С60 Fullerenes Diminish Dysfunctions in Rat Muscle Soleus With Chronic Alcohol Intoxication
Abstract
It is known that reactive oxygen species are important in the development of alcoholic myopathy, and their suppression by C60 fullerene nanoparticles, as potent antioxidants, is opening up a new direction for their use in biomedical nanotechnology. Here, for the first time, the biomechanical and biochemical parameters of the functioning of rat muscle soleus under alcohol intoxication at 3, 6, and 9 months were analyzed. C60 fullerene, as a therapeutic nanoagent, at an oral dose of 1 mg kg−1 was used along with alcohol throughout the experiment. The findings indicate that significant dysfunctions develop in the rat muscle soleus after chronic alcohol intoxication. However, these dysfunctions are significantly reduced due to exposure to C60 fullerenes, particularly with prolonged alcoholization of animals (9 months) and the development of high-intensity fatigue processes.
1. Introduction
Alcoholic myopathy is characterized by biochemical, physiological, and structural changes in skeletal muscles leading to a disorder of their functional activity [1]. This pathology is most pronounced in the fast-contracting muscles [2] and is characterized by progressive proximal weakness, pain, atrophy, and impaired mobility. The results of studies [3–5] show that chronic alcohol consumption causes a decrease in the mass of fast muscles and their physiological cross-sectional area. In contrast, the decrease in the cross-sectional area of slow fibers is insignificant under similar conditions of alcoholization [6]. Alcohol consumption for 14–16 weeks correlates with a decrease in maximal muscle tension and tetanic strength of fast muscles. In addition, in alcohol-fed mice, fast muscles developed significantly lower contraction force during high-frequency stimulation compared to slow muscles [5]. Chronic alcohol consumption also reduces the time to onset of muscle fatigue [7, 8].
The development of alcoholic myopathy increases the level of oxidative stress in the body. In addition to significant atrophy, the fast muscles of alcohol-fed rats exhibited a decrease in glutathione (GSH) levels, the activity of GSH reductase, superoxide dismutase (SOD), and glutathione peroxidase (GPx), as well as an increase in NADPH Oxidase-1 gene expression, indicating significant oxidative stress [9]. The authors [10] found high levels of cholesterol hydroperoxides in the tibialis anterior muscle (a fast muscle) compared to the vastus lateralis (a slow muscle), suggesting low antioxidant activity in alcohol-exposed muscles rich in Type II fibers [11]. However, by using the increased concentration of carbonyls as indicators of muscle protein damage caused by reactive oxygen species (ROS), the authors [12] found that Type II muscles exhibit a higher protective effect than Type I muscles during chronic alcoholization.
The use of the antioxidant procysteine attenuated the atrophy of alcoholic fast skeletal muscle, restored GSH levels, and increased catalase (CAT) mRNA expression but did not reduce the levels of other markers of oxidative stress and catabolic disorders. It is hypothesized that it minimizes muscle atrophy by inducing components of several anabolic pathways [9]. The use of antioxidants, such as vitamins C and E, exerted different effects on the two types of alcohol-exposed muscle tissue [13]. For example, in Type II muscle fibers, the effectiveness of antioxidant defense mechanisms in balancing increased ROS production was reduced, making them more susceptible to oxidative stress. This, in turn, led to impaired muscle contractile function, rapid fatigue, and injury.
It is known that biocompatible and bioavailable carbon nanoparticles such as C60 fullerenes [14] with antioxidant properties can effectively inactivate ROS in living systems [15–17]. Recent studies [18, 19] have demonstrated that the level of chronic alcoholic myopathy development in rat muscle gastrocnemius (a fast skeletal muscle) decreases when C60 fullerene is used as a therapeutic nanoagent. It was found that C60 fullerene reduces the level of liver damage in chronic alcohol intoxication of rats [20]. However, there is currently no data on the effect of this nanoantioxidant on the functional activity of slow skeletal muscle (muscle soleus) in rats during alcoholic myopathy.
Thus, the novelty of the current research lies in assessing the biomechanical and biochemical parameters of the functioning rat muscle soleus under chronic alcohol intoxication and exposure to C60 fullerenes over periods of 3, 6, and 9 months.
2. Materials and Methods
2.1. Sample Preparation and Characterization
A previously proposed ultrasonic method [21] was used to prepare a highly stable (for 18 months at a temperature of +4°C–25°C) C60 fullerene aqueous solution (C60FAS; initial concentration 0.15 mg mL−1) [20].
The structural organization of C60 fullerene particles in aqueous solution was investigated using atomic force microscopy (AFM; NT-MDT, Apeldoorn, Netherlands) with “RTESPA-150” probes (Bruker, Billerica, Massachusetts, USA) [20].
2.2. In Vivo Experiments
The research was conducted on male Wistar rats aged 1 month (at the beginning of the experiment) with a body weight of 170 ± 10 g [20]. The rats were housed under controlled environmental conditions (21°C, 12 h light–12 h dark cycle) with free access to water and a standard diet ad libitum [22]. All procedures with laboratory animals complied with the ARRIVE guidelines. The use of animals was approved by the Biomedical Ethics Committee of the ESC “Institute of Biology and Medicine” at Taras Shevchenko National University of Kyiv (protocol no. 2 dated September 2, 2022) and conducted in accordance with Article 26 of the Law of Ukraine “On the Protection of Animals from Cruelty” (no. 3447-IV, 21.02.2006), as well as European Union Directive of 22 September 2010 (2010/63/EU) for the protection of animals used for scientific purposes.
- -
Intact group (n = 30): Rats received 100% drinking water;
- -
Alcoholization group (control; n = 30): Each rat was placed in a separate cage and provided with alcohol (40% ethanol in drinking water). This meant that the animals did not have access to 100% water until they had consumed the dosed portion of ethanol [23]. The amount of ethanol administered was calculated as 0.5% of the animal’s body weight. The ethanol dose was recalculated every 24 h throughout the experiment. The duration of alcoholization was 3 (n = 10), 6 (n = 10), and 9 (n = 10) months.
The target concentration of 40% ethanol in drinking water was chosen because it reflects the blood alcohol concentration (BAC) reported in chronic alcoholics [24]. The BAC of the animals was determined using an alcohol analyzer (AM1, UK).
- -
Alcoholization + C60 (n = 30; 10 animals in each subgroup): These rats were administered C60FAS at a daily oral dose of 1 mg kg−1 along with alcohol. The C60FAS dose was recalculated every 24 h throughout the experiment. Control over the amount of C60FAS ingested was ensured by denying the rats’ access to drinking 100% water until they fully consumed the administered dose.
It should be noted that the dose of C60FAS used in this study was chosen as the most effective dose based on previously obtained results [18–20]. Additionally, this value is significantly lower than the LD50, which was found to be 600 mg kg−1 body weight when administered orally to rats [26].
Rats were anesthetized via intraperitoneal injection of Nembutal (40 mg kg−1), maintaining deep surgical anesthesia for at least 2 h [27]. The target skeletal muscles were isolated from surrounding tissues, tendons were transected distally, and ventral roots were severed at their spinal cord exit points [22, 27].
2.3. Biomechanical and Biochemical Analyses
The mechanokinetic parameters of muscle contraction were recorded using a National Instruments (USA) USB-6009, a 12-bit analog-to-digital converter (ADC) [27].
- -
Time to establish smooth tetanic muscle contraction (t1);
- -
Time for contraction force to decay to zero after stimulation cessation (t2);
- -
Integrated muscle power (area under the force curve; S) calculated numerically using Origin 9.4 software;
- -
The minimum force during a single contractile act (Fmin);
- -
Time to reach peak force response (tstart).
The pro- and antioxidant balance in muscle tissue, assessed by measuring reduced GSH level and the activities of selenium-dependent GPx, CAT, and SOD, was evaluated as markers of muscle damage [22, 27, 28]. Analyses were performed using biochemical analyzers: RNL-200 (Netherlands), ABX Micros ESV60, and Pentra C400 (France).
2.4. Statistical Analysis
The statistical analysis of the experimental results was conducted using the software package Statistica 8.0 (Dell, USA) through a mixed design analysis of variance (ANOVA) procedure [27]. Two between-group factors were considered: (1) alcoholization (with three levels: 3, 6, and 9 months of alcohol exposure) and (2) C60FAS treatment (with two levels: no treatment and C60FAS treatment). The Shapiro–Wilk W-test and Levene’s test were used to assess the normality and equality of variances across groups, respectively. Multiple pairwise comparisons between different groups and conditions were performed by the Bonferroni post hoc test. Differences between groups were considered statistically significant at p < 0.05. Each experimental force curve represents the average of 10 similar tests, and all biochemical measurements were repeated at least three times.
3. Results and Discussion
3.1. AFM Study
Since the size of nanoparticles affects their toxicity for biomedical applications [29, 30], we conducted AFM research on the resulting C60FAS.
As seen in the 3D AFM image (Figure 1), the C60FAS consisted of randomly located point-like objects, with heights reaching up to 3.5 nm. The typical width of their profiles was 10–15 nm. Taking into account that the radius of curvature of the tip of the used AFM probe was ∼12 nm, we believe that the nanoobjects had a spherical shape, corresponding to either a single C60 molecule (∼0.7 nm) or its nanoaggregates with diameters ranging from 1.4 to 3.5 nm. This was consistent with our previous theoretical results [31].
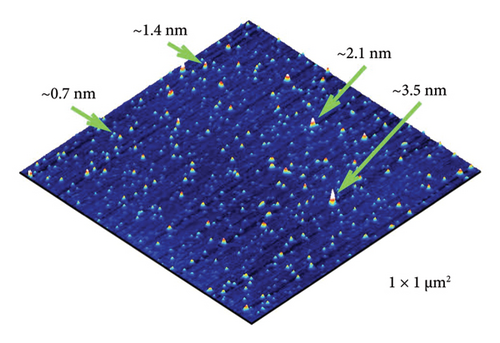
Thus, the detected nanoformation of the C60 fullerenes in an aqueous solution indicates their suitability for in vivo tests.
3.2. Muscle Contraction Analysis
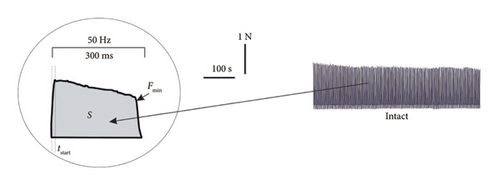
Figures 2(a), 2(b), and 2(c) show the mechanograms of rat muscle soleus contraction at the application of an increasing stimulation signal with a frequency of 0–50 Hz for a duration of 5 s for an intact group of rats in Figure 2(a), groups of alcoholized rats in Figure 2(b), and rats receiving C60FAS (1 mg kg−1) along with alcohol in Figure 2(c).

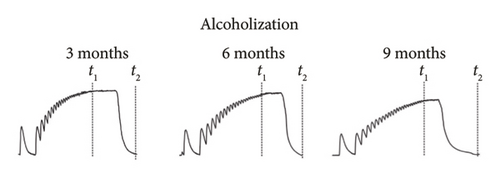
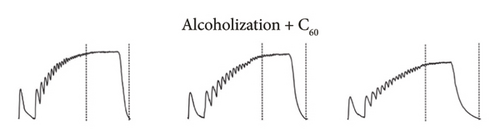
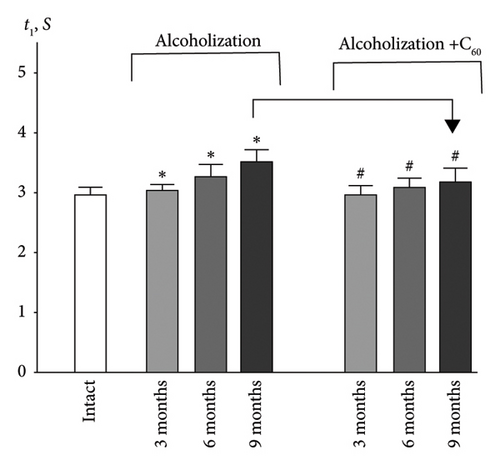
Analysis of smooth contractile reactions (smooth tetanus) provides insight into the disruption of force generation by individual motor units [19]. The use of stimulation pools increasing in frequency made it possible to determine the time of establishment of smooth tetanic muscle contraction (t1) in the experimental groups of animals (Figures 2 and 3(a)). In the intact group, the level of this index was 3.0 ± 0.2 s for the muscle soleus (Figure 3(a)). Three-month alcoholization did not significantly change the time to establish smooth tetanic contraction of muscle soleus (Figure 3(a)). At the same time, alcoholization during 6 and 9 months increased its level up to 3.2 ± 0.2 and 3.5 ± 0.2 s, respectively. The application of C60FAS reduced this time almost to the value in the intact group.
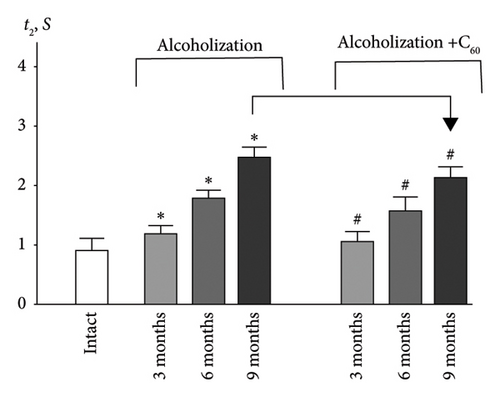
The time it takes for the muscle contraction force to decrease to zero after the cessation of stimulation (t2) (Figures 2 and 3(b)) is an important indicator of muscle stiffness increase, which is associated with the formation of connective tissue components in the muscle [32]. The increase in stiffness opposes the contraction of the muscle fiber and its physiological transverse thickening during contraction, thereby prolonging the time required for the muscle to return to its initial state. Note, however, that this index depends not only on the contractile components but also on the nature of the interaction between them and is therefore a marker of the development of myopathic disorders of muscle kinetics [1, 5].
Figure 3(b) shows that in the intact group, the time for the force to decrease to zero in the muscle soleus was 0.9 ± 0.1 s. Alcoholization led to a significant increase in this parameter, particularly at 6 and 9 months, reaching 1.8 ± 0.1 and 2.5 ± 0.1 s, respectively, indicating notable degenerative changes in the muscle soleus. The application of C60FAS reduced this index nearly to the intact group level after 3 months of alcoholization and to 1.5 ± 0.1 and 2.1 ± 0.1 s at 6 and 9 months of alcoholization, respectively. The most positive effect of C60FAS application was observed at 9 months, with 17 ± 1% improvement compared to the alcoholization group.
One of the effective approaches to identify the level of pathology in the muscle system is analyzing the development of fatigue processes during nonrelaxation stimulation of the muscle [18]. These processes play an important role in ensuring the precise positioning of movements of the musculoskeletal system. In this context, several biomechanical parameters were investigated as markers of muscle fatigue development [18, 19].
Figures 4(a), 4(b), and 4(c) show mechanograms of 500 consecutive contractions of muscle soleus in alcoholized rats when relaxation-free pools of 50-Hz frequency for a duration of 3 s each were applied for an intact group of rats Figure 4(a), groups of alcoholized rats Figure 4(b), and rats receiving C60FAS Figure 4(c).


Analysis of the integrated muscle power (S) (Figures 4 and 5(a)) allows us to evaluate the formation of muscle activity in the “force-external load” system, which is essentially a physiological analog of the performance of the muscle system [19]. In the group of intact animals, this index decreased from 100% at the 1st contraction to 94 ± 2% at the 500th contraction of muscle soleus (Figure 5(a)). Testing of the muscle soleus revealed a significant decrease in the integrated muscle power at 6 and 9 months of alcoholization: 22 ± 1% and 30 ± 1% (1st contraction) and 30 ± 1% and 44 ± 2% (500th contraction), respectively, relative to the intact group. Application of C60FAS increased this index, and at 6 and 9 months of alcoholization, its difference was 22 ± 1% and 37 ± 1% (500th contraction), respectively, relative to the alcoholization group (Figure 5(a)).
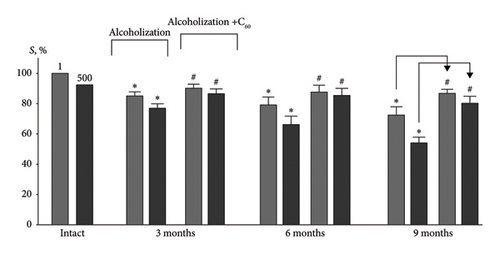
For comparison: In the case of the muscle gastrocnemius [19], the most positive effect of C60FAS was 26 ± 2% following 9 months of alcoholization of animals.
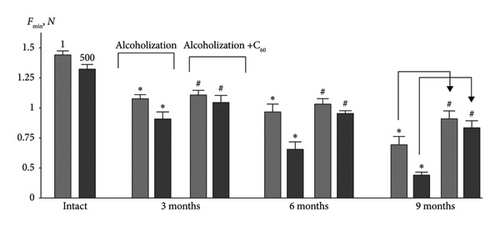
The change in the level of minimal contraction force (Fmin) (Figures 4 and 5(b)) is the most sensitive indicator of muscle dysfunction caused by pathological processes [18]. Thus, this index for muscle soleus decreased by 25 ± 1% and 38 ± 2%, 36 ± 2% and 50 ± 2%, 51 ± 2% and 62 ± 3% at the 1st and 500th contractions at 3, 6, and 9 months of alcoholization, respectively, relative to the intact group (Figure 5(b)). The application of C60FAS increased this parameter by 11 ± 1% (1st contraction) and 23 ± 1% (500th contraction) at 3 and 6 months of alcoholization, respectively. At 9 months of alcoholization, the increase was 11 ± 1% (1st contraction) and 37 ± 2% (500th contraction) relative to the alcoholization group.
For comparison: In the case of the muscle gastrocnemius [19], the most positive effect of C60FAS was 33 ± 3% following 9 months of alcoholization of animals.
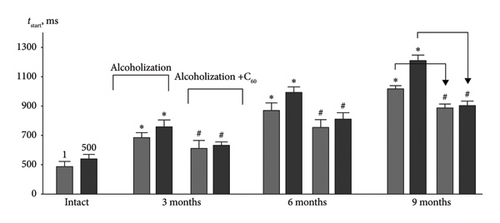
The increasing time to reach the maximum force response (tstart) (Figures 4 and 5(c)) is also an important marker of dysfunctions in the muscle’s excitation–contraction system, as it indicates the possibility of blocking the accurate positioning of the joint during movement. Thus, this index increased by 38 ± 2% and 30 ± 2%, 43 ± 2% and 45 ± 2%, 52 ± 3% and 55 ± 3% for the 1st and 500th muscle soleus contractions at 3, 6, and 9 months of alcoholization, respectively, compared to the intact group (Figure 5(c)). The positive effect of C60FAS was 14 ± 1% and 22 ± 1%, 12 ± 1% and 20 ± 1%, 15 ± 1% and 25 ± 1%, respectively, relative to the alcoholization group.
In summary, the most significant biomechanical effects of C60FAS (over 25%) occur at long-term alcoholization in animals (9 months) under the development of high-intensity fatigue in the muscle soleus, which agrees with the results for the muscle gastrocnemius [19].
3.3. Biochemical Analysis of Muscle Tissues
The BAC in alcoholized rats ranged from 140 ± 5 mg dL−1 (3 months of alcohol consumption) to 261 ± 6 mg dL−1 (9 months of alcohol consumption). These results agree well with previously reported data [23]. The administration of C60FAS alongside ethanol did not significantly alter the BAC values [18–20].
Antioxidant defense enzymes, particularly SOD and CAT, play a critical role in regulating ROS and peroxide processes [33]. Research [34] has demonstrated that under conditions of physical exercise, SOD activity in the skeletal muscles of animals increases predominantly in oxidative muscles without significant changes in mRNA expression. In our experiment, SOD activity in the muscle soleus increased by 11 ± 1%, 18 ± 1%, and 36 ± 2% at 3, 6, and 9 months of alcoholization, respectively, compared to the intact group (Figure 6(a)). The most pronounced positive effect of C60FAS was observed at 9 months of alcoholization, with an increase of 25 ± 1% relative to the alcoholization group.
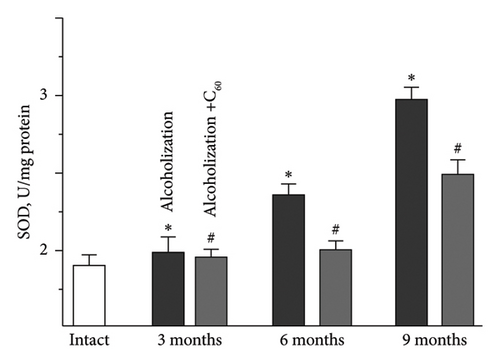
For comparison: In the case of muscle gastrocnemius [19], the most positive effect of C60FAS was 44 ± 2% following 9 months of alcoholization of animals.
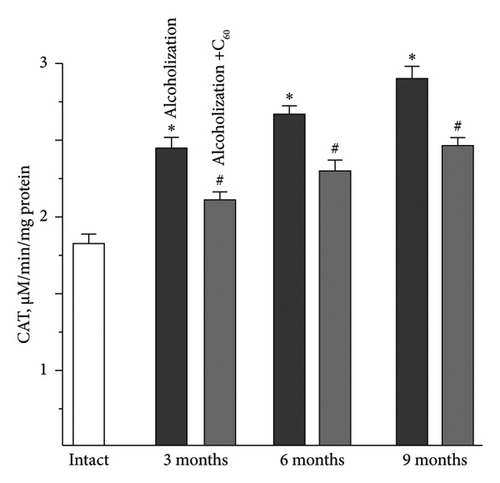
The increase in CAT activity in the muscle soleus was as follows: 21 ± 1%, 27 ± 1%, and 32 ± 2% at 3, 6, and 9 months of alcoholization, respectively, relative to the intact group (Figure 6(b)). The most pronounced positive effect of C60FAS was observed at 9 months of alcoholization with a 20 ± 1% increase relative to the alcoholization group.
For comparison: In the case of the muscle gastrocnemius [19], the most positive effect of C60FAS was 48 ± 2% following 9 months of alcoholization of animals.
Despite a significant body of research, data on CAT activity under conditions of intensive physical exercise and the development of pathologies in skeletal muscle remain contradictory. Thus, it was found [34] that chronic exercise does not alter CAT activity in slow muscle but reduces it in fast muscle. Research [35] reported a decrease in CAT activity in both oxidative and glycolytic types of muscle fibers. Finally, the authors [36] recorded an increase in CAT activity during exercise in both muscle types.
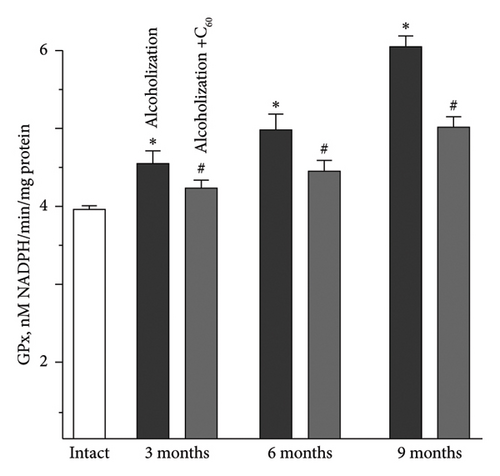
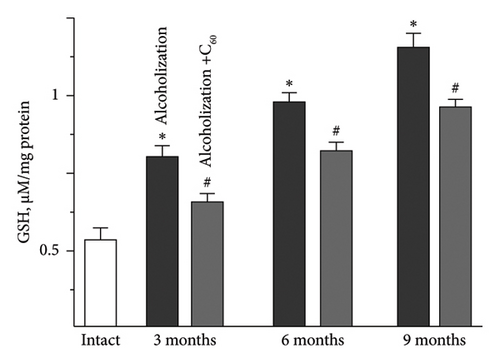
Along with antiradical enzymes, the GSH link is an active component of the antioxidant defense of the organism [33, 37–39]. The increase in GPx activity in the muscle soleus was 11 ± 1%, 18 ± 1%, and 27 ± 1% at 3, 6, and 9 months of alcoholization, respectively, relative to the intact group (Figure 6(c)). The most positive effect of C60FAS was 21 ± 1% relative to the alcoholization group for 9 months of alcoholization.
For comparison: In the case of the muscle gastrocnemius [19], the most positive effect of C60FAS was 39 ± 2% following 9 months of alcoholization of animals.
GSH content in the muscle soleus increased by 25 ± 1%, 33 ± 2%, and 99 ± 4% at 3, 6, and 9 months of alcoholization, respectively, relative to the intact group (Figure 6(d)). The most positive effect of C60FAS was 23 ± 1% at 9-month alcoholization relative to the alcoholization group.
For comparison: In the case of the muscle gastrocnemius [19], the most positive effect of C60FAS was 42 ± 2% following 9 months of alcoholization of animals.
Thus, the most significant biochemical effects of C60FAS (exceeding 20%) are observed during long-term (9 months) alcohol administration in animals, which agrees with the results for the muscle gastrocnemius [19].
In summary, the application of C60 fullerenes contributes to the reduction of oxidative processes in both the soleus and gastrocnemius muscles of rats. This can be attributed to the maintenance of a balance between the antioxidant defense system and pro-oxidants, which mitigates the negative effect of ROS on cellular and subcellular structures during the development of muscle fatigue in alcohol-exposed rats [27].
Possible molecular mechanisms describing the obtained data require further detailed study, since, for example, the use of such known antioxidants as carotenoids, vitamins, polyphenols, and flavonoids in treating chronic alcoholism demonstrates contradictory results [13]. For today, the following mechanism of C60 fullerene action can be considered. In alcoholic myopathy, ROS are formed in dysfunctional mitochondria [10], which leads to the destruction of cell membranes and functional disorders of their enzyme systems. So, a decrease in the activity of Na+/K+-ATPase inhibits the specific increase in Na+ conductivity that occurs in response to an adequate stimulus and thus prevents the development of excitation in myocytes. This can lead to a temporary imbalance in the time of establishment of smooth tetanus, which, in turn, increases the time required for the muscle to perform a force exercise. On the other hand, it is known that over 90% of alcohol in the body is metabolized through oxidative and nonoxidative pathways, resulting in the formation of highly reactive compounds that also actively generate ROS [40]. C60 fullerenes as powerful exogenous nanoantioxidants [16, 26, 41] with mitochondrial targeting [15] effectively inactivate ROS and thus reduce dysfunctions in muscle soleus of rats with chronic alcohol intoxication.
It has shown [42] that C60 fullerenes are capable of effectively adsorbing various aromatic molecules on their surface. It can be assumed that nanoaggregates of C60 fullerenes present in ethanol are capable of binding its molecules and, thus, entering the body, reduce the negative impact of alcohol on the muscular system functioning, which requires theoretical verification of this hypothesis.
4. Conclusions
The obtained biomechanical and biochemical data on the functioning of rat muscle soleus indicate that after 3, 6, and 9 months of alcoholization, the experimental animals develop significant dysfunctions in the skeletal musculature. A significant therapeutic effect of C60 fullerenes (at an oral dose of 1 mg kg−1 along with alcohol throughout the experiment) was observed in correcting the contractile activity of the alcoholized muscle soleus, as evidenced by the studied biomechanical and biochemical parameters (on average over 20%). This effect is attributed to the modification of ROS-dependent mechanisms, which play a critical role in alcoholic myopathy. These findings highlight the potential of C60 fullerenes for correcting pathological states of the skeletal muscles induced by alcohol intoxication.
Ethics Statement
The study protocol was approved by the Biomedical Ethics Committee of the ESC “Institute of Biology and Medicine” of Taras Shevchenko National University of Kyiv (protocol No. 2 dated September 2, 2022) and performed following Article 26 of the Law of Ukraine “On the Protection of Animals from Cruelty” (No. 3447-IV, 21.02.2006), as well as European Union Directive of 22 September 2010 (2010/63/EU) for the protection of animals used for scientific purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Olexandr Motuziuk and Dmytro Nozdrenko: methodology, biomechanical analysis, analysis of the data, and preparation of the manuscript. Svitlana Prylutska, Kateryna Bogutska, and Olena Dmytrotsa: biochemical analysis. Yurii Osyp and Peter Scharff: preparation and characterization of the samples and analysis of the data. Yuriy Prylutskyy: conceptualization, methodology, coordination of the research work, analysis of the data, preparation of the manuscript, writing – original draft preparation, and writing and review–editing. Uwe Ritter: coordination of the research work, preparation of the manuscript, and writing and review–editing.
Funding
We acknowledge support for the publication costs by the Open Access Publication Fund of the Technical University of Ilmenau, Germany.
Acknowledgments
We acknowledge support for the publication costs by the Open Access Publication Fund of the Technical University of Ilmenau, Germany.
Open Research
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request. Apart from the data sets used, no Supporting Information is available.




