Advancing Sustainable Gamma-Valerolactone Synthesis Using Homogeneous Catalysts: Catalytic Strategies, Mechanistic Insights and Transition to Eco-Friendly Biomass Processing
Abstract
Gamma-valerolactone (GVL) emerges as a pivotal platform chemical derived from the reduction of levulinic acid (LA), a hydrolysate of lignocellulosic polysaccharides. GVL is recognized as a viable substitute for fuels and chemicals generated from fossil fuels due to its properties. The reduction of LA to GVL in the presence of organometallic catalysts is discussed in detail in this review, including the challenges and opportunities associated with this catalytic transformation process. The organometallic complexes utilized for LA reduction are the main topic of this review, focussing on the catalytic superiority of Ir complexes over their Ru and Fe counterparts. The review investigates the feasibility of replacing noble metal complexes with cost-effective and abundant alternatives such as Fe, to enhance the ecological sustainability and cost competitiveness of GVL and its derivatives in comparison with traditional fossil-based alternatives. Furthermore, the review discusses the mechanistic intricacies associated with the transformation of LA to GVL, elucidation of the rate-determining steps and ideal hydrogen sources for this catalytic transformation. This review provides vital insights into the ongoing efforts aimed at making GVL manufacturing environmentally friendly and economically feasible by providing a complete exploration of various catalytic systems and sustainable management of biomass.
1. Introduction
Since the discovery of fossil fuels—namely coal, crude oil and natural gas—human civilization has undergone tremendous socioeconomic and industrial advancements. These energy sources have powered communication, transportation and global trade, driving development across nations. However, this progress has come with a cost. The global demand for fossil fuels has increased dramatically, with consumption rising by over 50% since 1980 and projected to exceed 600 × 1015 Btu by 2030 [1, 2].
This growing dependency on a finite, nonrenewable resource poses significant challenges. Fossil fuels are not only depleting but also unsustainable in terms of their environmental impact. Their combustion is the primary contributor to greenhouse gas emissions, including CO2, methane, nitrous oxides and sulphur oxides—key drivers of global warming and climate change. The rising cost of fossil-based fuels and the socioeconomic crises associated with energy shortages further underscore the urgency to find viable alternatives [2]. Renewable energy sources such as hydroelectric, geothermal, solar and wind energy have been introduced as substitutes, slightly offsetting fossil fuel usage as portrayed in Figure 1. However, these technologies come with limitations, including high capital investment, land use demands, energy storage issues and geographic dependency [3]. Furthermore, these systems cannot fully replace fossil fuels, as they do not offer the carbon backbone required for synthesizing many essential chemicals [4]. In 2011, 87% of the world energy needs were still supplied by fossil energies, showing that great efforts are needed to reduce the fossil fuel dependency [3].
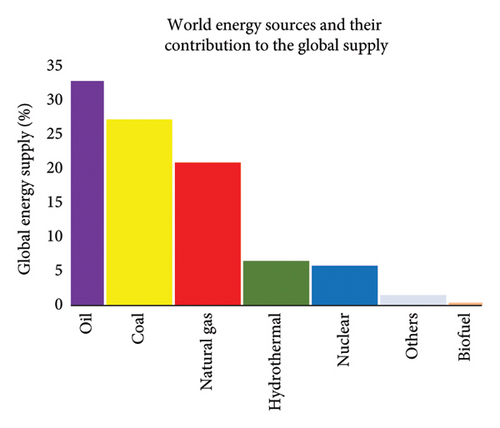
In this context, biomass has emerged as a compelling solution. Derived from living organisms—plants, animals and microorganisms—and their by-products, biomass represents the only renewable and carbon-rich alternative to fossil fuels [5, 6]. Unlike other renewables, it can be converted into both energy and valuable petrochemical commodities. In particular, the utilization of biomass waste from agriculture and forestry not only mitigates waste disposal issues and greenhouse gas emissions but also lowers fuel production costs [1, 2]. One of the most promising directions in biomass valorization is the production of bio-based platform chemicals, especially gamma-valerolactone (GVL) [7–9]. GVL is a renewable compound gaining attention for its role as a green solvent, fuel additive and liquid fuel. It offers numerous advantages, including high boiling point, low toxicity, full biodegradability and compatibility with existing fuel infrastructure, making it an ideal candidate to displace fossil-derived chemicals [7, 9, 10]. GVL is synthesized from levulinic acid (LA), which in turn can be derived from lignocellulosic biomass (LCB)—a cheap and abundant feedstock composed of cellulose, hemicellulose and lignin [6, 7, 10]. LCB contains fermentable sugars such as glucose and xylose, which can be transformed through hydrolysis and dehydration to produce LA [4, 11]. Utilizing LCB for GVL production supports a circular economy, enhances energy security and contributes to environmental sustainability. The catalytic conversion of LA to GVL can proceed via gas-phase or liquid-phase hydrogenation [4]. While gas-phase routes often involve expensive and thermally stable catalysts under harsh conditions, liquid-phase hydrogenation is more economically feasible. This pathway enables the use of less energy-intensive processes and more cost-effective catalysts [2, 4, 7]. Traditionally dominated by heterogeneous catalysis, this approach still faces challenges including low product selectivity and catalyst deactivation [12–14]. In contrast, homogeneous catalysis—especially using organometallic complexes of ruthenium (Ru), iridium (Ir) and iron (Fe)—offers promising solutions [15, 16]. These catalysts provide higher tunability, allowing for greater selectivity and efficiency in the transformation of LA to GVL. Yet, despite their potential, homogeneous catalytic systems remain underexplored in this context. Therefore, this review aims to examine the catalytic transformation of LCB to GVL using homogeneous catalysts. It will explore the physicochemical advantages of GVL, the significance of LCB pretreatment and fractionation, and the need for improved catalytic systems for LA production to aid in the commercialization of bio-derived GVL. Special emphasis is placed on the role of Ru-, Ir- and Fe-based homogeneous catalysts in achieving efficient hydrogenation pathways, ultimately supporting scalable and sustainable GVL production from lignocellulosic feedstocks [17, 18].
2. Structure of Lignocellulose
LCB structure is made up of cellulose (30%–50%), hemicellulose (15%–30%) and lignin (15%–30%) as the major components [17, 18]. There are also proteins, waxes, ashes and inorganic salts, though in low concentrations (Table 1).
| Lignocellulosic material | |||||
|---|---|---|---|---|---|
| Plants | Cellulose wt% | Hemicellulose wt% | Lignin wt% | Other wt% | |
| Grasses | Sugarcane bagasse | 46 | 27 | 23 | 4 |
| Wheat straw | 38.2 | 24 | 23.4 | 14.4 | |
| Rice straw | 34.2 | 24.5 | 11.9 | 29.4 | |
| Corn stover | 35.6 | 22.1 | 12.3 | 30 | |
| Hardwood | Birch | 38.2 | 19.7 | 22.8 | 19.3 |
| Willow | 43 | 29.3 | 24.2 | 3.5 | |
| Stems | 40–55 | 24–40 | 18–25 | — | |
| Softwood | Spruce | 43.4 | 18 | 28.1 | 10.5 |
| Pine | 46.4 | 22.9 | 29.5 | 1.2 | |
| Stems | 45–50 | 25–35 | 25–35 | — | |
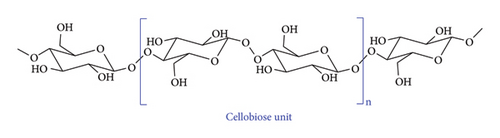
Cellulose is a linear homo-polymer made of D-glucose units, hence a glucan. This is the most abundant component of LCB in almost all plant species. The D-glucose units are joined together by glycosidic ester linkage in a β-1-4 fashion forming a dimer called cellobiose (Figure 2) [19]. Cellobiose units join to form linear microfibril chains which are further linked together by hydrogen bonds to form cellulose fibres. The resulting structure is highly crystalline due to the strong secondary interactions and linearity of microfibrils which affords uniform packing and reduction in free volume [20]. A single microfibril can contain thousands of glucose units, making cellulose an attractive component of LCB for the production of fuels and chemicals. Hemicellulose is another polysaccharide found in lignocellulose biomass and the second abundant component after cellulose. It is a heteropolymer made up of pyranose and furanose sugars, xylose, mannose, arabinose glucose and galactose, among others joined mainly by β-1-4 (Figure 3). This causes variations in hemicellulose structural content. Hemicellulose polymers are grouped into general classes (xylans, mannans, xyloglucans and mixed-linked β-glucans) based on the type of sugars making the main structural chain [21].

Since most of the hemicellulose polymers are highly branched and amorphous, the polymer easily dissolves in water, alkali and acid media with the latter leading to degradation. The difficulty in valorizing this component of LCB is also due to tightly wounded hemicellulose to cellulose and the lignin to the holocellulose complex making it difficult to access the cellulose structure.
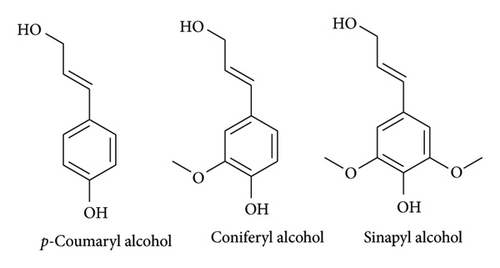
Lignin is a random-branched heteropolymer part of lignocellulose consisting of mainly 4-hydroxypropanol units called monolignols (Figure 4) [22, 23]. These monolignols are joined together by different linkages (acetyl ester, ether and carbon–carbon) at random positions making the structure complicated and diverse [22].
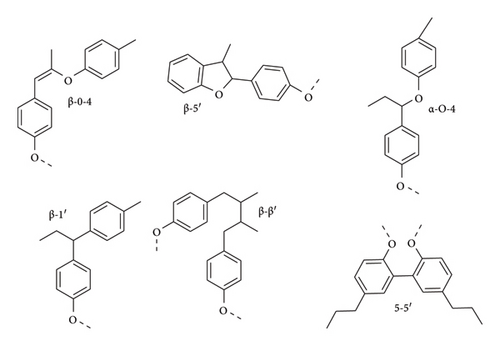
These linkages arise from different reacting sites of 4-hydroxypropanol radicals (resonance forms) produced by oxidizing enzymes (peroxidase or laccase) from the amino acid phenylalanine in the cytosol through the 4-hydroxyphenylpropanol pathway (Figure 5) [22]. These radicals randomly form complex and varied aromatic polymer chains through crosslinking. Cross-coupling of these monomers mostly occurs through oxygen and carbon free radicals, resulting in an ether linkage (C-O-C), and also through carbon radicals forming carbon–carbon linkages through a condensation reaction. It is important to note that the β-O-4 linkage is the dominating linkage in lignin structure and is also easily degradable.
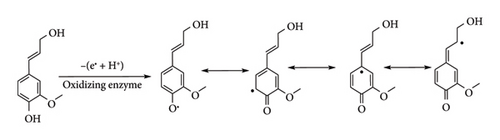
The variation in linkages results in three different monolignols with different relative abundancy in a specific plant species, hence different types of woods (hardwood, softwood and grass). Softwood lignin consists largely of guaiacyl and small quantity of p-hydroxyphenyl units which are derivatives of coniferyl alcohol and p-coumaryl alcohol, respectively [23, 24]. Hardwood is made up mostly of guaiacyl-syringyl (GS) units though trace amounts of p-hydroxyphenyl units can be isolated from it. Lastly, grass is made up of all the three monolignols together with aromatic acid residues. The native structure of lignin is still not known up to this day, as there is no perfect method to isolate lignin from the plant without causing structural change. The structure of lignin polymer not only depends on the wood species but also on extraction methods such as milling, organosolv, soda pulping and kraft. These techniques cause changes in the native structure of lignin by converting C-O-C to C-C through hydrolysis and condensation reactions.
3. Valorization of Lignocellulose
The complexity and recalcitrance of lignocellulose has motivated many researchers to develop better technologies to process it for the production of fuel and chemicals. As of present, there are two routes for biomass processing, being thermal processing and aqueous-phase processing as depicted in Figure 6. Thermal phase processing includes techniques such as pyrolysis and gasification, which thermally degrade biomass to intermediate molecules which are further transformed to fuels by catalytic systems [3]. The gasification converts solid biomass in the presence of gasifying agents such as air, steam and CO2 into mixture of gases. A common mixture is H2/CO which is normally termed synthesis gas (syngas) including CH4 and CO2 in small amounts. These gases are then converted to liquid fuels through the Fischer–Tropsch (FT) process. Gasification is a clean combustion and thermally efficient process, but it is an overly sensitive and complex to run as a continuous process.
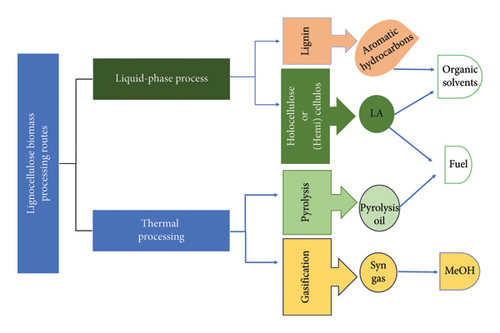
Pyrolysis is employed to convert biomass into bio-oil in the presence of syngas and biochar but in the absence of oxidizing agents [3]. The bio-oil produced by this method has poor quality, is complex and unstable to be used in the existing combustion engines and needs catalytic transformation making the process more expensive. Furthermore, the production of biofuels through this route is expensive. Therefore, much work must be done to reduce technoeconomic barriers faced using the pyrolysis process. Liquid/aqueous-phase process on the other hand offers an alternative way to get liquid fuels from biomass at relatively mild temperatures (≈200°C). This process is sometimes termed hydrolysis/solvolysis, as it employs solvents to fractionate lignocellulose prior to conversion to fuels or chemicals [3]. Liquid-phase processing enables amalgamation of different biomass conversion procedures and technologies to attain complete valorization of lignocellulose at low energy and mass loss in one facility (biorefinery). Such facilities are desirable as they maximize overall production chain value as fuel, power and platform chemicals can be obtained from biomass with minimal cost [1].
Biorefinery processing of lignocellulose is performed in three stages which are primary fractionation, secondary fractionation and catalytic upgrading of biomass (Figure 7). The primary fractionation stage separates lignocellulose into its individual components (lignin, hemicellulose, cellulose and ashes) through depolymerization reactions [6]. The process utilizes conversional techniques such as solvent extraction, distillation, filtration and supercritical solvents (H2O, ethanol and CO2) and catalysts [5, 25, 26]. The ability to separate LCB components offers a great advantage over the thermal process as high biomass conversion, and product selectivity can be attained.
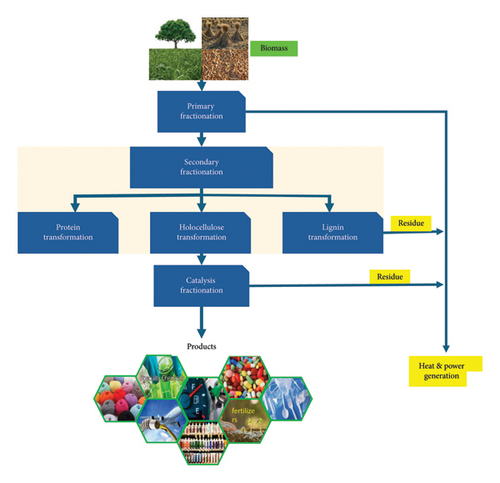
The polymers and intermediate compounds obtained from the primary stage are further processed into end products or platform molecules using thermochemical and biochemical techniques in the secondary stage [1]. Figure 7 depicts both end products and platform chemicals produced from each LCB fraction. For example, lignin is degraded into aromatic molecules such as phenol, vanillin, guaiacol, toluene and benzene, which are common organic solvents, pharmaceutical precursors and fuel additives [14]. On the other hand, holocellulose fraction can be used to produce fuels, nonaromatic acids, alcohols (bioethanol and bioethanol), ketones and aldehydes which are used in our everyday life in cosmetic, food, petrochemical and pharmaceutical industries [2, 5, 27].
In the last stage, platform chemicals are catalytically converted to more value-added compounds such as alkanes, aromatics and esters which are used as fuel (additives), pharmaceutical agents and cosmetics [7, 10]. In this stage, holocellulose fraction of LCB can be converted to GVL, a platform chemical to produce fuel-grade hydrocarbons such as butene. The residues from each stage can also be used as heat sources and other instances used to produce electricity.
Along with LA, hydroxymethylfurfural (HMF), furfural (FF) and furfural alcohol (FFA), which are thought to be potential biofuels and precursors for the synthesis of various organic compounds, GVL has been identified as one of the platform chemicals that can completely replace petrochemical feedstock in the future [7]. Therefore, this work aims to review the synthesis of GVL from LCB in the presence of homogeneous catalysts. First and foremost, a thorough explanation and discussion of GVL’s unique properties that make it an excellent chemical to replace fossil fuel feedstock in the fuel and petroleum industries are provided. This is followed by catalytic conditions for the treatment of lignocellulose for the production of LA, a GVL precursor and mechanistic pathways for the formation of the active species. Lastly, the review will discuss the catalytic transformation of LA to GVL in the presence of Ru, Ir and Fe complexes as catalysts precursors and the mechanisms involved.
4. Preparation of Lignocellulose for GVL Synthesis
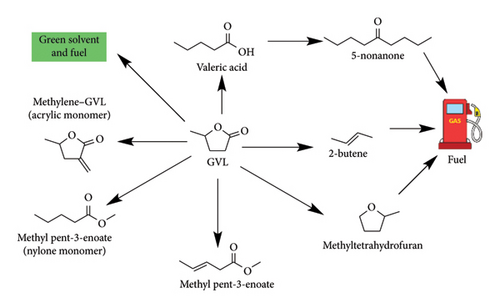
Currently, bioalcohols such as bioethanol are adopted as alternative fuels and green solvents to petroleum products in organic synthesis and cosmetics [7]. These chemicals are associated with high flammability (low flash point = 13°C) and are unstable in the presence of air, making it difficult to transport and store them in large quantities. This inspired research for alternative biofuels such as GVL. GVL is a penta-heterocyclic organic ester which exists as a colourless liquid at standard conditions. Table 2 shows interesting properties of GVL such as high boiling point, low melting point and high flash point, which makes it a desirable fuel additive, green solvent and liquid fuel comparable to ethanol [2, 8]. GVL has high water solubility and pleasant herbal aroma and is naturally produced by fruits (nontoxic), making it suitable for making perfumes, food additives and cosmetics. Different intermediates and platform compounds such as nonanone, alkanes, valeric acid, pentenoic acid, methyltetrahydrofuran (MTHF), methylene–GVL and pentanoates can be synthesized from a racemic mixture of GVL (Figure 8) [2, 8, 9].
| Terms | Ethanol | GVL |
|---|---|---|
| M (g mol−1) | 46.07 | 100.12 |
| Carbon (wt%) | 52.2 | 60 |
| Hydrogen (wt%) | 13.1 | 8 |
| Oxygen (wt%) | 34.7 | 32 |
| Boiling point (°C) | 78 | 207 |
| Melting point (°C) | −114 | −31 |
| Flash point (°C) | 13 | 96.1 |
| Density (gmL−1) | 0.789 | 1.0485 |
| Solubility in water (mg/mL) | Miscible | ≥ 100 |
| Octane number | 108.6 | — |
| Cetane number | 5 | — |
| dHvap (kJ mol−1) | 42.59 | −54.8 |
| dcH◦ liquid (kJ mol−1) | −1367.6 ± 0.3 | −2649.6 |
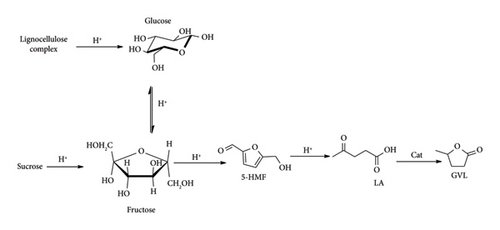
GVL is synthesized from LA, which is a sugar derivative as depicted in Figure 9. Currently, all the commercially produced LA depends on feedstock (starch and sucrose) derived from edible crops, causing competition and conflict between food production and energy. To resolve this issue, adoption of nonfood lignocellulose supplies such as agricultural waste, newspaper, pulp sludge, municipal waste and industrial wood waste is encouraged as they are rich in glucose [6]. This is also viewed to be economic as there is no need to invent a new plant (biorefinery) as glucose is a common intermediate in both edible sugar and LCB–LA production process as depicted in Figure 9.
5. Treatment of Lignocellulose for LA Production
Valorization of lignocellulose biomass for LA production requires an extra step of pretreatment compared to simple sugars due to its complexity and resistance to microbial and chemical agents. Lignocellulose biomass must first go through a fractionation process to separate undesirable lignin (aromatic polymer) from holocellulose and (hemi)cellulose and destroy the crystalline phases of cellulose to enable the release of sugars [2]. Pretreatment of biomass for LA production is commonly carried out in acidic conditions due to the desirable properties offered by the process. The acid acts as a solvent to dissolve lignin and also as a catalyst to cleave the lignin–carbohydrate bond (acetyl bond) and the glycosidic bonds between hemicellulose sugars [28]. This releases oligomers and simple sugars such as xylose, mannose, glucose and arabinose, as depicted in Figure 10 [11].
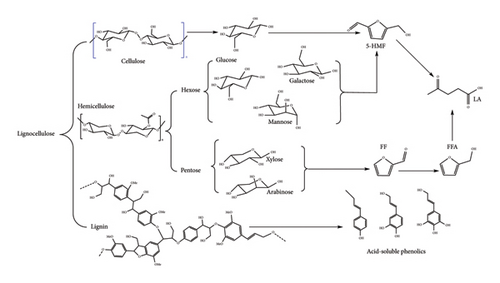
At this stage, cellulose structure is barely destroyed due to its high crystallinity, inhibiting acid attack (less free volume); hence, simple techniques like filtration can be used to separate the fractions. Cellulose is then mixed with dilute acid for a prolonged time to access the crystalline domains, while the amorphous regions are hydrolysed to glucose. The duration of this step is longer than the first as it is coupled with the hydrolysis of sugars to LA. Glucose, together with hexose derived from hemicellulose, is then converted to 5-HMF which is then degraded to LA and FA [7]. The pentose sugars are catalytically converted to FF which is then degraded to LA [11]. Acid pretreatment method is desirable as it promotes one-pot LA synthesis and utilizes the existing biorefinery set-up [29]. This process produces two types of lignin, namely acid-soluble and acid-insoluble lignin. The acid-insoluble lignin is formed due to re-polymerization and condensation reactions of lignin fragments during hemicellulose hydrolysis. This lignin is unreactive in an acidic environment due to the formed C-C bonds and hence allows hydrolysis of glucose with minimal contamination or side reaction promotion [30]. Table 3 shows the cellulose source and catalytic conditions to produce LA.
| Cellulose source | Conditions | LA yield (%) | Reference |
|---|---|---|---|
| Cellulose | H2SO, 2 h, 150°C | 60 | Girisuta [1] |
| Cellulose | H2SO4, 6 h, 150°C | 60 | Serrano-Ruiz et al. [19] |
| Bagasse and paddy straw | HCl, 220°C | < 83 | Yan et al. [5] |
| Water hyacinth plant | H2SO4/H2O, 0.5 h, 175°C | 53 | Girisuta et al. [31] |
| Cellulose | H2SO4/sulfolane/H2O, 1 h, 180°C | 73 | Wang et al. [29] |
| Cellulose | HCl/NaCl/GVL, 1.5 h, 155°C | 70 | Wettstein et al. [10] |
| Corncob |
|
|
Gurbuz et al. [28] |
| Poplar wood | PTSA/H2O, 1 h,162°C | 58 | Ji et al. [32] |
| Corncob | PTSA, 160°C, 0.75 h, then 180°C, 0.67 h | 61 | Ji et al. [33] |
| Cellulose | CrCl3/H2O, 3 h, 180°C | 67 | Peng et al. [26] |
| Wood pulp | CrCl3/H2SO4, 160°C, 1 h microwave heating | 56 | Lappalainen et al. [11] |
| Corncob | AlCl3/NaCl/H2O, 2 h, 180°C | 56 | Li et al. [20] |
Strong mineral acids, such as sulphuric acid and hydrochloric acid, are mostly used to delignify lignocellulose biomass due to their high dissociation (pKa < 2) values, giving high LA yield. For instance, Girisuta et al. [34] and coworkers demonstrated that cellulose can be hydrolysed to LA in the presence of sulphuric acids, giving 60% yield in less than 2 h at 150°C in the absence of solvents. In the following year, the same group showed that sulphuric acid can catalyse hydrolysis of lignocellulose biomass to LA in the presence of water hyacinth plant and water as a solvent, obtaining a yield of 53% in half an hour at 175°C [31]. Serrano-Ruiz et al. [19] reported a similar yield under similar conditions, though their system required a prolonged reaction time of 6 h. In a different study, pretreating eucalyptus at 150°C with 0.1 M sulphuric acid was observed to increase the glucan content of the biomass by 1.4 times, thus providing the LA yield [30]. HCl has also been reported by Yan et al. [5] to be an efficient catalyst for the hydrolysis of bagasse and paddy straw with an observable LA yield not higher than 83% at 220°C.
Two-step pretreatment using liquid hot water (LHW) followed by acid hydrolysis has proven effective for converting LCB into LA. In this process, the LHW step primarily solubilizes hemicellulose into simple sugars, while the subsequent acid hydrolysis breaks down holocellulose into LA. For instance, Rivas et al. [35] employed this strategy using Pinus pinaster wood, initially treating the biomass with hot water at 175°C, followed by sulphuric acid hydrolysis at 135°C, achieving an LA yield of 66%. Similarly, Madadi et al. [36] applied LHW pretreatment at 200°C for 40 min, which resulted in 91% hemicellulose solubilization and produced a glucan-rich solid with high cellulose accessibility. Subsequent heating of the hydrolysate at 190°C for 80 min in the presence of sulphuric acid yielded 72.49% LA.
The use of biphasic systems has also been shown to be effective in lignocellulose hydrolysis and increasing LA yield. Wang et al. [29] implored sulfolane/water solvent system to achieve full conversion of cellulose and 73% LA selectivity within 1 h at 180°C in the presence of H2SO4. Another improved yield was reported by Wettstein and coworkers [10] who carried out a HCl/NaCl/GVL biphasic system to achieve 70% LA yield from cellulose in less than 1.5 h at 155°C. This high catalytic activity was reported to be due to the high solvation properties and extraction efficiency of GVL. Since GVL is an organic solvent, extraction was more than 75% of hydrolysis products, LA and FA, from the aqueous phase, which inhibited side reactions. These reaction conditions are an example of an effective biphasic system for biomass hydrolysis to LA. Gurbuz and coworkers [28] managed to hydrolyse hemicellulose from corncob hemicellulose to xylose, which was then converted to LA in the presence of H2SO4 as a catalyst with SBP solvent, obtaining 72% yield in 2 h. They also demonstrated that other organic solvents such as PG and NHP can be employed for the process, though obtaining a slightly lower yield of 60%. Another study by Deng et al. [37] also reported acid hydrolysis of microcrystalline cellulose and α-cellulose to LA in the presence of HCl (0.8 M) and obtained low yield. Muranaka et al. [38] demonstrated that 59% LA can be obtained in the presence of high concentrated HCl (20 wt%). The production of LA either from simple sugars or complex lignocellulose polymers via mineral acid hydrolysis is reported to be accompanied by formic acid (FA) (hexose), water and insoluble compounds called humins as by-products [39]. FA is organic liquid which can be separated from the product together with water by fractionation process. Humins are polymeric materials resulting from aldol condensation of HMF and sugars. These compounds are undesirable as they reduce the product yield of LA. As of present, many protocols aimed at significantly reducing or inhibiting their formation are under development such as the use of biphasic systems and Lewis’s acids. The use of sulphur-containing acids is deemed unfavourable to use in the one-poor synthesis of GVL.
An alternative approach is the adoption of organic acids to hydrolyse LCB biomass to LA which can be effectively converted to GVL without the need for purification. Organic acids such as p-toluene sulphonic acid (PTSA) have also been reported to have high hydrolysis of lignocellulose biomass, such as corncob, to LA. For example, powdered poplar wood was hydrolysed using 0.95 M PTSA, yielding 58% LA in 64 min at 162°C in the presence of water as a solvent [32]. In another study, Ji et al. [33] demonstrated that LA can be synthesized from lignocellulose biomass in a one-pot synthesis, eliminating the need for extra steps, which are the removal of FF and lignin. The group observed that the synthesis temperature for FF and LA had a 50°C temperature separation, which allowed a two-step temperature synthesis protocol in the same batch reactor. They first hydrolysed corncob’s hemicellulose to FF at 160°C in 45 min using PTSA as the catalyst and converted it to GVL through the same catalytic pathway. They then increased the temperature to 180°C, promoting LA formation. This method provided advantages such as easy removal of FF by simple distillation before LA synthesis, as it is synthesized at its boiling point. It is also worth noting that FF can be converted to LA through metal catalysts, hence promoting the full utilization of biomass fraction. The group reported that a 61% LA ester (LE) yield was observed in this system as alcohols (C4–C8 alcohols) were used as extracting agents. The use of alcohol as an extracting agent is beneficial as it promotes the recovery of the catalyst, and the LE produced can be easily converted to GVL through the same LA pathway.
Lewis acids present another interesting catalytic system for LA production from LBC due to their ability to catalyse the isomerization of glucose to fructose. These acids are also advantageous as their hydrolysis yields Lewis acid [metal cation (Lewis acid)] and anions [Bronsted acid (anion)] which increases the acidity of the reaction mixture. This property of Lewis acids makes them ideal additives in the acid hydrolysis of cellulosic biomass processes, as their activity will be maintained. In 2010, Peng et al. [26] studied the activity of different transition metals (CrCl3, CuCl2 and FeCl3) and main group metals (Li, Na, K and Al) on the hydrolysis of cellulose to LA in the presence of water as solvent. Lewis acids were reported to have high catalytic activity compared to alkali metals, with CrCl3 being the best, obtaining a yield of 67% in 3 h at 180°C. Under the same experimental conditions, the activity of the transition metals was reported to increase in the order CuCl2 < FeCl3 < AlCl3 < CrCl3.
Lappalainen et al. [11] demonstrated the stability of CrCl3 salt in the presence of H2SO4 co-catalyst for hydrolysis of Swedish fibre sledge from a wood pulp mill under obtaining 56% LA yield even after four cycles. In the same study, similar LA yields (≈56%) were observed when CrCl3 was substituted with other transition metal chloride salts such as ZnCl2, CuCl2, NiCl2 and FeCl3. This demonstrated that expensive and hazardous materials like chromium salts could be replaced by cheap and naturally abundant iron salts. The high catalytic activity of these salts is also an attribute of the chloride ions. Cellulose is known to be resistant to the chloride ion that helps in solubilizing cellulose by disrupting hydrogen networks, which swells the polymer and allows penetration of the acid catalyst (Figure 11). Li et al. [20] observed high conversions of corncob when AlCl3 (97%) was used compared to Al2(SO4)3 (40%), degradation due to its hydrophilicity and high crystallinity (hydrogen bonding) [20, 40].
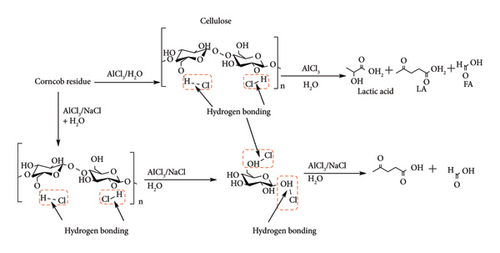
An increase in chloride concentration by the addition of NaCl, KCl and LiCl improved LA selectivity by more than 50%. Potvin et al. [40] also reported similar findings in the presence of NaCl as an additive at high temperature and pressure. A 5-fold increase in LA yield was obtained after the addition of NaCl when hydrolysing cellulose with Nafion (a solid acid catalyst). Li et al. [20] also demonstrated that changing the chloride ion with nitrites, carbonates, sulphates, bromides or fluorides either promoted the formation of lactic acid or inhibited cellulose hydrolysis, and hence, no LA yield was observed.
In fact, salts of carbonates and fluorides are deemed to make the reaction solution slightly alkaline, favouring C-C bond breakage of fructose, forming lactic acid via the retro-aldol pathway rather than LA. Due to the neutral nature of the chloride salts, they maintain the reaction pH, thus inhibiting side reactions [20]. The chloride ion is also believed to form strong hydrogen bonds with the C1 and C6 groups of both glucose and fructose, inhibiting retro-aldol reaction, hence promoting the formation of LA under metal chloride salts systems [20].
6. Mechanism for the Reduction of LA to GVL
The reduction of LA to GVL can be achieved through two pathways, namely gas (Path A) or liquid phase (Path B), with a catalyst as a pre-requisite in either case, as shown in Figure 12 [4]. In the gas-phase reaction, LA is converted to angelica lactone through reversible dehydration and cyclization of LA intermediate (pseudo-LA). Final step involves hydrogenation of C=C of angelica lactone yielding GVL. This path requires high temperatures to convert LA to gaseous phase prior to being catalytically reduced hence; thermally stable metal catalysts are a pre-requisite. Such catalysts are very expensive to prepare as they require a combination of precious metal and highly thermally stable supports which are rear or difficult to synthesize. Heterogeneous catalysts with low catalytic selectivity and tunability are the only option for GVL synthesis from biomass. The use of high temperature also promotes coking which causes catalyst deactivation [12]. Hence, make the gas-phase reactions expensive to be carried out in biorefineries.
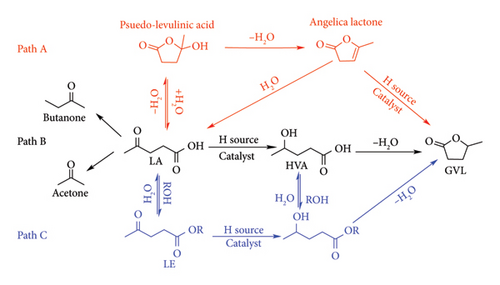
Compared to gas-phase reactions, the liquid phase offers ability to reduce LA to GVL without the need to first convert it to vapour, hence reducing energy costs. As shown in Figure 12, this path involves hydrogenation of the keto-group of LA forming 4-hydroxyvaleric acid (4-HPA) which spontaneously self-esterifies to GVL [4]. The reduction of LA in the liquid phase offers great advantages, such as the use of inexpensive metal such as Fe, Cu, Ni, Al and Zn as heterogeneous catalysts and metal complexes which are more selective and easily tunable [15, 41–43]. It also allows the use of additives such as acids, bases and alcohols to improve the activity and selectivity of the catalysts. The use of alcohols as solvents has also been pointed out to offer great advantage as they convert LA to LE, which is easier to hydrogenate through Path C [43].
This process has been dominated by heterogeneous catalysts due to their high activity, thermal stability and recyclability, as well as being compatible with common solvents. Though heterogeneous catalyst offers great advantages, such systems still require high operational temperatures and pressures and have low selectivity towards the product of interest [4, 44]. Over-reduction of LA to undesirable products such as 1,4-pentadinol and 2-MTHF is due to these harsh reaction conditions. In fact, 2-MTHF is considered hazardous as it can easily get oxidized in the presence of air, forming radicals even at ambient conditions [45]. They also present limited catalyst tunability, as few metals and supports have desired properties, hence prompting the development of homogeneous catalysts.
Homogeneous catalysts offer easier tuning of the catalyst to attain desired properties for a specific reaction or product, hence increased product yield [42, 46]. Moreover, this can be easily achieved as there is a pool of ligands and metal precursors already available or can be synthesized from renewable sources such as biomass. In the liquid-phase reactions, homogeneous catalysts play an important role in abstracting hydrogen from its source and using it to reduce LA to the 4-HVA intermediate [15]. This is achieved through two routes, namely direct hydrogenation and transfer hydrogenation, which are categorized by the formation of metal hydride species [41].
Direct hydrogenation follows a conversional molecular hydrogenation activation path to form either M-H2 or M-H, where M is the metal centre and H is the hydrogen [16]. The generation of metal hydride is generally observed to follow three steps as depicted in Figure 13. The first step involves ligand dissociation from the metal complex to create a vacant site that hosts the reductant molecule in the next step [41]. The success of this step depends not only on the presence of an easily liable ligand on the metal centre but also on external factors such as temperature, solvents and additives [45, 47, 48].
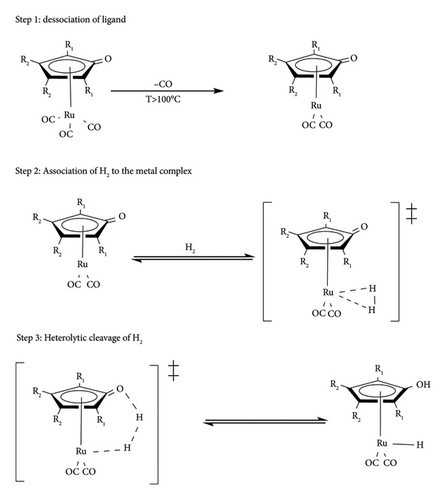
If the right conditions are not met, there could be reduced catalytic activity or no catalytic activity due to failure to activate the metal complex [41, 42]. In the second step, molecular hydrogen gets associated with the metal, forming metal dihydrogen species. Finally, the metal dihydride species gets into a transition state (TS) where molecular hydrogen is homolytically cleaved to form metal hydride species. In the presence of the Shvo catalyst and the iridium bipyridylamine precatalysts, the cleavage of the dihydride complex is shown to be assisted by additives and solvents (water, ethanol and conjugate bases) or LA (Figure 14) [41, 46, 49]. Such assisted hydrogen cleavages were observed to be thermodynamically favoured due to lower activation energy compared to dry cleavage [41, 49].
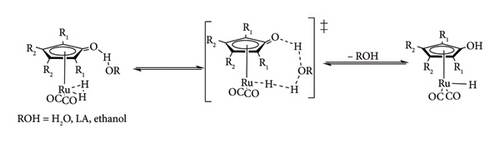
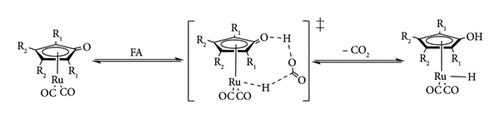
Under the transfer hydrogenation pathway, metal hydride species are formed using surrogate donors such as alcohols, organic acid and their conjugate bases as hydride sources [41, 42]. The most commonly used donor molecules are FA, sodium formate and isopropanol (IPA). The catalytic hydrogen transfer mechanism follows a similar route to the direct hydrogenation one, the only difference being the use of donor molecules, which go through a heterolytic cleavage process (Figure 15). The cleavage of donor molecules results in the formation of metal hydride and by-products such as CO2 or acetone when FA and IPA are adopted, respectively [41]. Transfer hydrogenation is increasingly attracting interest over direct hydrogenation, primarily due to its enhanced safety and long-term feasibility. Alcohols and organic acids serve as stable and nonexplosive hydrogen carriers, offering a safer alternative to gaseous hydrogen for storage and handling. Moreover, many of these compounds can be co-produced from biomass, reducing reliance on fossil-derived hydrogen and contributing to both economic viability and environmental sustainability [9].
7. Synthesis of GVL From LA Using Ruthenium (Ru) Complexes as Precatalysts
In recent years, there has been growing interest in the development and use of homogeneous catalytic systems for hydrogenation of LA to GVL. Of these, Ru complexes have shown a potential to offer excellent selectivity and efficiency in the conversion of LA to GVL at high yields under both transfer and direct hydrogenation pathways summarized in Table 4.
| Catalyst | Substrate/H2 source | Conditions | GVL yield (%) | Other products (%) | TON (TOF/h−1) | Reference |
|---|---|---|---|---|---|---|
| [Ru (Cl2) (p-cymene)]2 | H2O/LA/H2 | H2O | 100 | — | 995 | [50] |
| Ru-1a | LA/FA | Et3N, 16 h, 150°C | > 87 | — | 1980 | [42] |
| Ru-1b | H2O/LA/H2 | 16 h, 130°C | 92 | — | — | |
| Ru-2a | LA/FA | KOH, 16 h, 120°C | 100 | — | — | [51] |
| Ru-3a | LA/FA | KOH, 16 h, 120°C | 90 | 10 (4-HVA) | — | |
| Ru-2a | LA/H2 | KOH, 16 h, 120°C | 94 | — | — | |
| Ru-3a | 100 | — | — | |||
| Ru (acac)3/POct3 | LA/H2 | 100 bar | > 99 | — | — | [45] |
| Ru (acac)3/DPPB | LA/H2 | NH4PF6, 18 h, 160°C | 89 | 11 (1,4-PDO) | — | |
| Ru (acac)3/triphos | LA/H2 | PTSA, 18 h, 160°C | 58 | 39 (2-MTHF) | — | |
| Ru-L10 | LE/H2 | 50 bar, PTSA, 22 h,140°C | 95 | — | 30,400 (1382) | [52] |
| Ru-L8 | LA/H2 | 50 bar Sc (OTF)2 96 h, 140°C | 73 | — | 73,142 | |
| Ru (acac)3/PBu3 | NH4PF6 | 100 bar, 8 h, 135°C | 37 | — | — | [47] |
| Ru (acac)3/TPPTS | H2O/LA/H2 | 69 bar, 12 h, 140°C | 95 | — | — | |
| RuCl3.H2O/PPh3 | LA/FA | Py or Net3, 6 h, 200°C | 95 | — | — | [35] |
| RuCl3.H2O/PPh3 | LA/FA/H2O | KOH or Py or Net3, 12 h, 150°C | 90 (48) | — | — | |
| RuCl3.H2O/(PCy3 or DPPE) | Py, 150°C | 80 | — | — | ||
| RuCl3.H2O/PPh3 | H2O/LA/CO2/H2 | Py, 150°C | 100 | — | — | |
|
LA/FA/H2O | Py, 150°C | 50 | — | — | |
| RuCl3.H2O/TPPTS | DCM-H2O/LA/H2 | 45 bar, 1.3 h, 90°C, | 81 | 100 (170) | [53] | |
| Ru (acac)3/PBu3 | LA/H2 | 100 bar, 0.75 h, 140°C | > 99.9 | — | 6370 | [48] |
| Ru (acac)3/Bu-DPPDS | LA/H2 | 10 bar, 4.5 h, 140°C, | > 99.9 | — | 6370 | |
| Ru-7b | FF/EtOH/H3PO4/H2 | 30 bar, 7 h, 100°C | 84 | — | — | [13, 54] |
| Ru-7b | Xylose/EtOH/H3PO4/H2 | 30 bar, 48 h, 125°C | 73 | — | — | [13] |
| Hemicellulose/EtOH/H3PO4/H2 | 75 | — | — | |||
| Ru (acac)3/DPPB | LA/H2 | 100 bar, 0.6 h, 160°C, | > 99.9 | — | 12561 (21233) | [55] |
| Ru (acac)3/BINAP | LA/H2 | 100 bar, 1.8 h, 140°C | 98.6 | — | 12561 (6978) | |
| Ru-9 and Ru-8c | LA/H2 | 100 bar, 5 h, 100°C | > 99 | — | — | [15] |
In a different study, Tay et al. [50] demonstrated that [Ru(Cl2) (p-cymene)]2 is an efficient hydrogenation catalyst of LA to GVL when used under aqueous solvents or biphasic systems, obtaining 100% GVL selectivity and turnover number (TON) of 995. It is also worth mentioning that the activity of this complex was similar to Ru/C heterogeneous system (TON = 952). This high activity of that [Ru (Cl2) (p-cymene)]2 was attributed to its ability to dissolve in water, forming Ru nanoparticles (Ru-NPs). The stability of the Ru-NPs improved when Ru (p-cymene) was complexed with imidazole, forming Ru–carbene complexes (Ru-1 and Ru-2), attaining TOF values as high as 493 h−1 in 120 min [50]. Ru-1 and Ru-2 complexes obtained a high conversion rate of LA to GVL compared to Ru (p-cymene) dimer, with bidentate complexes being the best (k = 5.90 × 10−5 M/s) [50]. The substituents on the imidazole nitrogen were found to have an effect on the activity of the catalyst, as high GVL yields were observed when alkyl-substituents were used instead of aromatics. Tay et al. [50] observed a 40% drop in GVL yield in the presence of Ru-2 with N-benzyl substituent instead of the isopropyl wing.
Jansen et al. [56] reported a remarkable 80%–85% conversion of LA and methyl levulinate to GVL at 70°C, 50 bar in less than 17 h using Ru-1b under basic conditions (KOtBu/THF). Westerhaus et al. [57] demonstrated that Ru (p-cymene) complexes have the ability to hydrogenate alkyl ketoacids such as LA to GVL, obtaining yield in the range of 62%–80% after 6 h in the presence of [Ru (Cl2) (p-cymene)]/bisimidazolium salts (L1 and L2) (Figure 16) under basic conditions (KOtBu) using 1,4-dioxane as a solvent with complex [Ru (Cl) (p-cymene) (L1)] being the best performing catalyst. A slight increase in GVL yield (5%) was also reported by Westerhaus et al. [57] using bisimidazolium salts with C6H11Me (salt 1) and benzyl (salt 2). Further loss in activity was observed in the presence of an alkyl chain bridging the imidazolium rings was increased and also by the substitution of one of the imidazole rings (bidentate complexes) with pyridine (< 60% GVL yield). On the contrary, Wang et al. [42] reported that the replacement of imidazole salts with bispyridylamine resulted in cationic Ru (p-cymene) (Ru-3 and Ru-4), with high stability and selectivity towards the reduction of LA to GVL as compared to carbene complexes. For example, Ru-4/H2O was reported to attain 92% GVL yield under direct hydrogenation of LA at low pressure (5 bar), though at prolonged reaction time (16 h), whereas Ru-3 was reported to catalyse the reduction of LA to GVL under transfer hydrogenation conditions, obtaining yield above 87% under solventless conditions in excess FA and base.
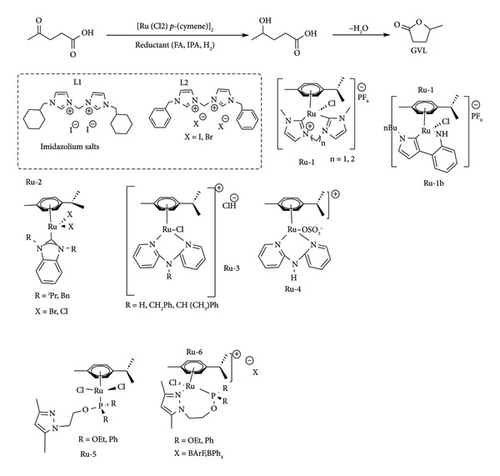
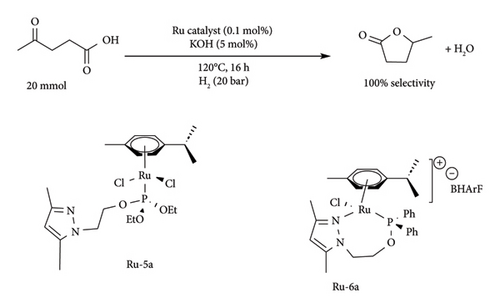
In this catalytic system, the substitution of the n-bridge had no effect on the catalytic activity of the complex as the complete conversion of LA to GVL yield was attained from both N-H and bulky N-CH (CH3) Ph-bridged complexes. Of these catalysts, the N-CH (CH3) Ph-bridged complex was reported to be stable and effective even at lower catalytic loading (0.05 mol%), achieving full conversion of GVL and a TON value of 1980 for more than 6 cycles under transfer hydrogenation. The work of Wang et al. [42] demonstrates the effect of the Cl− and OSO3− on the pathway taken by the metal complex to make the active species (Ru-H). In a different study, Amenuvor et al. [51] reported pyrazole phosphite and pyrazole phosphinite complexes of Ru (p-cymene), Ru-5 and Ru-6, to be effective in both transfer and direct hydrogenation of LA to GVL under solventless conditions.
Under transfer hydrogenation, all complexes were reported to achieve GVL yields above 84% though full conversion of LA was not achieved [KOtBu (20 mmol), FA (20 mmol), 120°C, 16 h]. Of these complexes, near-full conversion (96%) and selectivity (100%) were observed when Ru-5a was employed under solvent-free transfer hydrogenation reactions only [51]. In these systems, GVL was reported to be accompanied by 4-HVA, though at yields below 14%, which is thought to be an intermediate compound. Under direct hydrogenation reaction, all complexes were reported to have high conversion of LA and selectivity towards GVL (100%) except for Ru-5a, which only managed to convert 78% under identical conditions [51]. Ru-6a (Figure 17) was reported to achieve full conversion of LA to GVL in 16h at 20-bar H2 pressure. Furthermore, the activity was observed to be improved in complexes possessing BArF anion compared to BPh4. This was attributed to the polarity BArF anions, which increases miscibility of the catalyst and the substrate (polar LA) compared to nonpolar BPh4 [51].
Of the most available ligands, phosphine ligands are preferred due to their high thermal stability and catalyst activity.
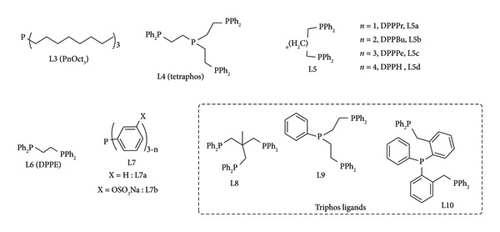
Chowdhury et al. [52] demonstrated that high yields of GVL can be obtained by the reduction of levulinic esters (methyl levulinate) in the presence of Ru (acac)3/THF with either L9, L10 or L4 phenyl phosphine ligands (Figure 18). The triphos complexes were reported to attain a GVL yield around 82% using PTSA as an additive, whereas tetraphos system required excess PTSA (5 equiv. instead of 1.75 equiv.) to achieve 77% [52]. The GVL yield under tetraphos system increased to 82% in the presence of Sc (OTF)3 under identical conditions to PTSA (50 bar, 22 h and 1.75 equiv.). A significant difference in the catalytic activity of triphos ligands (L9 and L10) was observed when the catalyst loading was reduced to 0.025 mol%. L8 system achieved a TON value of 3050 under 6 h, whereas L9 and L4 took 22 h to attain 3650 and 2850, respectively [52]. This signifies the ability of L10 to accelerate GVL production as compared to L7 and L2 (Figure 18).
The same study reported improved catalytic efficiencies under LA as compared to LE, as L4, L9 and L10 (Figure 18) reached TON values of 73,142, 59,265 and 2000, respectively, albeit at lower catalyst loading (0.001 mol%) [52]. The reduction in the activity of triphos catalyst was reported to be due to their poor solubility in LA and inhibition caused by THF. Water as a solvent was also reported to have the same effect on Ru–phosphine catalysts. Tay et al. [50] also reported the formation of Ru black particles under aqueous conditions using [RuCl (PPh3)3] complex for the reduction of LA to GVL in aqueous conditions and observed that the catalyst was insoluble and unstable due to the formation of Ru black particles in water, achieving a low TON of 137.
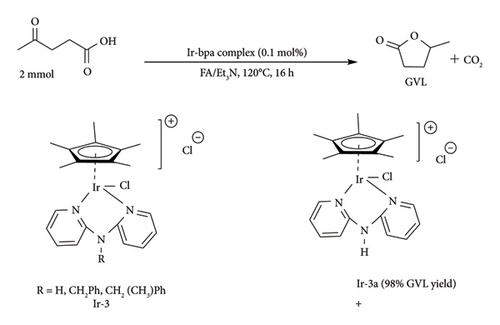
Though the Ru–phosphine complexes have shown great catalytic activity, they do have some negative properties, such as high volatility, water and oxygen sensitivity, hence limiting their application only to nonaqueous systems [48]. This is a problem as their usage requires elimination of water prior to catalysis, and even the presence of the slightest moisture could lead to catalyst deactivation [53]. Therefore, a need arises to develop water-soluble phosphine complexes to catalyse biomass either in biphasic or aqueous systems. To achieve this, highly polar substituents such as −COOH, −OH, −NH2 and −SO3H can be incorporated on the phosphine ligand [48]. These polar groups provide different pH conditions without catalyst deactivation. For example, −COOH and −NH2 phosphine complexes were reported to be stable in both basic and acidic conditions, while −SO3H gives the ability to work at all pH ranges [55]. Incorporating ionic fragments such as −SO3Na into complexes can minimize volatility while enhancing solubility and stability under polar conditions during catalytic reactions [47, 53, 59, 60]. Delhomme et al. [59] reported that Ru complexes with −SO3Na on phosphine ligands outperformed typical phosphine ligands (alkyl and aryl phosphine) in the presence of water as a solvent. Utilization of TPPTS with either [Ru (acac)3] or [RuCl3] catalysts obtained 99% and 74% conversion and 97% and 91% selectivity of GVL, respectively, at 140°C, 5 MPa H2, in 40 mL within 5 h with a catalytic loading of 0.2 mol%. Under the same conditions, the [RuCl2PPh3] catalytic system performed poorly, with just 22% conversion and 86% GVL selectivity. Mehdi et al. [47] reported that Ru (acac)3/TPPTS catalyst can be used to attain 95% GVL yield in 12 h from commercial LA in the presence of water as solvent and molecular hydrogen as a reducing agent at 140°C.
Chalid et al. [53] demonstrated the use of RuCl3.H2O/TPPTS complexes to catalyse hydrogenation of LA in biphasic systems (DCM/water) at mild conditions, obtaining yield as high as 81% in 80 min (45 bar, 90°C) ]. Ru–TPPTS catalytic system was also reported to be good in the synthesis of GVL from biomass through dehydration-transfer hydrogenation which allows a one-pot synthesis [39]. In this technology, the hydrolysis of sugars to LA occurs simultaneously with the catalytic hydrogenation of LA to GVL. RuCl3/TPPTS/NaI system was reported to obtain GVL yields around 23% and 40% from trifluoroacetic acid (TFA) hydrolysed glucose and fructose, respectively, during dehydration-transfer hydrogenation [39].
Alternatively, hydrous metal precursors could be utilized for in situ synthesis of Ru catalysts, which will allow in the cooperation of hydrophobic ligands in aqueous or biphasic synthesis of GVL without losing efficiency. Deng et al. [37] in cooperated RuCl3.H2O metal precursor for in situ generation of RuCl3–phosphine (PR3) complexes using water-insoluble DPPE (Figure 18, L6), PCy3 (Cy = cyclopentane) and PPh3 ligands. The RuCl3.H2O/py catalytic systems were reported to be stable, obtaining a GVL yield < 80% at 150°C. Hydrophilic triphenylphosphine (TPPTS) and TPPMS obtained a moderate yield (< 54%) under similar experimental conditions [37]. The H2O/PPh3/py system was also reported to produce GVL from LA/FA obtained from acid hydrolysis. A GVL yield of 48% was obtained (based on glucose) at 150°C, proving the system to be efficient, though there was a need for neutralizing and distilling the HCl hydrolysis products prior to GVL synthesis [37]. The same study demonstrated the possibility of hydrogenation of LA in the presence of CO2. The activity of RuCl3/PPh3 improved when CO2 (4 MPa) was added to the reaction mixture, resulting in full conversion of LA to GVL under H2 (4 MPa) conditions [37]. This positive impact of CO2 gas only holds at solvent (water) concentration below 75 wt%, exceeding this threshold results in low GVL yield.
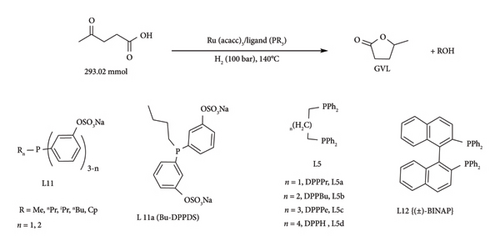
The issue of decomposition of homogeneous catalysts and low catalytic activity in solvents can be addressed by solventless synthesis. This is an attractive, cost-effective green chemistry process of attaining full conversion of LA to GVL [37, 52] For instance, Deng et al. [37] reported an effective system under both organic (py and NEt3) and inorganic (hydroxides of alkali metals) bases, obtaining yields above 72%. Of these bases, py and NEt3 were effective in obtaining GVL yields above 90%. RuCl3.H2O/PPh3/py was observed to be stable than RuCl3.H2O/PPh3/NEt3 (40%) as only 8% loss in catalytic activity was recorded after 3 cycles, demonstrating potential industrial applicability [37]. Chowdhury et al. [52] reported high TON values in solventless systems of tridentate phosphine ligands, L4, L9 and L10 (Figure 18) in the presence of appropriate additive. A high yield of 95% GVL was achieved in the presence of Ru(acac)3/PTSA/L10 system with TOF values of 1382 h−1 under 22 h at 140°C [50 bar cat. load (0.003 mol%)] [52]. Further reduction in catalyst loading (0.001 mol%) increased TON values above 60,000 in both triphos and tetraphos systems, though at very prolonged reaction time (> 4 days) [52]. In a different study, Geilen et al. [58] used phosphine ligands [PnOct3 (L3), DPPBu (L5b), triphos (L8)] (Figure 18) with Ru(acac)3/NH4PF6 as catalysts for the synthesis of GVL from LA. The activity of Ru (acac)3/phosphine catalyst was reported to increase in the order triphos < DPPBu < PnOct3 (GVL yield 8%–99%) at 160°C in 18 h [58].
Though the solventless synthesis of GVL is a promising technique to solve problems related to the use of solvents, it still has limitations such as catalytic inhibitions and deactivation caused by the additives used. Some additives cause a loss in selectivity of the desired product in other systems. For example, Geilen et al. [58] reported that the triphos/NH4PF6 system resulted in over-reduction of LA to undesired products, which are 1,4-pentanediol, 1-pentanol and MTHF at high yields (93%) instead of GVL. Similar reports of high conversions but low GVL selectivity (37%) of Ru catalyst under NH4PF6 additive were given by [47] using Ru (acac)3/PBu3 system. The use of the ionic liquids system was also observed to reduce the selectivity of Ru (acac)3/triphos towards GVL but promote over-reduction products. The activity of the Ru (acac)3/triphos was found to be improved to 50% when PTSA was used as an additive in place of NH4PF6 [52, 58]. The effect of different additives on the activity of Ru–phosphine catalysts towards GVL synthesis was studied by Chowdhury and coworkers [52] and observed that additives such as PTSA, H3PO4 and Sc (OTF)3 improve catalytic activity of Ru(acac)3/triphos/THF (< 58% GVL yield), whereas MSA, NaOTF and H3PW12O40 suppressed the performance of the catalyst (< 22%). This difference in catalytic activity indicates the important role played by additives on the selectivity of Ru homogeneous catalyst towards the desired product. Therefore, not only the right metal and ligand combination would give the best catalytic system for GVL in this technology, but also the right additive for that system is of great importance. Additives are believed to assist in stabilizing and regulating the reactivity of generated active metal species, as well as controlling pH of the reaction media [58].
In a different study, Tukacs et al. [48] reported the ability to catalyse hydrogenation of LA to GVL under neat conditions (absence of solvent and additive) using Ru/PR3 catalytic systems with high efficiency. Under neat conditions, Ru (acac)3/PBu3 catalyst was reported to achieve 6370 TON at 140°C, 100 bar in less than 0.75 h [48]. This indicates the negative effect solvents and additives had on the Ru/PR3 catalytic system. This approach is also attractive as it eliminates the use of expensive, hazardous solvents and additives, which require extra steps such as separation to obtain the product [55]. The activity of the Ru/PR3 complex system was influenced by the electronic and steric properties of the phosphine ligands [55]. This was demonstrated by using sterically bulky and poor electron donor TPPTS in neat conditions, attaining yields 10 times lower than PBut3 under identical conditions [48]. In the same study, the group also demonstrated that the activity of sulphonated phenyl phosphine can be improved by incorporation of one (DPPDS) or two alkyl (MPPMS) groups on the phosphine atom (Figure 18, L11) with TON values above 4000 [48]. Better performance was observed with DPPDS complexes even at low pressure (10 bar).
The performance of the complexes was observed to be controlled by both steric and electronic properties of phosphine ligands. This was demonstrated by the huge difference in yields achieved between Ru/iPr-DPPDS (< 50%) and Ru/Pr-DPPDS (> 99%) at identical conditions (10 bar, 140°C and 4.5 h) [48]. Even lower activity was observed when a bulkier cyclopentyl-phosphine ligand was used (< 40%) [48], whereas full conversion was observed with Bu-DPPDS (L11a) (Figure 19), attaining a TON value of 6370, which is similar to PBu3 at under 10 bar, 140°C and 4.5 h. The Ru (acac)3/Bu-DPPDS {butyl-P (C6H4-m-SO3Na)2} catalytic system was able to maintain its activity even after 6 consecutive cycles [48]. Apart from steric and electronic properties, the concentration of the ligand was also found to play a role in the yield observed. An increase in the amount of Bu-DPPDS at constant metal precursor was reported to accelerate the conversion rate until it reaches 0.015 mol where further increase resulted in low yields.
Bu-DPPDS (L11a) was also reported to improve the performance of heterogeneous catalysts when used as an additive in the catalysis of LA to GVL in a continuous flow reactor. Tukacs et al. [61] reported an increase in activity of Ru/C catalyst from 83% to 99% using Bu-DPPDS (L11a) as an additive at flow rate of 1 mL/min (100°C, 100 bar). The high activity of the Bu-DPPDS surface-modified Ru/C catalytic system achieves more than 3 runs for a 3-h continuous flow process. The conversion of LA to GVL was reported to be accelerated with an increase in temperature to 160°C, and any further increase resulted in deactivation of the catalyst [48, 55, 61].
In another study, Tukacs et al. [55] synthesized bidentate phenylphosphine ligands for the synthesis of GVL from LA in the presence of Ru (acac) precursor under neat conditions. The length of the alkyl chain was observed to affect the activity of the in situ generation of the complex. The activity increased with the number of CH2 up to four (< 99% GVL yield with DPPB), and further increases resulted in a yield drop [48]. Similar behaviour was observed when monodentate P (C6H5-OSO3Na) was used for LA reduction [48] under similar conditions. This high activity could be maintained even after 8 cycles, demonstrating its effectiveness and stability. To achieve high conversion of LA to GVL in these systems, there is a need to optimize H2, to overcome mass transfer limitations [48, 55, 61]. In the same study (±), BINAP also demonstrated to be an efficient ligand for the synthesis of in situ Ru complexes, as it managed to convert 98.6% LA to GVL (TON = 12,561, TOF = 6978 h−1) under 1.8 h, 140°C and 100 bar.
Another effective ruthenium phosphine catalyst for GVL production is the Ru–PNP complex (Ru-7a c), which has shown great potential in converting biomass and its derivatives directly to GVL in a one-pot system, as shown in Figure 20. Koranchalil et al. [13, 54] reported that these complexes can convert FF to GVL under acidic hydrogenation conditions using phosphoric acid, achieving high yields of over 84%. The catalytic activity followed the trend Ru-7a < Ru-7c < Ru-7b, with GVL yields of 58%, 75% and 84%, respectively, when ethanol was used as the solvent [54]. However, the type of acid used had a major impact on the outcome. Acids like sulphuric acid, hydrochloric acid, oxalic acid, PTSA, KHSO4 and FA led to no GVL formation with Ru-7a and Ru-7c, and only low yields (below 46%) with Ru-7b, likely due to catalyst deactivation or side reactions [54]. Notably, using FA led to methanol as a product, indicating it was reduced instead of FF. Water was also found to be unsuitable as it promoted humin formation [54].
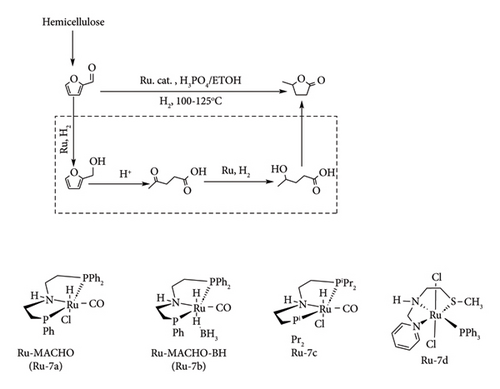
Additionally, Ru-7b (Ru-MACHO-BH) showed the ability to convert simple sugars (like xylose, glucose and fructose) and holocellulose to GVL with up to 75% yield [13]. However, when raw biomass sources such as sawdust, potato flour or chitin were used, the yield dropped to around 26%. Stadler et al. [62] achieved a 77% yield of GVL using the Ru(NNS) (PPh3) Cl2 complex (Ru-7d), where NNS = 2-(methylthio)-N-(pyridin-2-yl-methyl)ethan-1-amine. The reaction was conducted in methanol as a solvent under 30 bar of H2 for 2 h [13]. Moustani et al. [63] studied nonphosphine compounds that catalyse the conversion of LA to GVL in aqueous settings. It was reported that ruthenium complexes with different nitrogen-containing ligands outperformed sulphonated phosphine complexes in reducing LA to GVL. Among these complexes, Ru/BPhDS ((BPhDS)), Figure 21, had exceptional catalytic activity, attaining up to 3000 h−1 (turnover frequency) with good selectivity and 100% conversion to GVL [63]. It was also reported that temperature, catalyst loading and prolonged reaction time led to the over-reduction of LA to 1,4-pentanediol with 0.1% selectivity.
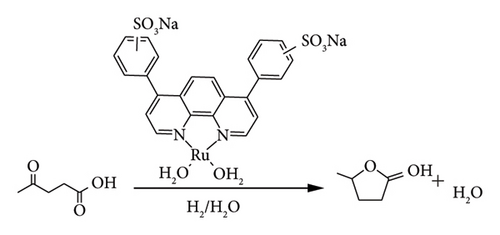
One of the most attractive homogeneous catalysts is the Shvo catalyst Ru-8 {[Ph4(η5-C4CO)2H]} Ru2(CO)4 (m-H)} (Figure 22), due to its high catalytic activity in the reduction of carbonyl, acids, unsaturated hydrocarbons and imines. This is achieved through direct hydrogenation (H2) and transfer hydrogenation in the presence of additives such as alcohols, acids and amides. The efficiency of this catalyst gained attention of researchers such as Fabos et al. (2014), Van Slagmaat et al. and Qi et al.[15, 41, 64, 65] for the selective transformation of LA to GVL under direct hydrogenation and transfer hydrogenation. Fabos et al. [64] studied the influence of substituents on the 4 and 3 positions of the cyclopentenone ligand of Shvo catalyst using complexes, MeO (Ru-8a), MePh (Ru-8b) and Ph (Ru-8c) (Figure 22).
The activity of the complex was observed to increase in the order MeO (Ru-8a) > MePh (Ru-8b) > Ph (Ru-8c). This indicated that electron-donating groups improved the performance of the catalyst. The rate of LA conversion to GVL was observed to be sluggish when (Ru-8b) and Ph (Ru-8c) were used as compared to (Ru-8a) [64]. For example, GVL yields of 69%, 59% and 48% were observed when (Ru-8a), (Ru-8b) and (Ru-8c) were used in transfer hydrogenation of LA (8.6 mmol) in the presence of FA (7.2 mmol) under identical conditions, respectively (100°C, 1h) [64].
Nonetheless, full conversion of LA to GVL was observed in less than 5 h for all the catalysts under these conditions. A similar report was given by Van Slagmaat et al. [41] on the activity of Shvo catalyst, obtaining < 95% GVL yield, albeit at prolonged time using complex Ru-8c under IPA (neat), FA and H2 (toluene solvent), and hydrogenation. Fabos et al. [64] observed that the catalytic activity of Shvo complex was temperature-dependent, as no conversion of LA was recorded at temperatures below 80°C. From this observation, the group conducted controlled experiments where the catalyst was exposed to H2–hydrogenation conditions in the absence of the substrate at 21°C at 13 bar [64]. Under these circumstances, they detected a trace amount of mononuclear complex Ru-I, indicating the slow dissociation of Shvo catalyst. The rate of dissociation was observed to be directly proportional to temperature, as an equivalent amount of the dimeric species and monomeric species was observed using NMR [64]. This equilibrium could not be altered even at prolonged time (80°C, 36 h).
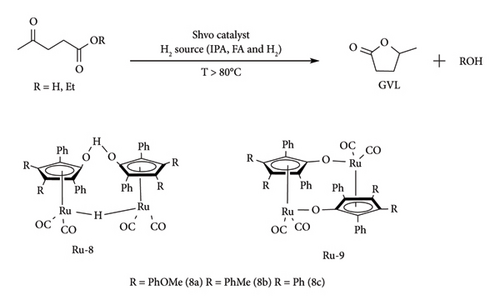
The same observation was made under inert conditions (N2) [64]. All this information indicates that a Shvo dimer needs 80°C to be transformed into its active species (metal hydride). Similar observations were made under FA–hydrogenation conditions, albeit with the production of CO2. Using this information together with the reported literature, the authors proposed that the dimeric Shvo catalyst dissociates in solution, forming hydroxy-Shvo (Ru-I) and keto-Shvo monomers (Ru-II). As shown in Figure 23, Ru-I is converted to Ru-II by hydrogen acceptors (A) [64]. On the other hand, Ru-II is converted to Ru-I by gaining hydrogen from donors (AH2) such as alcohols (IPA), acids (FA) and H2 [49, 64]. Therefore, equilibrium exists between these two species, which is regulated by the concentration of hydrogen donors or acceptors.
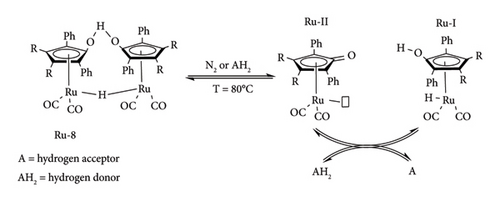
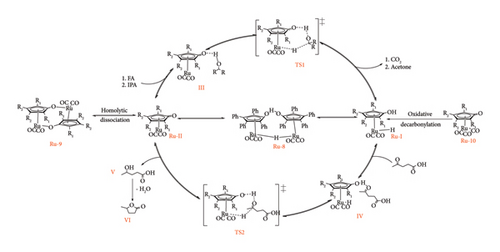
Currently, there are no crystal structures of these monomers, but their existence has been proved by different experiments using solution NMR studies and trapping agents. Ru-I has been characterized by NMR, whereas the structure of Ru-II has been studied using trapping agent such as PMe, which revealed it to be a coordinative unsaturated intermediate [49, 64]. Other than the interchange between these two species, they can undergo heterodimerization transformation. Ru-I or Ru-II can dimerize to form Ru-9, which is another resting state of Shvo catalyst [64, 66].
Complex Ru-9 has been synthesized using the same procedure as for Ru-8c though in the presence of aprotic solvents such as dry heptane. The complex was prepared by refluxing Ru (CO)12 and 1,2,3,4-tetraphenylcyclopentadienone in the presence of dry aromatic solvents such as mesitylene benzene and toluene, under inert conditions (N2) for 48 h giving monomeric tricarbonyl Ru compound Ru-10. After isolation, Ru-10 was left at 100°C for 40 h in dry heptane to dimerize into Ru-9 [67]. Van Slagmaat et al. [41] has demonstrated that Ru-9 can still be obtained by omitting Ru-10 preparation under similar conditions (Figure 24). It was reported that Ru-9 has similar catalytic activity as Ru-8c in conversion of LA to GVL under solventless conditions obtaining yield < 99% in 5 h at 50 bar and 100°C.
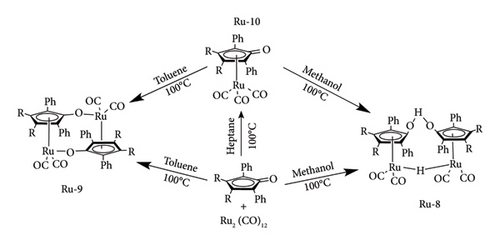
In the same work, they examined the monomeric tricarbonyl Ru complex, Ru-10 activity towards selective reduction of LA to GVL with the view that it opened doors for improving the catalytic activity of Shvo catalyst through substitution of bulky groups at the C2 and C5 position of cyclopentadienone ligand, which is impossible with stereo-sensitive dimers [41]. They reported that Ru-10 was able to catalyse the reaction, though it was sluggish compared to its dimeric counterparts. Near-full conversion was observed with the binuclear Ru-9 and Ru-8c in 1 h at 120°C, whereas Ru-10 required 5 h under similar conditions [41].
The difference in performance of the dimeric and monomeric Shvo catalysts was argued to be ease of forming the active species by Ru-I and Ru-II dimeric complexes through a dissociation mechanism as depicted in Figure 25. Due to the existence of Ru-I and Ru-II in solution, the reduction of LA and reformation of the reducing species (oxidation of donors) can occur simultaneously from the onset of the reaction, thus speeding the reaction [41]. Monomeric Ru-10 goes through mono-decarbonylation to form Ru-II, followed by H2 split to form reducing hydride species Ru-I, which requires time and is also energy-consuming. In fact, it was observed to have low performance (63% GVL yield) at temperatures below 100°C [41].
Though Ru-10 presented such an interesting performance, recyclability under direct hydrogenation proved to be difficult in the presence of water as an extractive solvent, as it led to catalyst deactivation [41]. Van Slagmaat et al. [32] reported the Shvo catalyst Ru-8c to be inactive in the presence of water and DMSO as solvents, whereas, in the presence of common organic solvents such as alcohols, ethers, esters, hydrocarbons, furans, ketones and even chlorobenzene, it showed improved and excellent activity, obtaining 99% GVL yields in toluene and chloroethane after 16 h at 50 bar under direct hydrogenation conditions [15]. This observation inspired Van Slagmaat et al. [41] to switch LA with EL, which co-produces ethanol instead water. Under these new conditions, Ru-10 was able to maintain its activity for more than 2 cycles, giving < 90% yield, which was similar in the presence of LA [41].
Qi et al. [65] proposed the use of GVL as a solvent in its own synthesis to eliminate the need for product separation since the product is also the solvent. This also addressed the environmental issues associated with the disposal of organic solvents. This was also viewed to be possible by Qi et al. [65] who demonstrated the inactivity of Shvo catalyst towards GVL reduction to form undesirable products, 1,4-pentadinol and 2-MTHF, under FA-transfer hydrogenation conditions. Qi et al. [65] were able to obtain a yield of 55% (based on initial fructose) and 99% selectivity of GVL in 2 h using Ru-8a in one-pot synthesis of GVL from biomass using H2SO4/GVL biphasic media using a step-down temperature protocol. In this protocol, hydrolysis of fructose in the presence of H2SO4 was first achieved at 130°C, achieving LA (69%) and FA (71%) followed by LA and FA catalysis to GVL. This also showed the stability of the catalyst in the acidic environment [65].
The mechanistic studies of the hydrogenation of LA to GVL by the Shvo catalyst have been studied through computational calculations [68] and spectroscopic experiments under both transfer and direct hydrogenation conditions [49, 66, 69] through isotopic labelling, NMR spectroscopy and trapping experiments of Ru-I and Ru-II metal species. The catalytic cycle for the Shvo catalyst was reported to start with the formation of either Ru-I or Ru-II (or both), depending on the Shvo precursor under investigation [64]. In Figure 25, the cycle begins with the formation of Ru-II by heterolytic or homolytic dissociation of Ru-8 and Ru-9, respectively [41, 64]. Under transfer hydrogenation conditions, this is followed by the association of donor molecule (FA and IPA) to the metal complex via the cyclopentadienone ligand (II), leading to transition state 1 (TS1) [41, 68].
This TS is eliminated by concerted heterolytic transfer of hydrogen from the donor to the metal complex, resulting in a metal-H species (Ru-I) and loss of residual molecules such as CO2 (FA) and acetone (IPA) [15, 68]. It should also be noted that Ru-I could result from either oxidative decarbonylation of Ru-10 or during heterolytic cleavage of Ru-8, with the latter case suggesting simultaneous existence with Ru-II from the onset of the catalytic cycle [64, 65]. The formation of metal-H is followed by the association of LA (substrate) to Ru-II through an outer sphere fashion, leading to the second transition state (TS-II) [15]. This is followed by concerted hydrogen transfer from both the metal and the cyclopentadienone ligand of the complex to the keto-group of LA, forming 4-HVA. 4-HVA eventually dissociates (V) and self-cyclizes to GVL with loss of water or alcohol if levulinic ester is used as substrate (VI). In this process, the oxidizing form of Shvo catalyst (Ru-II) is regenerated to restart the cycle [64].
The direct hydrogenation of LA using the Shvo catalyst follows the same pathway as the transfer hydrogenation, the only difference being the route for the formation of metal hydride [41]. The onset of this catalytic cycle is marked by the association of molecular hydrogen to Shvo catalyst (Ru-II), forming a metal dihydride complex (II) as depicted in Figure 26. This is followed by the association of water to the cyclopentadienone ligand through hydrogen bonding (III) [69]. The complex then forms a TS1, in which one hydrogen is transferred to the water while water loses its hydrogen to the cyclopentadienone ligand in a concerted manner [41]. After the transfer of hydrogen is complete, the water molecule is dissociated from the complex, resulting in a ruthenium metal hydride (Ru-I) formation [69]. From here onwards, the cycle follows the same path as the transfer hydrogenation path to reduce LA to GVL. An alternative path can be taken for hydrogen splitting in which LA is used instead of water.
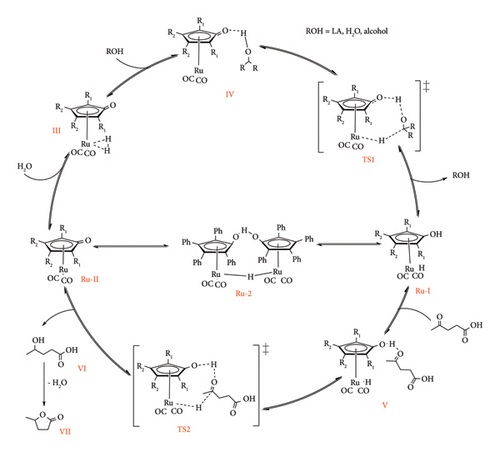
According to this mechanism, the association of H2 to Ru-II is followed by LA associating with cyclopentadienone through the acidic proton (II) [41]. Thereafter, a TS is formed, leading to one of the hydrogens of the dihydride being adopted by the oxygen (sp3) of LA while transferring a proton to the cyclopentadienone ligand. Elimination of LA results in the formation of a metal hydride, which reduces the keto-group [41]. It is argued that the two coexist as their energetic pathway (Ea (Ru-H) = 5.6–6.8 kcal/mol) and mechanistic atom interaction are similar. Casey et al. [69] also proposed that ethanol could be used as an H-relay agent following a similar path for Ru-I formation. It is also worth noting that in all of Van Slagmaat et al. [41] mechanisms (transfer and direct hydrogenation), the rate-limiting step was observed to be the transfer of hydrogen to LA (TS2) [41]. It was reported that the LA mediated by H2 had a lower reaction rate compared to FA and IPA donors under optimized solventless conditions (0.1 mol% Ru, 100°C), with the rate increasing in the order hydrogen donor H2 < IPA < FA [41]. These observations were further backed up by computational thermodynamics studies, which revealed a spontaneous formation of metal-H (Ru-I) when FA is used as a hydrogen donor, whereas IPA and H2 required activation energies of 5.4–6.3 and 5.8–6.7 kcal/mol (ΔG), respectively, under the identical experimental conditions [41].
8. Conversion LA to GVL Using Iridium (Ir) Complexes
Another noble metal that is well-suited for converting lignocellulose-derived LA to GVL in a single-pot system is iridium (Ir). Its favourable catalytic qualities include air, water and acid stability. The complexes of Ir have been reported to have high catalytic activity in both transfer hydrogenation and hydrogenation processes by groups of Deng et al. [44], Li et al. [70] and Wang et al. [16, 42], as shown in Table 5. For example, Li et al. [70] reported the application of Ir tridentate pincer complexes generated from [Ir (COE)2Cl]2, where COE = cyclooctene, with various tridentate P^N^P, P^N^N, N^N^N, P^C^P ligands, towards direct hydrogenation of LA to GVL (Figure 27, Ir-1a). The complexes were reported to have excellent catalytic activity with < 99% GVL yield with TON = 71,000 under mild conditions (100°C, 50 bar, 15 h), with the pyridine-based pincer complexes being the best [70]. The activity of the complex reduces in the order P ^ N ^ P > N ^ N ^ N > P ^ N ^ N > P ^ C6P of pincer ligand type.
| Catalyst | Substrate/H2 source | Conditions | GVL yield (%) | TON (TOF/h−1) | Reference |
|---|---|---|---|---|---|
| Ir (COE)2Cl2/1c | LA/H2 |
|
99 | 71,000 | Li et al. [70] |
| Ir-2a | LA/H2 |
|
98 | — | |
| Ir-3a | LA/FA | Et3N, 16 h, 120°C | < 98 | — | Wang et al. [16] |
| Ir-4a | LA/FA | H2O, 130°C, 72 h | 87 | 174,000 | Wang et al. [29] |
| Ir-5a | LA/H2 | H2O, 120°C, 36 h | 78 | 78 000 (2167) | Li et al. [70] |
| LA/FA | 99 | 9900 (2475) | |||
| Ir-8 | LA/H2 | H2O, 5 bar, 16 h, 110°C | 100 | — | Wang et al. [42] |
| LA/FA | H2O, 16 h, 110°C | ||||
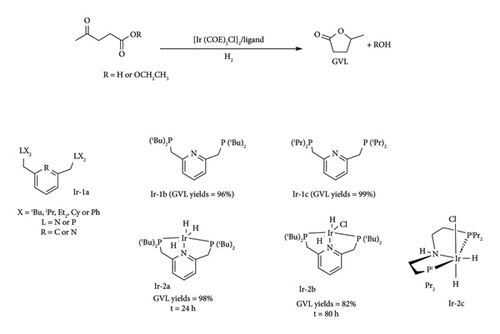
The high activity of the electron-rich pyridine-based complexes compared to PCP was highlighted to be due to their possession of a methylenic spacer group [70]. This group is easily deprotonated, leading to a dearomatized pyridine ligand, which assists in the activation of dihydrogen to form active M-H species which is necessary for LA reduction [71]. This also explains the need for strong bases, KOH, NaOH and LiOH, to achieve high yields [46]. For instance, GVL yields as high as 96% can be achieved when KOH is used as a base additive, while yields above 5% are observed with weak bases such as Et3N and K2CO3, under identical conditions (100°C, 50 bar, 15 h) [70]. The use of electron-donating groups [R = tbu or ipr (Figure 27, Ir-1b and Ir-1c respectively)] on the phosphine and amine moieties where also found to improve the catalytic activity of the complex compared to alkyl phenyl groups, attaining 96% and 99% GVL yields. The catalytic system was also reported to be solvent dependent, as high yields were obtained using polar protic solvents such as alcohols and water (Ir-1b, 96% GVL yield), rather than toluene and THF (< 21%) [70].
Padilla et al. [72] reported over 99% conversion of ethyl levulinate to GVL using the Ir-2c catalyst. The system also demonstrated high efficiency at elevated substrate loads, achieving 93% conversion and a TON of 9300 from a mixture of 40 mmol methyl levulinate and 70 mmol ethyl levulinate over 24 h at 25°C and 25 bar H2—performance that is comparable to that of Ru-based catalysts.
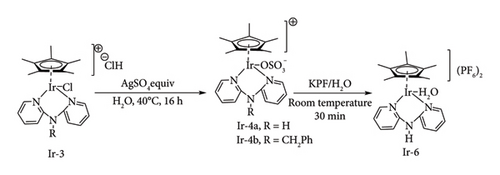
The group of Wang demonstrated the efficiency of Ir dipyridylamine (Ir-bpa) complexes, Ir-3 (Figure 28) on the transfer hydrogenation of LA to GVL affording < 98% in 16h, 120°C though excess amount of Et3N additive and FA are required to be driven reaction to near completion [42]. The product yield was found to drop to 33% when the bridging amine substituted with phenyl groups was used. The system showed to be stable to be used in continuous processing of LA to GVL as it was able to maintain its activity for more than 3 cycles [42]. Despite their remarkable activity, cationic Ir chloride complexes with a bipyridylamine ligand are ineffective direct hydrogenation catalysts because they lack the free coordination site necessary to create the metal dihydride intermediatory state that gives rise to M-H active species [16]. Also, the use of chloride liganded complexes necessitates the use of bases to help in neutralizing the HCl acid formed during the catalytic process, which increases the cost of GVL production [51]. Even under direct hydrogenation conditions, the chloride ligand shows catalytic retardancy in Ir catalytic systems.
This was demonstrated by Li et al. [70] by comparing the direct hydrogenation activity of Ir pincer complexes with trihydride Ir-2a and chloride ligand Ir-2b. They observed near-full conversion of LA to GVL using trihydride complex Ir-2a (98%) within 24 h, whereas 82% yield was reduced with chloride complex Ir-2b in 80h under identical conditions (Figure 27). In this work, the efficiency of Ir-2a was also noted to be increased at lower catalytic loading (0.001), achieving a TON of 71,000 within 2 days, highlighting the need for a nonchloride ligand to achieve high catalytic activities by Ir complex systems. This work has inspired Wang et al. (2017) [16] to synthesize and investigate the activity of Ir bipyridylamine complexes with sulphonate ligand, Ir-4a and Ir-4b, on the reduction of LA to GVL (Figure 29). These complexes were obtained by the abstraction of the Cl ligand of Ir bipyridylamine Ir-3 with Ag2SO4, resulting in zwitterionic complexes. These chloride-free complexes were reported to achieve GVL yields as high as 94% in 16h, 4 MPa at 130°C in the presence of water [44]. Ir bipyridine complexes were also reported to have similar catalytic activity, achieving near complete conversion (98%) on LA to GVL in less than 4h at lower hydrogen pressure (1.01 MPa) under base-free conditions [44].
The performance of these catalysts was observed to be influenced by the electrostatic properties of the pyridine ring, as drastically reduced yields were observed with electron-withdrawing groups such as COOH compared to strong electron donors such as OH and OMe (Figure 30) [44]. These groups are also required to be in sterically unhindered positions to maximize the efficiency of the catalyst as it is sensitive to steric pressure. For example, 96% GVL was observed using [Ir-bipy-H2O] with hydroxy groups on the para position, whereas having the hydroxy groups on the ortho position resulted in a drastically dropped yield (21%) [44]. In fact, the ortho-hydroxy-substituted bipyridine Ir complex was observed to dehydrogenate GVL to LA when a control experiment was carried out under identical conditions as LA hydrogenation using GVL. This demonstrates that the catalyst has GVL dehydrogenation properties rather than hydrogenation of LA [70]. Apart from structural properties, external factors such as temperature, H2 and the amount of solvent can influence the catalytic efficiency of the system. Hence, the optimization of these conditions is a necessity to obtain high GVL yield and TON. Direct hydrogenation of LA to GVL using base-free Ir systems was observed to require relatively mild conditions (< 1.01 MPa, < 120°C and 4 mL of water) [16, 70].
Full conversion of LA to GVL was observed at very low pressures and water volume (0.2 MPa, 110°C, 2 mL) with Ir-4a in 16 h. This high catalytic activity could also be maintained even at 0.01 mol% catalyst loading, albeit at prolonged reaction duration (72 h), whereas a slight drop in GVL yield (average 87%) was observed at the stingiest conditions (0.0005 mol%) with TON of 174,000 at 130°C and 72 h [16]. Bipyridine Ir-5a system is also reported to have improved efficiency (TON = 78,000) at lower catalytic load (0.001 mol%), 120°C and 36 h [70].
These Ir-bpy and Ir-bpa complexes were also reported to be efficient in the transfer hydrogenation reaction using FA and not IPA. Unlike their Ir-Cl counterparts, Ir–sulphonate complexes prefer base-free conditions to give a satisfactory yield [16, 70]. For example, Wang et al. [16] observed > 28% GVL yield drop by substituting Cl- ligand with OSO3- of the Ir–bipyridylamine complexes using Et3N as a base. The yield was observed to increase to 85% when base-free conditions were used, albeit at high temperatures (140°C) using Ir-4a. They also demonstrated that the reaction could be driven to completion with the addition of water as solvent, affording 100% GVL yield and TON of 9000 (110°C and 16 h). Ir-5a was also reported to be efficient and stable when water was used as a solvent, giving 99% GVL yield in 4 h [70]. Wang et al. [16] have also synthesized another monologue of Ir-3, [Ir-bpa-OH2] Ir-6, through anion metathesis from [Ir-bpa-OSO3] as depicted in Figure 29. This complex was reported to be efficient in both transfer and direct hydrogenation of La to GVL, yielding 66% and 85%, respectively. These results emphasized the efficiency of the sulphonate complex [16].
These complexes are also reported to be active under acidic conditions and, hence, could be used to produce GVL from LA and FA derived from acid hydrolysis of LCB without the need for evaporation, separation and pH adjustment. Li et al. [70] demonstrated that 61% and 45% GVL could be obtained by using a FA and LA mixture obtained from H2SO4 acid hydrolysed fructose and glucose, respectively, using Ir-5a, though with the assistance of external H2 (1.01 MPa) in less than 4h at 120°C. Ir-4a was also reported to have the ability to directly synthesize GVL from glucose-derived LA and FA, giving 44% yield. The activity of the catalyst was reported to be enhanced with the addition of 05 MPa external H2 (53% GVL yield) and increased by 4% when fructose was used instead, as depicted in reaction (Figure 30) [16].
The catalytic activity of Ir complexes for transfer hydrogenation under base-free conditions has been reported to be affected concentration of both FA and solvent (water) [16, 70]. Catalytic activity under this system is observed to be enhanced by the use of excess FA (≤ 2 molar ratio) or the addition of external H2 as a supplement. Li et al. [70] observed a decrease in GVL yield by more than 15% when the concentration of FA was dropped below a 0.5 molar ratio. The yield could not be improved even with the rise in temperature under these conditions, signifying the importance of FA.
Wang et al. [16] studied the effect of water as solvent under base-free transfer hydrogenation using Ir–bipyridylamine complexes Ir-4a (Figure 31). They observed an increase in GVL yield by 33% with an increase in volume of water from 0.33 to 1 mL, while a sharp drop in GVL yield (30%) is observed when 2.0 mL. They concluded that water volume must be between 0.5 and 1 mL to obtain full conversion of LA to GVL. The complexes were reported to be easily recovered using simple phase separation techniques, as they are water soluble [42]. In a different study, Wu et al. [73] demonstrated that pH significantly influences the hydrogenation of FF and LA, with optimal catalytic performance achieved using the Ir-5b catalyst (Figure 32). Under acidic conditions, particularly at pH 1.0, FF could be directly converted into GVL in a one-pot reaction with a 55% yield. Using FA as a hydrogen source further boosted catalytic activity, reaching TOFs over 12,000 h−1.
The superior performance of Ir-5b was linked to its hydroxyl groups, which enhance electron donation—an effect lost when replaced with methoxy groups in Ir-5a. Additionally, formate ions from FA improved catalyst–substrate interaction, making homogeneous transfer hydrogenation more effective than direct biphasic systems.
Unlike the pyridine-based pincer complexes which are the active species, the Ir-bpy and Ir-bpa complexes are reported to be precursors used to generate metal hydride responsible for the hydrogenation of LA [16, 42, 70]. This metal hydride can either be a dihydride or monohydride complex in nature, depending on the pathway taken to split dihydrogen associated with the precursor. The latter is formed through base-catalysed heterolytic cleavage, whereas homolytic cleavage is involved in the former. The nature of Ir–metal hydride species generated by half-switched complexes has been studied by the group of Wang et al. [16] through NMR experiments using Ir-4a under direct hydrogenation conditions. They reported the appearance of an Ir-H species, Ir-7, without any indication of a dihydride species, indicating that the system uses monohydride species.
Isolation of complex Ir-7 for crystallographic examination and further testing was observed to be difficult, even when alternative reductants were used, such as FA and NaBH4. The stable Ir-H species could only be obtained when a method used to synthesize complex Ir-6 was adopted, in which PF6 is observed as an uncoordinated counter ion (Ir-8), as presented in Figure 33 [16]. Complex Ir-8 was further tested for the catalysis of LA GVL to prove its intermediary under both direct and FA-transfer hydrogenation, giving quantitative yields [100% GVL in 16 h, 110°C, H2O (1 mL)]. The same activity was observed to be maintained under transfer hydrogenation at lower catalytic loading (0.05 mol%), and hydrogenation proved to be more effective than Ir-4a (77% GVL) [16].
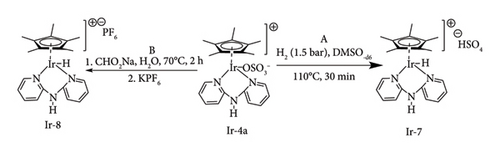
Wang et al. [16] proposed a mechanism where the Ir-bpa reduces LA to GVL (Figure 33). As depicted in Figure 34, the pathway of hydrolysis of Ir complex by water leads to an aqua cationic complex with sulphate counter ions, B. This is followed by association of dihydrogen to the aqua complex, resulting in M-H2, C. Since the M-H2 complex is acidic in nature, it easily goes through acid–base reactions with the sulphate ion, leading to a metal monohydride complex E [16]. Another alternative path to form the metal hydride is when the aqua ligand is used to deprotonate the complex through sigma-bond metathesis, D [16]. This is followed by LA association to then reduction of complex F and subsequent hydrogen transfer to the keto-group forming an Ir-alkoxy complex G. An intramolecular reaction of the alkoxy ligand assisted by the metal complex to produce GVL (I) with reformation of B was also suggested. This step could be coupled with hydrogen regenerating either as a dihydride complex or as active species to close the loop [16].
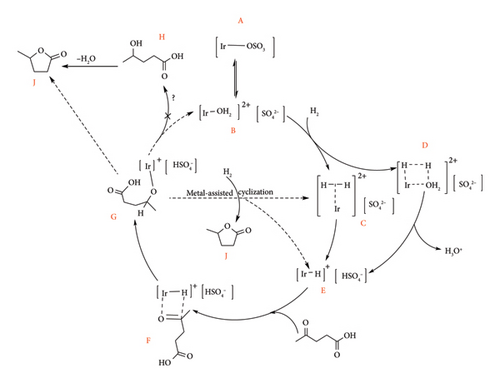
The idea of metal-assisted intramolecular cyclization is rather contrary to that of the alkoxy ligand getting hydrogenated and dissociated, as 4-HVA generates GVL via self-lactonization [15, 41, 64, 74]. Wang et al. [16] supported this claim based on the fact that 4-HVA has never been observed during in situ synthesis of GVL from LA when monitored by 1H NMR and IR as reported by Amenuvor et al. [51] and Chalid et al. [53] under direct hydrogenation. Chalid et al. [53] argued that the rate of intramolecular lactonization of metal-free 4-HVA was too rapid to be detected by the instrument. This is also backed up by density function theory (DFT) calculations performed by Van Slagmaat et al. [41] which indicated the release of 4-HVA from the metal and its cyclization to be exergonic (−9.2–10.2 kcal/mol), supporting the spontaneous self-cyclization.
All these claims are contrary to the experimental observations made by Amenuvor et al. [51] and Tukas et al. [55] who were able to identify 4-HVA as a co-product of GVL during the hydrogenation of LA. Tukas et al. also reported the transformation of GVL ring to 4-HVA under acidic conditions (1 M HCl) when a blank experiment was run in in situ 1H NMR. Qi et al. [65] reported the existence of equilibrium between 4-HVA and GVL at low pH, which ceases to exist at neutral conditions. Acids were also reported to catalyse ring opening of GVL (formation of 4-HVA), which greatly facilitates hydrogenation of LA beyond the ester in the Ru (acac)3/triphos systems [45]. This information might explain the observations made by Amenuvor et al. [51] since activation of the pyrazole phosphite and pyrazole phosphinite ruthenium complex resulted in HCl production, while Tukas et al.’s [55] catalytic system (Ru–BINAP–HCl) involved the use of acid as an additive. In fact, 4-HVA was only observed under FA reduction systems, which raises the suspicion that FA only acts as a hydrogen source or also plays a role in catalysing the formation of 4-HVA from GVL. Therefore, more work must be done to uncover the truth surrounding the existence of 4-HVA intermediary in GVL formation from LA hydrogenation.
9. Conversion of LA to GVL Using Iron (Fe) Complexes
Research on hydrogenation of LA to GVL is currently populated by noble metal catalysts which are rare and expensive due to their high catalytic performance (TON = 17,400) [75]. Hence, there is a need to shift towards catalysts with metals that are inexpensive and abundant such as Fe. This approach enables industrialization of GVL synthesis from LA using affordable homogeneous catalysts. Fe complexes have been previously reported to catalyse reduction of carbonyl such as ketones, CO2 and esters obtaining high catalytic activity [76–79]. Other complexes have been reported to have ability to dehydrogenate FA and methanol with high TOF (9425 h−1) and TON (92,000) under lower temperatures [15]. This makes Fe complexes appropriate when it comes to synthesis of GVL from crude biomass extracts, as LA is always accompanied by FA in equimolar ratios. This inspired several researchers to conduct research on the reduction of LA to GVL using Fe complexes. Fu and coworkers [74] utilized Fe-triflate precursors with phosphine ligands (Fe-1) (Figure 35) for the hydrogenation of ethyl levulinate to GVL in the presence of FA as reductant in additive-free conditions. The use of additive-free conditions promoted the recyclability of the solvent and ligand through distillation and column chromatography.
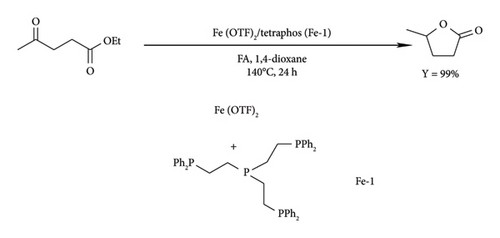
A high yield was observed including 99% conversion of LA with 98% selectivity for GVL, in the presence of Fe-1 catalyst at 140°C, 2.0 eq. FA and 1,4-dioxane without a base within 24 h [74]. Furthermore, attempts to reduce EL to GVL under H2 hydrogenation in the presence of Fe (OTF)2/tetraphos (Fe-1) were futile as no yield was observed at 3 MPa H2 [74].
This suggests that the metal-H responsible for the reduction of the keto-group of EL can only be formed by the abstraction of hydrogen from FA by Fe-1 catalyst and not through decomposition of FA as depicted in Figure 36 [74].
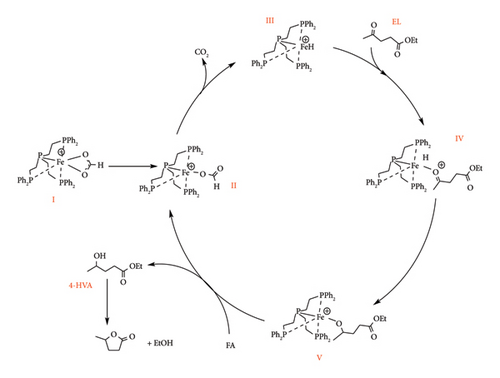
The catalytic cycle of Fe-1 starts by the addition of Fe (OTF)2 and tetraphos ligand, with subsequent substitution of triflate with formate anion, forming complex I. The easy liability of the triflate is considered to be due to weak coordination to Fe. This is followed by the transformation of I to II through switching the binding mode of the formate ligand from bidentate to monodentate, which aids in the dehydrogenation of formate ion. The dehydrogenation of formate ion forms a metal hydride [74], III and CO2, which dissociates from the metal. Thereafter, EL coordinates to Fe-H (III) through the keto oxygen, forming complex IV. Carbonyl groups form weak interactions with Fe (II), thus easily displaced by strong anions. This explains the observation made when NaCl was used as an additive [74]. Complex IV then transfers hydrogen to EL (V), followed by protonation and replacement of the alkoxy anion by formate, resulting in II and 4-HVA. 4-HVA self-lactonizes to GVL with the release of ethanol [74].
Pincer Fe complexes are another type of catalyst with high activity for LA and LE hydrogenation to GVL. Of interest is complex Fe-2 and Fe-3 (Figure 37) which were used by Yi et al. [46] with a pyridine-containing pincer ligand. The group reported high GVL selectivity (> 90%), TON and frequency above 1800 and 900 h-1, respectively, in MeOH in less than 2 h at 50 atm and KOH as a base. The system was also reported to have low activation energy compared to Ru and other noble metal systems as high catalytic activity (95% GVL) was reported at room temperature [46]. Fe-3 catalyst was reported to be effective in the presence of different protic solvents such as IPA, FA, H2O and even the biphasic H2O/THF, achieving yields as high as 90% compared to the aprotic system (neat THF = 15%). Similar performance was observed when hydroxides, carbonates, tert-butoxides of alkali and alkaline earth metals (< 91%) were used as no yield was observed in the presence of amine and hydrocarbon bases [46]. This demonstrates the dependency of the pincer Fe complexes on strong bases and protic solvents to assist in the formation of meta-H species responsible for reducing LA to GVL [46].
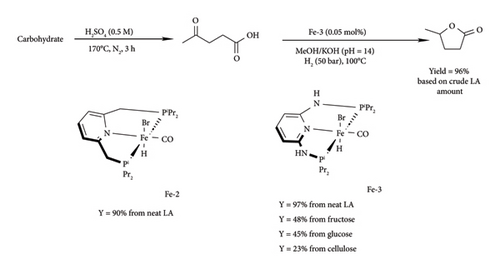
The catalytic activity of Fe-3/KOH/MeOH system was also reported to be enhanced at low catalyst loading, resulting in a TON and frequency of 23,000 and 1917 h−1 under harsh reaction conditions [46]. The same activity was reported when water was used as a solvent instead of MeOH under similar conditions. This is a desired system as it eliminates organic solvents, which are expensive and environmentally unfriendly. It is also worth noting that Fe-3/KOH/MeOH system is capable of hydrogenating LE, such as methyl levulinate, to GVL (85% yield, 1700 TON, 141 h−1 TOF) in 12 h, 50 atm and 0.05% catalyst loading [46]. Similar to the LA reduction, the catalytic activity was improved by reducing the catalyst loading to 0.005 mol. The high activity of this system was also observed when the work was extended to carbohydrate-derived LA, achieving 96% GVL yields (based on crude LA) as depicted in Figure 37.
Another interesting Fe complex is Fe3 (CO)12, reported to achieve a yield of 92% GVL under transfer hydrogenation conditions with FA as a hydrogen source and imidazole base [80]. The catalytic activity of this system was reported to increase with temperature and time, though a threshold of 180°C, 15 h exists where the catalyst deteriorates [80]. The complex also proved to be active in the presence of pyridine as a solvent, giving 87% GVL yield. It is also worth noting that the catalytic system was able to transform LA derived from sugarcane bagasse to GVL (50%) without the need for the separation process, which is impressive, as most catalytic systems would require nonacidic conditions [80].
Fe counterpart of the Shvo catalyst was synthesized by Knolker in its monomer form (Fe-4). This complex has been utilized for the hydrogenation of LA in both catalytic transfer hydrogenation and direct hydrogenation, but was observed to be inactive in both cases [15, 43]. Thus, the complex requires activation to form the active hydride species, Fe-6, by the addition of additives such as bases/acids as presented in Figure 38 before utilization (routes A and B) [15, 43]. In such cases, high activity was observed in the CHT process using IPA (95%) and moderate with pentanol (58%) and molecular hydrogen (2%) as reductants in the presence of LE as substrate [43]. Hence, it was concluded that Fe-6 is not a good indirect hydrogenation for the CHT process.
Van Slagmaat et al. [15] observed that these procedures for synthesizing Fe-6 are not compatible with in situ designs as the base additive has a high affinity for LA rather than the metal complex, resulting in no catalytic activity. The negative results encouraged them to conduct control experiments, in which LA was found to change from colourless to brown liquid when stoichiometric addition of Me3ON was performed. NMR and FT-IR of the brown liquid revealed a shift in the acidic proton and disappearance of the carboxylic acid vibrational band of LA, respectively, indicating an acid–base reaction between LA and Me3NO, forming hydroxylammonium levulinate ionic liquid (Figure 39) [15].
The authors then adopted the Moulin method, in which Fe-4 was reacted with acetonitrile in the presence of Me3NO at room temperature for 3 h, resulting in a mono-acetonitrile analogue of Fe-4, complex Fe-5. Complex Fe-5 has been demonstrated to be stable under and easily transforms to Fe-6 without the use of bases such as Me3NO [15]. The in situ synthesized Fe-6 was able to promote LA hydrogenation under H2 conditions, though the activity depended on the solvents as compared to CHT [15, 43]. Organic solvents such as alcohols, ethers, hydrocarbons and chlorine-based solvents were found to improve the activity, with high performance observed in alcohols. The presence of water, acetonitrile and DMSO was found to reduce the activity of the catalyst [15]. The catalyst quickly decomposed under solventless conditions with no LA conversion (100°C, 5 h, 50 bar) [15].
A Fe–Shvo catalyst is considered to follow the same pathway as the Ru counterparts, with the loss of acetonitrile by Fe-5 (Figure 40) [15]. Such claims are supported by the high liability of a similar mechanistic pathway for Fe–Shvo for LA transfer hydrogenation in the presence of FA was reported by Assary and Curtiss [68] using DFT. In their calculations using the dimeric Fe–Shvo catalyst (Figure 40, Fe-7), they reported that catalysis required high energy (15.3 kcal/mol) to dissociate to the monomeric form as compared to its Ru analogue (10.8 kcal/mol) which could present a barrier in its utilization. Nonetheless, the intrinsic activation energy to reduce LA by its monomeric form was found to be close to that of Ru–Shvo 16.5 and 19.4 kcal/mol, respectively, making it a promising cheaper alternative for Ru–Shvo catalyst under transfer hydrogenation [68]. A computational modelled mechanism for LA transfer hydrogenation under FA was also reported by Assary and Curtiss [68] using DFT.
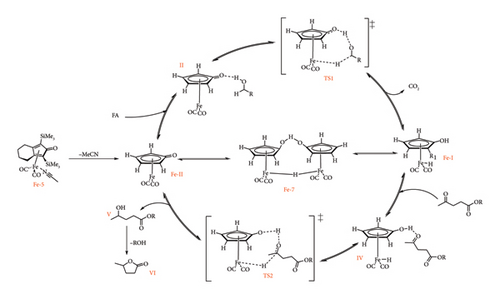
10. Future Outlook
The utilization of homogeneous catalysts in the production of GVL from biomass and LA is another option biofuels and bioenergy-related products production. In this growing discipline, significant consideration must be given to feedstock selection, catalyst selection and process optimization [27, 81]. A wide range of feedstock alternatives is available for this process, ranging from agricultural, forest biomass to municipal solid wastes and energy crops such as corn and sugarcane. The selection criterion of feedstock should be based on accessibility, low manufacturing costs and consistence in the supply of feedstock. Great emphasis is placed on waste streams with little or negative value, such as agricultural, municipal and industrial waste with high holocellulose components, such that the feedstocks are consistent with sustainable waste management of a recovery goals. When considering the homogeneous catalysts and catalysts precursors for the catalytic transformation of LA to GVL, emphasis is on adherence to green chemistry principles together with catalyst stability, reusability and cost-effectiveness of the green chemical pathways.
Integration of sustainability tools is crucial for the assessment of GVL manufacturing process sustainability and economic feasibility. Many tools have been used to assess the sustainability of biofuels. The tools use indicators such as net energy balance, net energy ratio, renewability and exergy analysis [81, 82]. Exergy analysis has been successfully used to the evaluate quality and quantity of energy generated. The combination of exergy analysis and life cycle assessment (LCA) which quantifies sustainability provides a comprehensive assessment of environmental consequences of each flow, components of bioenergy production plant, overall sustainability and cost flow in the system and optimization attempts to increase efficiency [27, 81]. Technoeconomic analysis (TEA) is another approach for conducting process design and evaluating economic viability and income-generating capacity of the production of biofuels from the renewable feedstocks [81].
The integration of these sustainability methods not only uncovers trade-offs, but also suggests ways to enhance biofuel systems [82]. As a result, early inclusion of sustainability assessment tools in the developmental stages of GVL production processes is critical. They aid in decision-making, optimization operational parameters and speed-up investment planning, for GVL commercialization as a sustainable fuel additive and platform chemical to replace fossil fuels. Ongoing research on GVL production must include sustainability evaluations to examine the environmental and economic viability of proposed processes, guiding feedstock selection and process development towards maximum sustainability. Evaluating both the manufacturing of LA from biomass and its reduction to GVL is critical for increasing consumer and legislators’ trust in GVL as an alternative fuel. In the future, customized case studies should be performed on biomass feedstocks such as lignin, proteins and waxes to encourage biorefineries companies to adopt the catalytic transformations of LA into GVL. Investigators are encouraged to concentrate on nonprecious metals such as Cu, Ni, Zn and Fe in the synthesis of the homogeneous catalysts. Exploring mechanistic routes, notably for 4-HVA production with FA, and the function of CO2 in LA to GVL reduction in aqueous settings, can give useful insights for tailoring exceptionally efficient catalysts.
11. Conclusion
In conclusion, this comprehensive review provides an extensive analysis of the utilization of organometallic catalysts in the reduction of LA to GVL and highlights the challenges and opportunities associated with this catalytic process. The discussed novel methods, such as solvent-free and additive-free synthesis, as well as the use of GVL as a solvent in its own synthesis from biomass, not only boost process efficiency but also contribute to the process’s environmental friendliness and cost-effectiveness. The in-depth investigation of metal complexes as homogeneous catalysts for the conversion of LA to GVL revealed that Ir-based catalysts perform better in terms of product selectivity and catalytic efficiency when compared with Ru- and Fe-based catalysts. Furthermore, the prospect of replacing costly noble metal catalysts with more plentiful and cost-effective options such as Fe offers an alternative for sustainable lower manufacturing costs, making GVL and related products stronger competitors against fossil-based alternatives.
The mechanistic studies mentioned in this review provide a clear understanding of the steps involved in the catalytic reduction of LA to GVL, identification of the rate-determining step and selection of the most suitable hydrogen sources for the catalytic process. An additional key component to the subject matter is the idea of implementing collaborative sustainability approaches, specifically LCA, to critically analyse the overall sustainability and economic feasibility of GVL manufacturing processes. This emphasis on the environmental and economic sustainability of proposed processes together with knowledge regarding feedstock selection and catalytic processes is critical for driving the research towards the most sustainable solutions.
Ultimately, this review not only adds to scientific comprehension of GVL manufacturing but also give a road map for its commercialization. The commercial feasibility of GVL as an alternative fuel is dependent on its environmental sustainability, economic viability and competitiveness with traditional fossil-based alternatives. This review offers a solid framework for increasing GVL production as a significant factor in the transition to more sustainable and economically viable fuel alternatives by incorporating novel methodologies, homogeneous catalysts and catalytic conditions.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Acknowledgements
The authors would like to thank the University of Botswana for research resources.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.




