is listed in Table 3. The table includes the UniProt accessions for the proteins, functional descriptions for them, and the IDs assigned throughout the manuscript. The set of potential targets listed in Table 3 contains proteins with different functions such as citrate synthesis, malate metabolism, kinase activity, and the processing of thymidylate.
| UniProt accession | Description | ID |
|---|---|---|
| A5DAK2 | Citrate synthase | CS |
| A5DES7 | Protein kinase | PK |
| A5DF16 | dTMP kinase | TMPK |
| A5DH28 | Malate dehydrogenase | MDH1 |
| A5DGY9 | Malate dehydrogenase | MDH2 |
| A5DE02 | Malate dehydrogenase | MDH3 |
| A5DPY2 | Thymidylate synthase | TS |
During molecular docking calculations, two possibilities were explored for TS. One of these included the presence of the dUMP cofactor, while docking in the absence of any substrate was considered too. Docking experiments were conducted on 6 compound 6-protein complexes with aggregated scores larger than 1 to be further analyzed. All complexes show meaningful ligand-receptor interactions, with the chemical occupying the binding cavities. The docking scores obtained for these molecular docking solutions are provided as Supporting Information in Table S1.
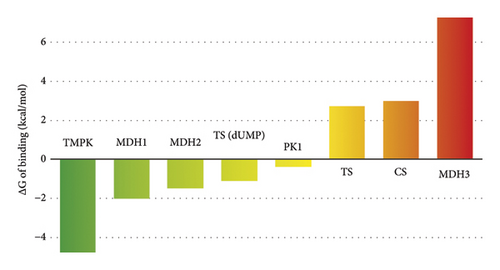
The docking-generated binding hypotheses were subject to MD simulations, from which the free energies of binding were computed. The obtained binding energies are provided in Table S2 of the Supporting Information file and represented in Figure 4. Only the best (lowest) binding energy per target is included in the figure. The MD-based free energies of the binding show that the most probable target of compound 6 in C. guilliermondii is the TMPK protein, followed by two malate dehydrogenases (MDH1 and MDH2) and TS in the presence of dUMP. To get additional insights into the potential binding of compound 6 to TMPK, its predicted binding mode to this enzyme was further analyzed.
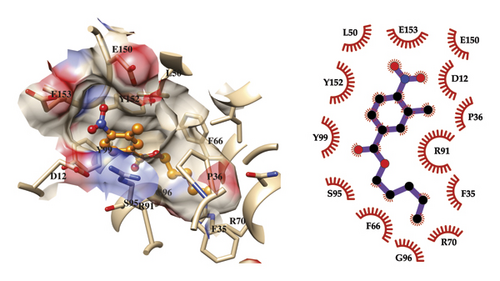
The predicted binding mode of compound 6 to TMPK and the observed network of ligand-receptor interactions are presented in Figure 5. The represented ligand conformation was selected by clustering the 100 MD snapshots employed for the free energy of binding calculations. Our model shows that the complex is mainly stabilized by hydrophobic and van der Waals interactions, with the pentyl tail at the bottom of the cavity. The region that interacts with this part of the ligand includes F35, P36, F66, R70, and G96. The carbonyl oxygen of the central ester linker orientates toward S95. On the other hand, the substituted benzene ring occupies the entrance of the binding cavity. This moiety stacks perpendicularly to Y99 and Y152, while also making contacts with the side chains of D12, L50, R91, E150, and E153.
TMPKs are involved in the synthesis of DNA in the cell through the production of thymidine diphosphate. This step is essential for DNA replication and repair processes and its inhibition can lead to cell death. This characteristic has been leveraged in the design of therapeutic agents targeting TMPK in various human pathogens. In the case of Candida species, no inhibitor of TMPK has been reported in the scientific literature. However, this protein is a validated molecular target for the development of antifungal agents as it is essential for the growth of C. albicans [75, 76].
This previous research, together with the modeling results, suggests that TMPK could be the principal target of compound 6 in C. guilliermondii, potentially contributing to fill the gap in the discovery of TMPK inhibitors against Candida fungi. The results herein presented will be a valuable tool for designing additional experiments that could fully clarify the antifungal mechanism of action of nitrobenzoic acid derivatives. Moreover, modeling results can guide the synthesis of additional derivatives with improved inhibitory potency against TMPK. For example, modifications that exploit the hydrogen bonding potential of the acid residues at the entrance of the binding pocket could be introduced in newly synthesized derivatives. Another aspect that deserves attention in future investigations is the selectivity of nitrobenzoic acid derivatives against Candida TMPKs, since there exists the possibility of binding to off-targets that also bind nucleotides. Any future optimization campaign should pay attention to this later aspect to avoid undesirable binding to other proteins.
4. Conclusion
All the 11 prepared nitrobenzoate derivatives presented bioactivity against at least one of the assayed Candida strains. The derivatives methyl 3-methyl-4-nitrobenzoate (1) and pentyl 3-methyl-4-nitrobenzoate (6) showed the best results for the strain of C. guilliermondii 207. Further, the bioactivity of compound 6 was slightly higher as well as it presented bioactivity against three of the four tested strains (C. guilliermondii 207, C. krusei 34,125, and C. albicans 90,028).
The SAR preliminary revealed the importance of alkyl side chains to potentiate the antifungal activity of this series against the strains under evaluation. In contrast, the insertion of benzyl groups or terpenic substituents does not influence the antifungal effect. The modeling studies suggest that compound 6 can interact with the TMPK protein, a molecular target for the development of antifungal agents, and that from the synthesis of new derivatives, it is possible to explore different interactions, such as hydrogen bonds. Thus, our findings suggest that the structure of the obtained nitrobenzoate derivatives needs to be further optimized to afford more insight into the structure-activity relationship of this class of compounds as potential antifungal agents. From the results obtained, it is possible to prepare larger collections of compounds with greater structural diversity to optimize biological action. The planning of potentially bioactive molecules to be synthesized can be carried out based on simulations of the interaction with biological targets via molecular docking. In this way, the possibility of synthesizing compounds with a better antifungal profile will increase.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work received financial support from Brazilian government agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Acknowledgments
This work was supported by the Brazilian agencies: National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). The authors declare that most of the data in this article are available in the dissertation [69] free of charge from https://repositorio.ufpb.br/jspui/handle/123456789/13649.
Supporting Information
Chemical data of synthetic derivatives. Tables S1 and S2 potential targets of compound 6.
Open Research
Data Availability Statement
The spectroscopic and spectrometric data used to support the findings of this study are with the author by correspondence.




