Effects of a Magnetic Field on the Nucleation and Growth of Silver Nanoparticles Prepared via Chemical Reduction
Abstract
This study investigates the effects of external magnetic fields on the synthesis of silver nanoparticles and the modulation of their properties. Silver nanoparticles were synthesized via liquid-phase chemical reduction. Their composition, micromorphology, and surface properties were characterized using scanning electron microscopy (SEM), differential scanning calorimetry, infrared (IR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). The results show that external magnetic fields significantly influence the morphology, size, distribution, and optical and electrical properties of the silver nanoparticles. Specifically, applying a magnetic field during the reduction of silver ions to monomeric silver using formaldehyde as the reducing agent promotes anisotropic growth. This research proposes a novel magnetic field–assisted method to control silver nanoparticle synthesis. In addition, it establishes a theoretical and experimental basis for their applications, particularly in sensors, catalysts, and electronic devices. For example, in sensor applications, the ability to precisely control the properties of silver nanoparticles using magnetic fields can enhance both sensitivity and selectivity, enabling more accurate detection of target substances. Theoretically, this work deepens the understanding of magnetic field effects on nanoparticle growth dynamics. These insights can help optimize nanoparticle properties for advanced applications, such as electronic packaging and energy storage devices.
1. Introduction
Silver nanoparticles possess the intrinsic properties of metallic silver along with unique features derived from nanoscale effects, such as exceptional catalytic [1, 2], thermal [3–6], and electrical characteristics. These distinctive properties make silver nanoparticles invaluable in various applications, including antimicrobials [7–11], sensors [12, 13], and thin-film electrodes. The preparation of silver nanoparticles is typically categorized into chemical [14–17], physical [18–22], and biological [1, 23–25] methods, depending on the underlying reaction mechanisms. Among these, green synthesis methods have emerged as an environmentally friendly and sustainable approach to silver nanoparticle production [26–30]. By utilizing natural reducing agents such as plant extracts, amino acids, and proteins, this method avoids the toxic reagents commonly used in traditional chemical synthesis.
Among these natural reducing agents, tryptophan has garnered attention for its dual role as both a reducing and stabilizing agent in silver nanoparticle synthesis. Methods employing tryptophan include photoreduction [31], chemical reduction [32], and microwave-assisted synthesis [33]. Tryptophan acts as both a reducing agent (for silver ions) and a stabilizer for the nanoparticles. The resulting silver nanoparticles show outstanding antibacterial activity and optical properties, enabling their use in biomedical and sensor fields [34].
Green synthesis offers cost advantages, low pollution, and scalability, making it ideal for large-scale production. An important aspect of green synthesis is the self-assembly of silver nanoparticles into thin films, which is crucial for applications in optics, catalysis, and biomedical fields. Surface-active agents or stabilizers can guide the orderly arrangement of silver nanoparticles, forming stable and uniform thin-film structures essential for their efficient application in various devices [35–39].
Seedless synthesis has emerged as a promising approach that does not require traditional seeds or templates, directly generating nanomaterials through chemical reactions. For example, Zaheer and Aazam [40] developed a simple chemical competition approach to synthesize Ag@Au bimetallic nanoparticles. In this method, silver nitrate (AgNO3), sodium chloride (NaCl), and sodium cyanide (NaCN) were employed as reactants, with cetyltrimethylammonium bromide (CTAB) acting as a structure-directing agent. Through the competitive coordination between Cl− and CN− ligands, flower-like nanoparticles were successfully obtained. Seedless synthesis techniques provide new possibilities for preparing nanocomposites with specific optical properties. By adjusting reaction conditions, such as metal ion concentration and the types of additives, nanoparticle morphology and optical characteristics can be precisely controlled [41, 42].
Despite significant advancements, key challenges remain in nanoparticle synthesis, including unstable particle sizes, irregular morphologies, and agglomeration. These issues, such as poor size control and time-dependent instability, hinder performance in catalysis and sensor technologies, underscoring the need for improved synthesis strategies.
In nanomaterial research, external stimuli play a critical role in influencing the size, shape, structure, and crystal growth of materials. Parameters such as stress [43], temperature [44, 45], pH [46], electric fields [47], and magnetic fields [48] can be strategically utilized to control material characteristics. Furthermore, studies have highlighted that the growth and stability of silver nanoparticles can be optimized by controlling synthesis conditions such as pH, temperature, and reducing agent concentration. Silver nanoparticles are known for their outstanding antimicrobial activities, effectively inhibiting a wide range of Gram-positive and Gram-negative bacterial strains, as well as Candida fungus [49–51].
Among these, magnetic fields have garnered substantial attention due to their ability to promote anisotropic growth in various metallic nanoparticles, including nickel dendrites [52], cobalt ball flux links [53], silver dendrites [54], and even protein crystals [55]. Over the past decades, magnetic fields have emerged as effective tools for modulating crystal growth [56–59], enabling applications such as the preparation of magnetic cobalt nanocrystals [60] and the alignment of < 110 >-oriented Fe3O4 nanowires [61]. These findings underline the significance of magnetic fields in both basic and applied chemistry research [62–64].
In recent years, the synthesis of silver nanoparticles has gained significant attention due to their unique properties, with external fields such as magnetic fields emerging as promising tools to influence nanoparticle growth and assembly [45]. Previous studies have demonstrated that magnetic fields can induce the formation of silver nanochain-like structures during chemical reduction, as evidenced by Zhang et al., who reported the generation of dendritic silver nanoparticles under the influence of magnetic fields [65]. This suggests that magnetic fields play a critical role in restricting diffusion within the liquid phase while directing the one-dimensional assembly of nanoparticles into superstructures by modulating the reaction system’s energy. In addition, it has been shown that altering reaction energy, such as applying high or low potentials during electrochemical deposition, can yield multitwin crystal particles or regularly arranged structures, respectively. A similar mechanism is hypothesized in the presence of magnetic fields, where they control the diffusion and aggregation dynamics of nanoparticles, guiding the formation of uniform, anisotropic structures [66, 67].
Despite these advancements, challenges persist in synthesizing silver nanoparticles with precise shape control, stable particle sizes, and enhanced morphologies. Current synthesis methods often face difficulties in overcoming issues such as irregular morphology and agglomeration. External field-assisted physical methods, including microwave-assisted synthesis [68], radiation synthesis, magnetic field control, photoinduction [69], and ultrasonic-assisted synthesis [70], have shown potential in addressing these issues. However, these techniques still require further investigation to fully exploit their capabilities [71].
In this study, we propose an innovative approach to improve the synthesis of silver nanoparticles by leveraging a more refined control of the external magnetic field and reaction parameters. By optimizing the magnetic field intensity and reaction conditions, we demonstrate enhanced control over nanoparticle morphology, size uniformity, and dispersibility, which are crucial for their applications in advanced electronic packaging. This approach not only addresses the challenges posed by traditional synthesis methods but also provides a new avenue for the controlled assembly of silver nanoparticles, opening up new possibilities for their use in various technological fields.
2. Experimental Work
2.1. Materials and Test Equipment
Silver nitrate (AgNO3) and stearic acid (C18H36O2) were purchased from Sinopharm Chemical Reagent Co. Formaldehyde (HCHO) was purchased from Shanghai McLean Biochemical Technology Co., Ltd. Anhydrous ethanol (C2H5OH) was purchased from Tianjin Fuyu Fine Chemical Co. Ammonia (NH3·H2O) was purchased from Shanghai McLean Biochemical Technology Co., Ltd. All chemical reagents were used as received without further purification. Sodium hydroxide (NaOH) was synthesized in the laboratory. Deionized water (H2O) was used for all experiments.
The instruments used in this study include electronic balance (FA2004, Shanghai Sunyu Hengping Scientific Instrument Co., Ltd.), constant speed electric stirrer (JJ-1B 200W, Changzhou Surui Instrument Co., Ltd.), circulating water vacuum pump (SHZ-D, Shanghai Kesheng Instrument Co., Ltd.), ultrasonic cleaner (KQ-3200E, Kunshan Meimei Ultrasonic Instrument Co., Ltd.), and vacuum drying oven (DZE, Beijing Yongmingming Medical Instrument Co.).
2.2. Preparation of Silver Nanoparticles
First, 1.08 g of silver nitrate was weighed and added to 360 mL of deionized water to form a solution. Thereafter, the solution was stirred using an electronic stirrer under the application of a magnetic field. Afterward, 4.5 mL of ammonia and 2 mL of 20 g/L sodium hydroxide were injected into the silver nitrate solution to adjust the pH value to ∼11. Next, 0.06 g of stearic acid was dissolved in alcohol and added to the solution under continuous stirring. We then rapidly added 4.8 mL of formaldehyde, which caused the solution color to transition from brownish-yellow to black. The mixture was stirred for 2 h to form a black suspension. After filtration through a Büchner funnel and two ethanol washes, the product was vacuum-dried to yield micro/nanoscale silver powder. This powder was used for the initial experiments (Figures 1(a) and 1(b)).

In this study, we systematically varied the magnetic field configuration by using different numbers of magnets (1–4). During chemical reduction, we monitored the field strength in the reaction beaker by using a Gauss Meter. The measurements revealed field strength variations that depended on both the number of magnets and their spatial orientation (horizontal vs. vertical), consistent with the magnets’ anisotropic properties.
2.3. Preparation of Silver Nanoparticles on Silicon Single-Crystal Wafers
Silicon wafer (100) was cut into 1 cm × 1 cm × 1 cm pieces, and a measured 4.8 mL formaldehyde was added dropwise using a pipette. Thereafter, the silver nitrate solution with a concentration of 3 g/L was configured on the single-crystal wafer such that the silver nanoparticles were directly deposited on the silicon single-crystal wafer. After 2 h, the silicon single-crystal wafer was collected and three or four sets of experiments were performed in this manner.
2.4. Characterization of the Sample Performance
For each test method, we used three samples prepared under the same batch conditions. The micromorphology of the nanomaterials was observed using a scanning electron microscope (Nova NanoSEM 230, FEI Czech Republic s.r.o., Czech Republic), and the particles were structurally analyzed and characterized using Fourier transform infrared (IR) spectroscopy (model Nicolet iS50, Thermo Scientific, United States). The sample surface was qualitatively, quantitatively, and structurally analyzed using X-ray photoelectron spectroscopy (XPS) (ESCALAB 250Xi, Thermo Fisher Scientific, United States). Thermogravimetric IR gas chromatography (PerkinElmer TG8000-FTIR-GCMS-ATD, PerkinElmer, United States) was used to determine the thermophysical properties of the sample with respect to temperature or time to characterize the molecular structure of the substance, structure of the polymer space, changes in molecular motion to determine the solid–liquid interface, and other relevant features of the substance.
3. Results and Discussion
Recently, the growth of metallic nanomaterials (e.g., nickel dendrites, cobalt spherocrystals, and silver dendrites) has attracted substantial attention as it can be induced by the application of a magnetic field. Moreover, most of the components used in previous experiments comprised magnetic materials, such as Ni, Co, and Fe. These substances possess strong magnetizing properties. This renders them effective for promoting chemical reactions even under applied magnetic field conditions. Numerous studies have shown that magnetic fields can affect reactant diffusion and electrochemical reaction rates, potentially accelerating the reduction of silver ions under suitable magnetic field conditions. In addition, the magnetic field also governs surfactant adsorption and molecular arrangement, consequently regulating silver nanoparticle morphology and size. This regulation depends on field parameters (strength/direction), reaction conditions, and nanoparticle properties.
3.1. Microstructure Analysis
In the macroscopic analysis, all samples obtained via the reaction under the application of different magnetic field strengths were earthy yellow suspensions with negligible variability. After extraction and filtration, the samples were obtained as silvery mixtures, which were washed to yield gray–black powders. At the microscopic level, scanning electron microscopy (SEM) images reveal different particle sizes and morphologies under different magnetic field conditions. When a magnetic field was not applied (Figure 2(a)), the sample surface was rough with small particles at the edges. After the application of a horizontally oriented magnetic field of H = 18.07 mT (Figure 2(b)), the particle surfaces are smooth without unevenness. The particle surfaces are smoother and uniformly distributed under the condition of H = 28.65 mT (Figure 2(c)). Under the conditions of H = 38.71 and 49.27 mT, the surfaces are smooth but possess a few small particles (Figures 2(d) and 2(e)). Overall, SEM observations demonstrated that the samples were formed via the mesoscale assembly of primary nanoparticles, which possessed a spherical or spherical-like morphology, with diameters of 10–100 nm.
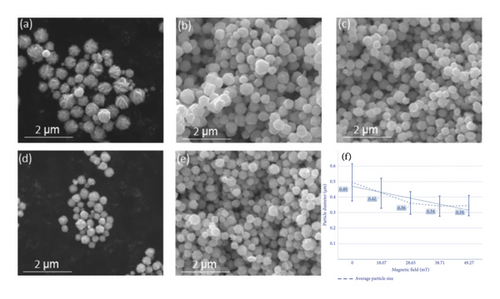
The SEM analysis results of the samples are presented in Figure 2(f). Particle size analysis demonstrated that increasing magnetic field strength during synthesis reduces the average silver nanoparticle size from 490 to 340 nm while narrowing the size distribution. The range decreases from 240 to 140 nm. The related data are presented in the form of a line graph in Figure 2(f), indicating that the magnetic field affects the nucleation and growth of the powder particles during the preparation of silver nanoparticles via stirring and injection.
Based on the sample preparation method described in Section 2.2, a magnetic field was applied underneath the beaker and the reaction was performed via the stirring and injection method. Afterward, the silver nanoparticle samples were extracted, filtered, and cleaned. The SEM images of the samples shown in Figure 3 reveal that the particle size and morphology of the silver nanoparticles obtained under different conditions vary. In Figure 3(a), the sample surface is rough under the condition without a magnetic field. In Figure 3(b), after applying a magnetic field of H = 63.06 mT in the vertical direction, the particle surface is smooth and the particle size distribution is uniform. In Figure 3(c), after applying a magnetic field of H = 114.21 mT in the vertical direction, the silver nanoparticles are agglomerated, with smooth surfaces and a uniform particle size distribution. After applying a magnetic field of H = 157.04 mT in the vertical direction (Figure 3(d)), a small number of particles with small particle sizes and smooth surfaces appear. Figure 3(e) presents a smooth surface of the powder after the application of a vertically oriented magnetic field of H = 180.78 mT.
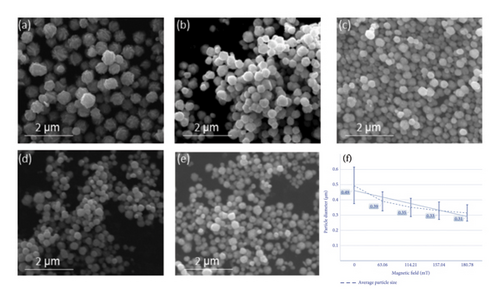
The experimental results reveal that with an increase in the strength of the magnetic field during the reaction process, the average particle size of the prepared silver nanoparticles exhibited an overall decreasing trend and the particle size distribution of the powder particles became narrower. The specific data are presented in the form of a line graph in Figure 3(f). These findings are consistent with the experimental results obtained with the application of the magnetic field in the horizontal direction, i.e., when the strength of the magnetic field increases, the size of the as-prepared sample particles gradually decreases. The width of the distribution of the powder particle size distribution decreases.
3.2. Analysis of IR Spectral Results
Figure 4 presents the IR spectra of the first and second groups of samples. The sample spectra demonstrated flatter peaks at 3450.03 and 1654.62 cm−1. The spectra of the nine groups of experimental samples revealed small peaks at approximately 3450 and 1650 cm−1, which were attributed to O–H stretching vibration and H–O–H bending vibration of water present in the free state in the tested sample powders [72, 73]. Interestingly, in Samples 3 and 4, prepared under magnetic fields of 28.65 and 38.71 mT, respectively, an additional absorption peak near 1100 cm−1 was detected. This peak is commonly assigned to C–O or C–N stretching vibrations, often originating from residual organic species such as citrate ions or other capping/reducing agents used during synthesis. The selective appearance of this peak suggests that the applied magnetic field in this range may have altered the nanoparticle surface structure or growth dynamics. Specifically, moderate-intensity magnetic fields could influence the diffusion of reactants and the alignment of growing crystal facets, possibly resulting in a more developed or porous surface morphology. Such changes may increase the surface area or active sites available for organic species to adsorb, making their IR signatures more detectable. In contrast, samples without a field (sample 1) or with very low (18.07 mT) or high (49.27 mT) magnetic field strengths may undergo different growth regimes. In these conditions, the particle surfaces may be either too compact (leading to minimal surface adsorption) or too kinetically controlled (limiting organic residue binding), hence the absence of the 1100 cm−1 peak.
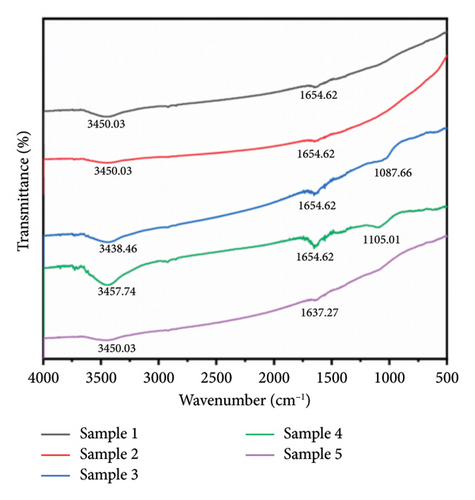
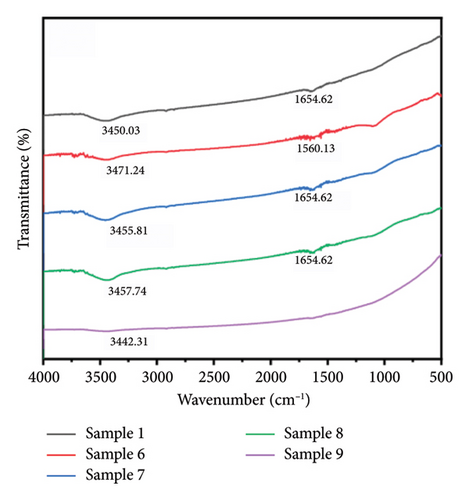
In addition, a weak but consistently observed absorption band around 2900 cm−1 was detected in all IR spectra, regardless of the applied magnetic field. This region corresponds to the C–H stretching vibrations of methylene (–CH2–) and methyl (–CH3) groups. The ubiquitous presence of this signal suggests trace amounts of stearic acid from the synthesis process, which remain weakly adsorbed on the silver nanoparticle surfaces.
3.3. Analysis of XPS Results
To determine the surface valence of the prepared silver nanoparticles, the experimental samples were analyzed via XPS. Figure 5 depicts the XPS full spectra of the silver nanoparticles obtained under the conditions of no magnetic field and horizontally oriented magnetic fields with different strengths. Ag 3d peaks are detected at 373.76 and 367.80 eV, which are attributed to 3d3/2 and Ag 3d5/2, respectively. The XPS full spectra reveal the presence of Ag 3p1/2, Ag 3p3/2, C 1s, O 1s, and N 1s signals.
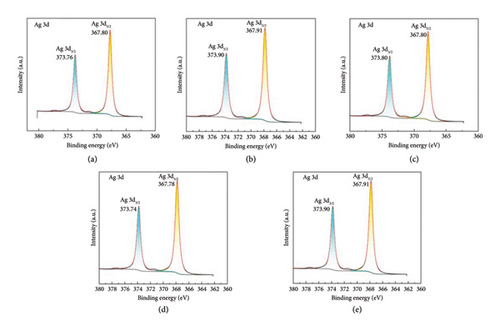
In the XPS full spectra of all aforementioned five sets of images, strong Ag 3d3/2, Ag 3d5/2, Ag 3p1/2, Ag 3p3/2, and C 1s peaks were observed. Furthermore, peaks corresponding to N and O elements were observed in the XPS full spectra. The peak of the O element was noted at ∼531.60 eV. An analysis of the XPS database revealed that the prepared silver nanoparticles were partially oxidized to form AgO. In addition, all peaks of the N element appeared at ∼399.40 eV. Compared with those in the standard Ag 3d3/2 spectrum, the Ag 3d3/2 binding energy in all aforementioned Ag spectra increased and all the peaks shifted toward high binding energies. These findings indicate that the generation environment of the silver nanoparticles in the various aforementioned reaction systems differently changed such that the density of the outer electron cloud decreased, resulting in a considerable reduction in its shielding effect on the inner electrons. Furthermore, the binding energy of the inner electrons considerably increased. The peak in the 1s energy spectrum of element N shifts toward low binding energies, indicating that N atoms have gained electrons during the preparation process of the silver nanoparticles.
XPS and IR analyses together provide a comprehensive understanding of the silver nanoparticles’ surface chemistry. XPS revealed partial oxidation to AgO, with the O1s peak at ∼531.60 eV, indicating oxidation and changes in the electronic environment of the silver. IR spectra, showing peaks at 3450 and 1650 cm−1, identified water molecules adsorbed on the nanoparticle surface.
In the chemical reduction method, the role of strong mechanical stirring in diminishing the impact of magnetic fields on silver nanoparticle nucleation is significant [74, 75]. The agitation caused by stirring disrupts the alignment of nanoparticles in the magnetic field, which weakens the magnetic influence during the initial nucleation phase. As a result, we hypothesize that the magnetic field plays a limited role in the early stages of nanoparticle formation, where the nucleation process is primarily driven by chemical reduction without substantial interference from the magnetic forces.
However, the situation changes during the growth phase of silver nanoparticles, where aggregation becomes a key issue. In the absence of a magnetic field, nanoparticles tend to aggregate due to Brownian motion, van der Waals forces, and mechanical stirring [76, 77]. These interactions lead to the formation of larger particle clusters, which can negatively impact the material’s uniformity and performance. This is particularly important when considering applications that require precise control over particle size and dispersion, such as in electronics and catalysis.
When a magnetic field is introduced, the situation changes dramatically. Silver atoms and nanoparticles experience Lorentz forces, which act perpendicular to both their direction of motion and the magnetic field. The exact direction of these forces depends on the orientation of the magnetic field relative to the stirring motion. When the magnetic field aligns parallel to the stirring direction, the silver atoms are pushed toward the top of the container, whereas, if the magnetic field is perpendicular to the stirring direction, the atoms are directed toward the container’s exterior [78–80]. These deviations from the original stirring motion result in a more controlled trajectory for the nanoparticles, reducing their interaction frequency with other particles. As a consequence, the reduced frequency of atomic and particle collisions diminishes the likelihood of nanoparticle aggregation [81]. This mechanism is particularly advantageous in preventing the formation of larger clusters, which often leads to broad-size distributions and compromised material properties. Interestingly, the extent of this effect is directly correlated with the strength of the applied magnetic field: the stronger the magnetic field, the more pronounced the deviation in particle trajectories, which enhances dispersion and results in smaller, more uniform particle sizes.
The application of magnetic fields in chemical reduction for the synthesis of silver nanoparticles offers substantial advantages in terms of particle purity, uniformity, and size control. By improving dispersion and reducing aggregation, this technique enhances the overall quality of the synthesized nanoparticles, making them more suitable for advanced applications in electronics, catalysis, and antimicrobial treatments. Precise control of particle growth and size distribution optimizes material performance while enabling tailored property adjustments for specific applications.
Looking to the future, further optimizations in magnetic field control could lead to even more significant improvements in the synthesis process. Potential breakthroughs in this area may unlock new possibilities in the development of high-performance materials for use in advanced electronics, next-generation catalysts, and cutting-edge medical treatments. These advancements could pave the way for more efficient, sustainable, and customizable nanoparticle-based technologies, underscoring the critical role of magnetic fields in controlling nanoparticle synthesis.
4. Conclusions
- (1)
The applied magnetic field affects the particle size of the produced silver nanoparticles. Within the range of the magnetic field strengths applied in this experiment, an increase in magnetic field strength led to a gradual decrease in particle size and a more concentrated particle size distribution. This is because the magnetic field made the atoms’ trajectories deviate within the initial stirring motion, diminishing the frequency of collisions between atoms and particles. This reduction in collision frequency decreases the likelihood of forming larger clusters leading to smaller nanoparticles.
- (2)
The IR spectra of the nine groups of experimental samples showed small peaks around 3450 and 1650 cm−1, attributed to the free-state water present in the tested sample powders. Peaks corresponding to other functional groups were negligible. This indicates that the silver powder obtained through filtration and twice alcohol cleaning possesses high purity, with negligible amounts of organic matter attached to the surface.
- (3)
It is found in XPS that the generation environment of the silver nanoparticles in the various aforementioned reaction systems caused a reduction in the density of the outer electron cloud, significantly diminishing its shielding effect on the inner electrons. Consequently, the binding energy of the inner electrons increased substantially. In addition, the peak in the 1s energy spectrum of nitrogen (N) shifted toward lower binding energies, suggesting that N atoms gained electrons during the preparation process of the silver nanoparticles.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zhirong Li and Yumeng Wang contributed equally to this work.
Funding
This work was supported by the Natural Science Foundation of Hunan Province, China (Grant no. 2019JJ50813) and the National Defense Pre-Research Foundation of China (Grant no. 61409220415).
Acknowledgments
This work was supported by the Natural Science Foundation of Hunan Province, China (Grant no. 2019JJ50813) and the National Defense Preresearch Foundation of China (Grant no. 61409220415).
Open Research
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the authors.




