Mesoporous Silica Particles Functionalized With Poly(Glycidyl Methacrylate) Brushes Cross-linked With Ethanolamine to Potentially Enhance Pollutant Removal From Contaminated Water Systems
Abstract
Water pollution caused by synthetic dyes and industrial effluents poses a significant environmental challenge. Among these dyes, methyl orange (MO), widely used in the textile and printing industries, stands out as one of the most persistent and toxic pollutants due to its complex aromatic structure and resistance to biodegradation. Its presence in aquatic environments leads to serious ecological and health concerns, underscoring the urgent need for developing efficient and selective adsorbent materials for effective wastewater treatment. In this study, mesoporous silica particles (MSPs) were synthesized and functionalized with poly(glycidyl methacrylate [GMA]) (PGMA) brushes cross-linked with ethanolamine (MSPs-PGMA-x-ETA) to enhance dye adsorption performance. The surfaces of silica particles were first modified with (3-glycidoxypropyl)trimethoxysilane (GPTS) and treated with concentrated hydrochloric acid to produce hydroxyl groups. Then, the surface was reacted with 2-bromo-2-methylpropionyl bromide to produce atom transfer radical polymerization (ATRP) initiator sites. A high grafting of PGMA brush was obtained, followed by cross-linking with ethanolamine (MSPs-PGMA-x-ETA), as an adsorbent. The newly developed sorbent was subject to thorough characterization through a variety of physicochemical techniques, including FTIR and TEM. These analyses provided detailed insights into the structural and chemical attributes of the sorbents. The adsorptive performance of the sorbent was evaluated under a range of experimental conditions, focusing on parameters such as the pH of the solution, the contact time with the dye, and the initial concentration of MO. The optimal adsorption capacity observed was ca. 63 mg·g−1 of MO at a dye concentration of 100 mg·L−1, with a contact time of 150 min being sufficient for effective dye removal. Extensive adsorption studies indicated that the process adhered to the Langmuir adsorption isotherm, suggesting that adsorption occurs via a monolayer formation on distributed sites across the MSPs-PGMA-x-ETA’s surface. Furthermore, the kinetics of the adsorption were best described by the pseudosecond-order model, indicating that the rate-determining step might involve chemisorption.
1. Introduction
The world’s growing population has led to faster industrialization, which has resulted in a lot more industrial waste being dumped into the environment and water sources [1, 2]. Water pollution is now considered a serious global issue [3, 4]. Organic dyes from industrial waste are concerning because they can cause serious health problems such as cancer, poisoning, and kidney and brain damage [5, 6]. Among the various methods used to decontaminate organic pollutants from water, adsorption, which uses materials to capture and remove pollutants, is considered a highly effective physicochemical method [7, 8]. It is widely used as its affordable, simple, environmentally friendly, and very effective [9]. There are many different materials used for adsorption in water purification, agricultural waste [10], activated carbons [11], metal-organic frameworks (MOFs) [12], clays [13] and mesoporous silica particles (MSPs) [14, 15].
MSPs possess several remarkable characteristics, including a thermal stability, tunable pore size, high surface-to-volume ratio, and eco-friendliness. Furthermore, surface of MSPs can be easily modified to significantly improve adsorption efficiency and selectivity [16–18]. The hydroxyl groups on the MSPs’ surface facilitate functionalization with various materials, such as surface-active agents and polymers [19–21]. Usgodaarachchi et al. showed that mesoporous silica nanoparticles (MSNs) synthesized from rice husk with cetyltrimethylammonium bromide (CTAB) and functionalized with (3-aminopropyl)triethoxysilane (APTES) had morphological changes, exhibited higher adsorption capacity for methylene blue, fit the Langmuir model well with a maximum capacity of 19.26 mg g−1, and followed a pseudosecond-order model, demonstrating efficacy in methylene blue removal from water [22]. Beagan synthesized MSPs modified with cysteine and used it to enhance methylene blue removal efficiency with adsorption capacity increasing from around 70 mg g−1 in acidic conditions to approximately 140 mg g−1 in basic conditions, fitting the Freundlich isotherm and the pseudosecond-order kinetic model for adsorption elucidation [23].
Polymer brush-coated MSPs have been used as adsorbents. Magnetic MSPs modified with a cationic polymer brush have been synthesized and applied as nanoadsorbents for the removal of methyl orange (MO) and bromothymol blue dyes, achieving high removal efficiency for both dyes [24]. MSPs-poly(2-(tert-butylamino)ethyl methacrylate) (PTBAEMA) brush nanoparticles have been reported to efficiently remove 2,4,5-trichlorophenoxyacetic acid (TCA) with a high adsorption capacity of 290 mg/g over a wide pH range (3–7) [25]. Alswieleh reported the synthesis of PTBAEMA–SO3H@MSPs for efficiently removing Rhodamine B and crystal violet from water, with optimal conditions identified and adsorption processes fitting the Langmuir model, showing maximum capacities of 147.78 mg g−1 for Rh B and 343.57 mg/g for CV [26].
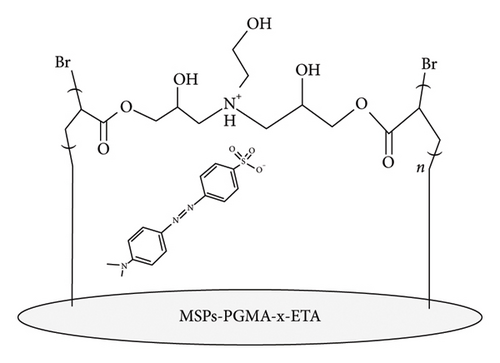
The objective of this research project was to synthesize and characterize MSPs with poly(glycidyl methacrylate [GMA]) (PGMA) brushes cross-linked with ethanolamine (MSPs-PGMA-x-ETA) and evaluate their adsorption efficiency and kinetics for MO dye removal from aqueous solutions, as depicted in Scheme 1. The MSPs were synthesized and surface-modified using (3-glycidoxypropyl) trimethoxysilane (GPTS), followed by the introduction of atom transfer radical polymerization (ATRP) initiator sites and subsequent grafting of PGMA chains. Ethanolamine was employed as a cross-linking agent owing to its dual functionality, hydroxyl and amine groups, which react efficiently with the epoxide groups of PGMA. Moreover, the presence of hydrophilic moieties enhances water compatibility and provides additional active sites for dye adsorption through hydrogen bonding and electrostatic interactions. The cross-linking step resulted in the formation of MSPs-PGMA-x-ETA adsorbents. The characterization of these particles was conducted using transmission electron microscopy (TEM) and Fourier-transform infrared spectroscopy (FTIR) to analyze morphology and chemical properties. Adsorption studies were performed using UV-visible spectrophotometry to measure the adsorption capacity, and the efficiency was analyzed through Langmuir and Freundlich isotherm models as well as kinetic models such as pseudofirst-order and pseudosecond-order. The project aimed to optimize dye removal conditions and analyze the kinetics to develop an efficient, stable, and reusable adsorbent for wastewater treatment, contributing to environmental sustainability and pollution control.
2. Experimental Works
2.1. Materials, Measurement, and Characterization
Deionized water was sourced using an ELGA Pure Nanopore system. A comprehensive list of chemicals was procured from various reputable suppliers. Hexane (99.9%), dichloromethane (DCM) (HPLC grade), copper(I) chloride (CuCl) (≥ 99%), copper(II) bromide (CuBr2) (≥ 99%), 2,2′-bipyridyl (Bipy) (≥ 99%), ammonium hydroxide (32 wt%), and N-CTAB (98%), methanol (99.8%), α-bromoisobutyryl bromide (BIBB) (98%), GMA (≥ 97%), GPTS (98%), ethanolamine (ETA) (≥ 98%), and ethanol (99.8%) were obtained from Sigma-Aldrich. Ammonium nitrate (NH4NO3) (99%) and pyridine (analytical grade) were obtained from Fisher Scientific. Tetraethyl orthosilicate (TEOS) (98%) was received from Tokyo Chemical Industry (TCI). Triethylamine (99%) was purchased from Loba Chemie. All chemicals were used as received.
The structural details and surface characteristics of the synthesized nanoparticles were meticulously analyzed using TEM using a JEOL JEM-1230, providing high-resolution insights into the particle morphology, for understanding the surface properties that influence adsorption behaviors. To further investigate the chemical properties of the nanoparticles, FTIR was employed using a Thermo Scientific Nicolet iS10 instrument. The analysis was conducted across a broad spectrum range from 4000 to 400 cm−1, allowing for a detailed assessment of the functional groups present on the particle surfaces and confirming the successful modification of the particles with organic molecules. The efficiency of the particles in adsorbing the MO dye was quantitatively measured using a UV-visible spectrophotometer, model Shimadzu UV-2600. This analysis involved determining the concentrations of MO in an aqueous solution before and after the adsorption process.
2.2. Preparation of MSPs
CTAB (1.0 g) was dissolved in an aqueous solution, followed by a 10-min sonication. At a temperature of 38°C, 7 mL of NH4OH was incorporated into the solution. Subsequently, a combined solution of 5 mL of hexane and 3 mL of TEOS was gradually added to the reaction flask while stirring continuously for 15 h. The particles were then separated via centrifugation, thoroughly rinsed several times with deionized water and methanol, and dried at a temperature of 110°C for a day.
To create channels within the particles, the CTAB template was eliminated by dispersing the synthesized particles in a solution of ammonium nitrate dissolved in ethanol (8 mg·mL−1). The mixture was stirred and refluxed for a day. Afterward, the MSNs were isolated and subsequently cleaned with ethanol to ensure purity.
2.3. Preparation of GPTS-Modified MSPs
The surface of MSPs was modified using GPTS to enhance their functionality. Initially, 1.5 g of MSPs was uniformly dispersed in a solution of 40 mL of toluene and 0.4 mL of GPTS. The mixture was then heated to 130°C and maintained under reflux conditions overnight. After the reaction period, the GPTS-coated MSPs were separated using centrifugation and repeatedly washed with methanol to remove any unreacted silane and residual solvents. The particles were dried at a temperature of 120°C for 3 h.
Subsequently, GPTS-modified MSPs were resuspended in 40 mL of a 1 M hydrochloric acid solution. This mixture was then heated to 100°C while stirring continuously for 24 h. The final product, designated as MSPs-OH, was isolated through centrifugation and subjected to thorough washing with water and methanol to ensure purity. The particles were dried at a temperature of 120°C for 3 h.
2.4. Synthesis of the ATRP Initiator Functionalized MSPs (MSPs-Br)
To introduce ATRP initiator sites, 1 g of MSPs-OH was suspended in a mixture of 40 mL of DCM and 0.3 mL of triethylamine. To the suspension, 0.2 mL of 2-bromo-2-methylpropionyl bromide dissolved in 5 mL of DCM was added under constant stirring at 25°C and continued overnight. After completion of the reaction, the product, MSPs-Br, was filtered, followed by multiple washes with both DCM and methanol to ensure the removal of any impurities on the silica surface. The particles were dried at a temperature of 70°C for 3 h.
2.5. Preparation of PGMA Brushes on the MSPs Surface (MSPs-PGMA)
The process began by dispersing MSPs-Br in a mixture of methanol and water (ratio 3:1), followed by the addition of 2.5 mL of GMA under a nitrogen atmosphere. After 45 min of prepolymerization mixing, the catalytic components, 1.92 mg of CuBr2, 18.2 mg of CuCl, and 70.5 mg of Bipy were introduced into the suspension. The mixture was then subjected to polymerization at ambient temperature for 2 h. MSPs-PGMA was thoroughly washed using water and methanol to remove residual monomers and catalysts. Drying of the particles was carried out at 70°C for 3 h.
2.6. MSPs-PGMA-x-ETA
The cross-linking of the PGMA brushes present on the MSPs was achieved using ethanolamine. Initially, 0.5 g of MSPs-PGMA was suspended in 20 mL of ethanol to ensure complete dispersion. A solution of ethanolamine (5 mL, 3 μM concentration) prepared in ethanol was gradually added to the suspension over 3 h under reflux at 90°C. The reaction mixture was maintained at 90°C and stirred continuously overnight. Following the reaction, the cross-linked particles (MSPs-PGMA-x-ETA) were isolated through multiple wash cycles with water and methanol. Drying of the particles was carried out at 70°C for 3 h.
2.7. Adsorption Studies
2.8. Adsorption Kinetics
2.9. Adsorption Isotherms
3. Results and Discussion
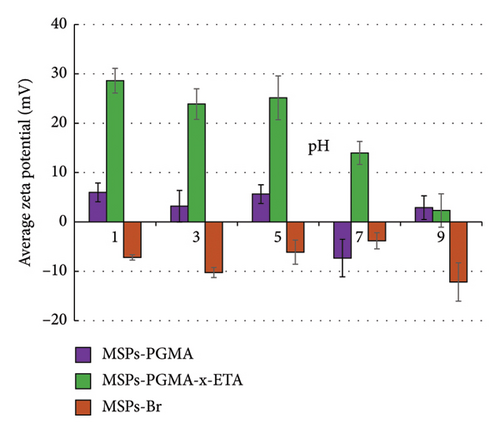
The surface modifications of MSPs with CTAB, MSPs without CTAB, MSPs-Br, MSPs-PGMA, and MSPs-PGMA-x-ETA were assessed through FTIR spectroscopy to confirm the surface structural changes, as illustrated in Figure 1. The FTIR analysis revealed that peaks between 1250 and 1050 cm−1 and at 810 cm−1 were linked to Si–O bond stretching in the silica network. In MSPs with CTAB, peaks of C-H stretching bands were identified at around 2900 and 1430 cm−1, indicating the presence of hydrocarbon chains present in CTAB molecules. The spectrum of MSPs-Br after CTAB extraction exhibited a peak at approximately 1440 cm−1, attributed to –CH2– stretching within the propyl group in the silane initiator. For MSPs-PGMA, characteristic peaks were observed at about 1730 cm−1, which was associated with carbonyl stretching in the polymeric chains. After reaction with ETA, an increase in the peak at around 1650 cm−1 corresponded to the introduction of the amine group in PGMA brushes.
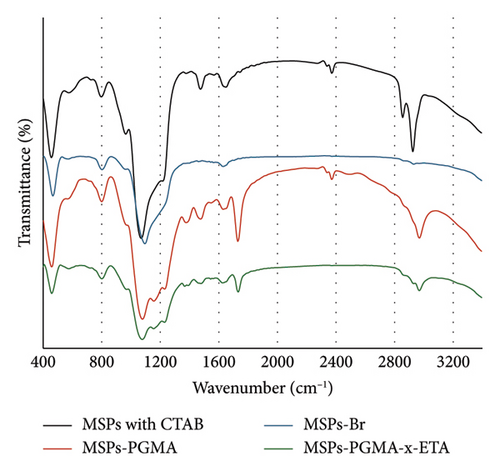
Figure 2 displays the average zeta potential of three types of modified MSPs, MSPs-Br, MSPs-PGMA, and MSPs-PGMA-x-ETA, varied with pH. The zeta potential for MSPs-Br remained relatively stable in the range between −4 and −13 mV, across the pH level. MSPs-PGMA particles had a relatively low zeta potential across all pH levels, indicating a consistent surface charge. MSPs-PGMA and MSPs-Br exhibited more stable potentials, indicating that their surface modifications are less sensitive to pH changes. MSPs-PGMA-x-ETA exhibited significantly positive zeta potentials, especially at lower pH values. This high positive charge indicates strong repulsive forces between particles, enhancing stability in suspension. The strong negative charge of MSPs-PGMA-x-ETA at low pH is likely due to protonation of amine groups on the surface, which decreases with increasing pH.
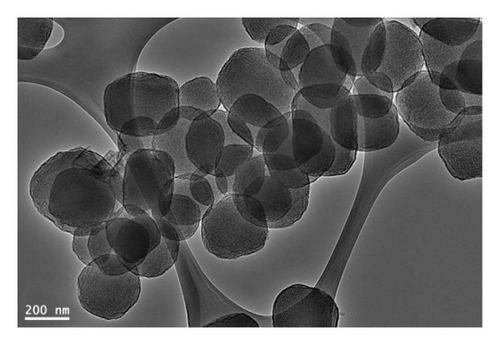
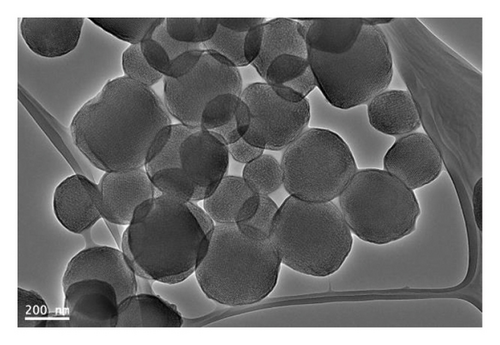
The morphological structures of MSPs-Br and MSPs-PGMA-x-ETA were analyzed using the TEM technique (Figure 3). This image depicts MSPs with an average diameter of approximately 250 nm. The porous structure, with pore sizes around 5 nm, is clearly visible, highlighting the material’s high surface area and potential for applications such as environmental applications.
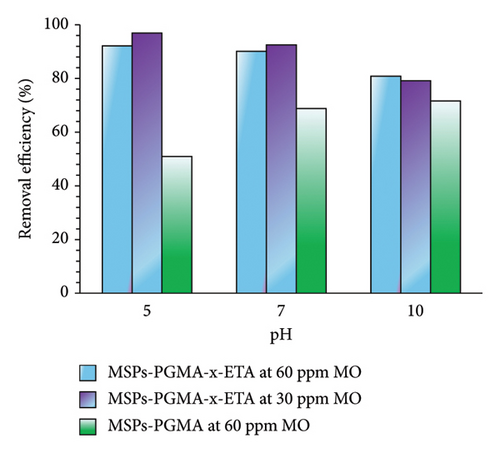
The impact of the pH of the solution on the adsorption of MO onto MSPs-PGMA and MSPs-PGMA-x-ETA was investigated at different concentrations and pH levels of 5, 7 and 10. In these experiments, the adsorbent dosage remained constant at 10 mg per 10 mL solution, with an exposure time of 150 min at 25°C. Figure 4 illustrates that the adsorption efficiency of MO onto MSPs-PGMA and MSPs-PGMA-x-ETA exhibited a slight increase in acidic media, whereas MSPs-PGMA exhibited a slight increase in basic conditions. The presence of amine groups in MSPs-PGMA-x-ETA imparted a positive charge to the surface of the adsorbent due to the reaction between ETA and epoxy groups present in PGMA brushes. At pH levels above 4.5, MO exists in a negative charge, leading to the highest adsorption efficiency at pH 5. At pH greater than 7, the MSPs-PGMA-x-ETA acquires an uncharged state, while MO retains its ionic characteristics.
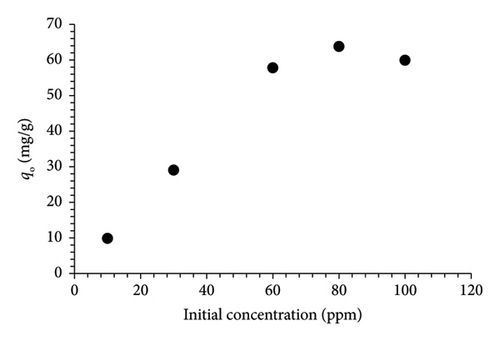
An investigation into the utilization of MSPs-PGMA-x-ETA for purifying water contaminated with MO involved studying various dye concentrations (10, 30, 60, 80, and 100 ppm) at 25°C and pH 5. Figure 5(a) illustrates that the adsorption capacity of MSPs-PGMA-x-ETA increased proportionally with higher dye concentrations. The adsorption capacity of the adsorbent was ca. 63 mg·g−1 within the dye concentration range of 80–100 mg·L−1.
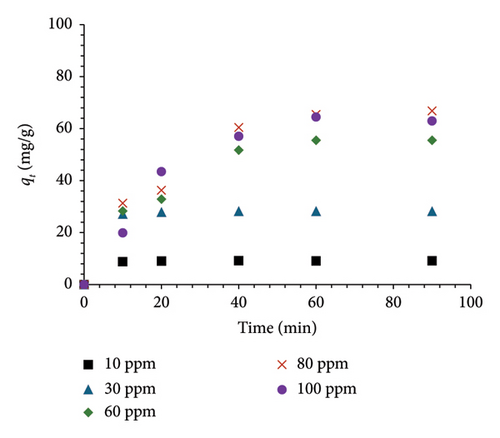
Exploring the impact of exposure time on MO adsorption capacity by MSPs-PGMA-x-ETA across intervals of 10, 20, 40, 60, and 90 min, Figure 5(b) showed a gradual increase in MO adsorption capacity, reaching equilibrium after 20 min for concentrations below 30 ppm. At a concentration of 60 ppm and above, a slower rise in adsorption rate occurred after 40 min, with equilibrium reached at 60 min. Notably, with increasing initial MO concentrations, the adsorption capacity also increased, peaking at approximately 66 ppm at 80 ppm and 100 ppm.
The enhanced MO removal rate following surface modification with ETA is attributed to the electrostatic interactions between the dye molecules and the functionalized surface. MO carries a negative charge, while the surface of the material remains positively charged at pH values below 7, as shown in the surface zeta potential data in Figure 3. Therefore, the optimal removal efficiency is expected in the pH range of 5–7, where favorable electrostatic attraction occurs. Scheme 1 illustrates the schematic representations of the surface of the adsorbent and the molecular structure of MO.
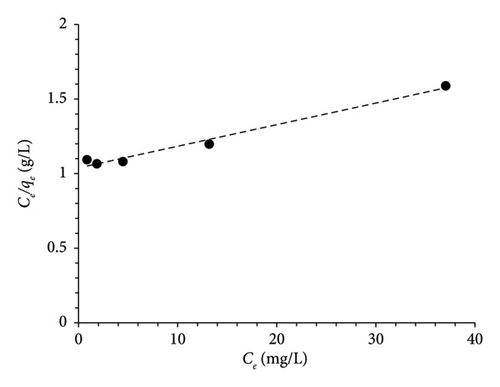
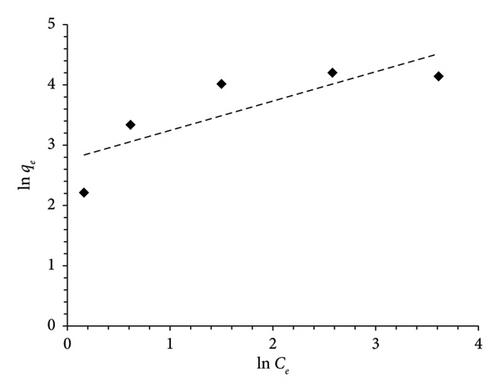
The Langmuir and Freundlich isotherms were employed to study the distribution of MO on MSPs-PGMA-x-ETA surface, as illustrated in Figure 6. Based on the analysis of the linear regression coefficient (R2), it is evident that the adsorption of MO molecules onto the adsorbent aligns more closely with Langmuir’s model than with Freundlich’s model. This observation indicates that the MO molecules have predominantly occupied uniform and specific sites within the surface of MSPs-PGMA-x-ETA, forming a complete monolayer on its surface rather than multiple layers. This behavior points toward an effective and predictable adsorption process governed by specific interactions between the MO and the MSPs-PGMA-x-ETA surface. The parameters obtained from the Langmuir and Freundlich equations are presented in Table 1.
| Isotherms | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg·g−1) | 69.1 |
| KL (L·mg−1) | 0.014 | |
| R2 | 0.98 | |
| Freundlich | KF ((mg·g−1)/(mg·L−1)1/n) | 15.77 |
| n | 2.05 | |
| R2 | 0.67 | |
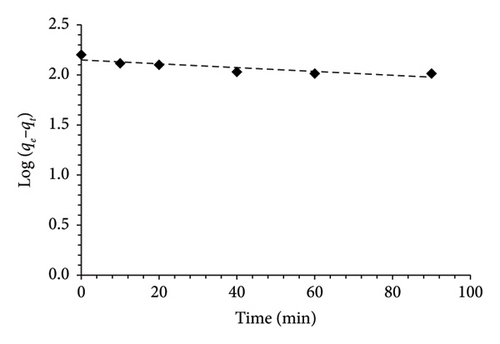
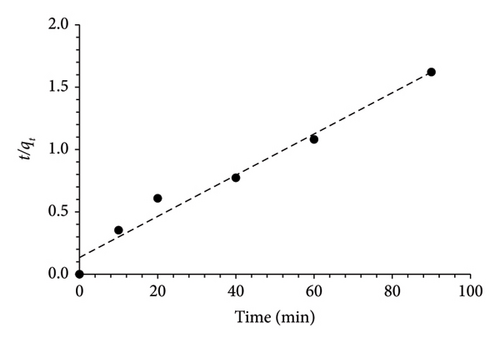
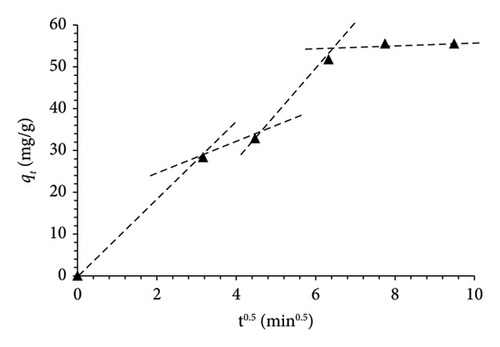
Three kinetic models were employed to elucidate the adsorption mechanism of MO by MSPs-PGMA-x-ETA. Figure 7 illustrates the plot curves for the pseudofirst-order, pseudosecond-order, and intraparticle diffusion models at various dye concentrations. Notably, based on the R2, it is evident that the adsorption of MO onto MSPs-PGMA-x-ETA predominantly adapted to the pseudosecond-order model at 60 ppm. This finding verified that the rate-limiting step involves a chemisorption process. When the intraparticle diffusion model was employed, it provided further detail on the mass transfer process within the adsorbent particles. The adsorption of MO onto MSPs-PGMA-x-ETA was characterized by three steps. The first step was a fast-step adsorption mechanism when MO molecules moved from the bulk solution to the external surface of the nanoadsorbent. Subsequently, MO dye diffused into the internal pores, enhancing adsorption capacity. Finally, internal diffusion of MO within particle structures occurred, reaching various adsorption sites. The parameters derived from these kinetic models are presented in Table 2.
| Kinetic models | Parameters | Value |
|---|---|---|
| Pseudofirst-order | qe (mg·g−1) | 8.58 |
| k1 (min−1) | 0.004 | |
| R2 | 0.76 | |
| Pseudosecond-order | qe (mg·g−1) | 60.55 |
| k2 (min−1) | 0.002 | |
| R2 | 0.97 | |
Numerous materials have been investigated for the removal of MO dye from water. Table 3 presents a comparison between the adsorption performance of MSPs-PGMA-x-ETA and various previously reported adsorbents. As shown, MSPs-PGMA-x-ETA exhibit a higher adsorption capacity than many other materials reported in the literature. Due to their facile synthesis, effective surface modification, and superior adsorption efficiency, MSPs-PGMA-x-ETA represent a promising and efficient adsorbent for the removal of ionic dyes from wastewater.
| Materials | qe (mg·g−1) | Ref |
|---|---|---|
| Starch cryogel–integrated mesoporous silica nanoparticles | 18.9 | [27] |
| Chitosan–silica nanocomposite | 7 | [28] |
| Hybrid spherical silica | 45 | [29] |
| Silica nanoparticles functionalized with multihydroxyl-containing Gemini surfactant | 401.8 | [30] |
| Quaternary ammonium polyethylenimine–modified silica nanoparticle | 105.4 | [31] |
| Fe MSPs-PGMA-x-ETA | 63 | This study |
4. Conclusions
In conclusion, the synthesis and characterization of MSPs-PGMA-x-ETA have shown promising results for enhancing pollutant removal in wastewater systems. The multistep modification process, involving surface functionalization with GPTS, ATRP initiator attachment, and subsequent PGMA brush grafting and cross-linking, yielded MSPs-PGMA-x-ETA as an efficient adsorbent. Thorough characterization using FTIR, DLS, and TEM provided valuable insights into the structural and chemical properties of the sorbent. The adsorption studies revealed an optimal adsorption capacity of approximately 63 mg·g−1 of MO at a dye concentration of 100 mg·L−1, with an effective contact time of 150 min. The Langmuir adsorption isotherm model suggested monolayer adsorption on distributed sites of the MSPs-PGMA-x-ETA surface, highlighting the specificity and efficiency of the adsorption process. Moreover, the kinetics of adsorption followed the pseudosecond-order model, indicating a chemisorption-dominated mechanism with a rate-limiting step likely involving strong chemical interactions between the dye molecules and the sorbent surface. These findings underscore the potential of MSPs-PGMA-x-ETA as a promising adsorbent for pollutant removal applications, emphasizing its effectiveness, specificity, and controlled adsorption behavior.
Conflicts of Interest
The author declares no conflicts of interest.
Funding
This study was supported by the King Saud University, ORF-2025-239.
Acknowledgments
This research was supported by the Ongoing Research Program (ORF-2025-239) of King Saud University, Riyadh, Saudi Arabia.
Open Research
Data Availability Statement
All data generated or analyzed during this study are included within the article.




