Synthesis of Cu-Doped Fe3O4 Nanocomposites for Bacterial Disinfection
Abstract
In this work, copper-doped Fe3O4 magnetic nanocomposites were synthesized via the solvothermal method. The synthesized catalysts were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, transmission electron microscopy, inductively coupled plasma mass spectrometry, and electron paramagnetic resonance. The performance of the catalysts in the Fenton-like reaction was evaluated through the analysis of their antibacterial activity in the presence of hydrogen peroxide. Electron paramagnetic resonance results indicated that •OH and •OOH, generated from hydrogen peroxide, were the primary active components in the reaction system. The Cu2+-Fe3O4 nanocomposites showed stronger antibacterial activity towards Escherichia coli than Fe3O4, possibly due to the presence of Cu2+ which accelerated the circulation between Fe2+ and Fe3+ ions and enhanced the utilization efficiency of hydrogen peroxide. Furthermore, inductively coupled plasma mass spectrometry analysis demonstrated that the concentration of released copper ions in aqueous solution remained within safe limits. Herein, we may provide a promising environmentally friendly catalyst for water disinfection treatment.
1. Introduction
Water pollution-induced adverse health consequences continue to pose a significant threat to public health in China [1]. According to a comprehensive literature survey, the current global population lacking access to clean and safe drinking water amounts to approximately 785 million individuals [2]. This results in a significant number of fatalities annually, thereby presenting a substantial challenge to humanity. Among all the water contaminants, the pathogenic microorganism Escherichia coli is typically present [3]. E. coli is capable of causing various diseases, including gastroenteritis, parenteral infections of the urinary tract, bloodstream, and central nervous system infections [4]. These pose a significant threat to global health security, as the diarrheal diseases result in numerous deaths worldwide, with a particularly high toll among children under the age of five [5]. Thus, it is crucial to eliminate pathogenic microorganisms from contaminated water. The disinfection technology of harmful microorganisms has been widely studied by researchers.
Traditional water disinfection technologies, such as chlorine disinfection [6], ozone disinfection [7], and ultraviolet disinfection [8], have disadvantages such as high cost, low disinfection efficiency, and the production of toxic by-products [9]. Advanced oxidation processes (AOPs) are significant, promising, efficient, and environmentally friendly water treatment methods with great potential for the removal of pollutants from water or wastewater [10]. Fenton oxidation is one of the AOPs that employs hydrogen peroxide (H2O2) to react with ferrous ions, generating highly reactive hydroxyl radicals (•OH). The strong oxidative capacity of these radicals is capable of disrupting bacterial cell membranes/walls, ultimately leading to the inactivation of pathogenic microorganisms [11].
Compared with the homogeneous Fenton process, the heterogeneous Fenton process exhibits numerous benefits, including minimal iron ion leaching, efficient Fe3+ and Fe2+ cycling, reduced iron sludge generation, a broad operational pH range, and the recyclability and long-term stability of the catalysts [12]. The cyclic conversion of Fe3+ and Fe2+ under the action of H2O2 constitutes the primary mechanism of the Fenton reaction [13]. Fe3O4 serves as a widely utilized heterogeneous Fenton catalyst, as it not only facilitates the Fenton effect through the alteration of iron ion valence, but also exhibits excellent magnetic separation properties and recovery capabilities [14].
However, Fe3O4 is still unavoidably restricted by pH and its catalytic activity is significantly inhibited in neutral to alkaline water environments [15, 16]. To broaden the pH application spectrum of catalysts and expedite the redox cycling process of Fe3+ and Fe2+, metal doping is a frequently employed strategy [17]. Guo et al. [18] prepared Cu-doped Co3O4, which has a significant degradation effect on metronidazole within a pH range of 3.0–9.0, and they pointed out that the activation of H2O2 by copper ions can occur over a wide pH range [19]. During Fenton’s reaction, copper ions serve as electron intermediation centers to accelerate oxidation reaction based on the Cu(II)/Cu(I) couple [20, 21]. In addition, the reduction of Fe3+ by Cu+ is thermodynamically favorable, contributing to the redox cycles of Fe3+/Fe2+ and Cu2+/Cu+ [22, 23].
In this study, we report an innovative Cu-doped Fe3O4 magnetic nanocomposite for Fenton-like reaction aimed at disinfection. Analysis by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) demonstrated that the copper was successfully doped into the nanocatalyst. The Cu2+-Fe3O4 nanocomposites possessed an enhanced disinfection effect against the Gram-negative bacterium E. coli compared to pristine Fe3O4. Furthermore, inductively coupled plasma mass spectrometry (ICP-MS) analysis demonstrated that the concentration of copper ions in the aqueous solution did not exceed 0.2 mg/L. This is in full compliance with the international standards. This study provides a novel approach by integrating Cu doping into Fe3O4 nanocomposites, not only enhancing the catalytic efficiency through accelerated Fe3+/Fe2+ redox cycling but also addressing critical challenges such as pH versatility, environmental safety, and reusability. This study is also focused on the direct mechanistic validation of reactive oxygen species generation, while demonstrating sustained antibacterial performance and minimal ion leaching, making this system uniquely suited for real-world water disinfection applications.
2. Materials and Methods
2.1. Materials
Copper(II) chloride dihydrate (CuCl2·2H2O), with a copper content of 99%, and sodium hydroxide (NaOH) were purchased from Shanghai Aladdin Reagent Co., Ltd. Iron(III) chloride hexahydrate (FeCl3·6H2O), with 99% purity, was obtained from American Sigma Reagent Co., Ltd. Absolute ethanol was purchased from Sinopharm Group Chemical Reagent Co., Ltd. Potassium dihydrogen phosphate (KH2PO4) and disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O), both with a purity of 99%, were purchased from Tianjin Fengchuan Chemical Reagent Co., Ltd. All reagents mentioned above were of analytical grade and were used without further purification. Luria–Bertani (LB) medium and nutrient agar were purchased from Beijing Auboxing Biological Reagent Co., Ltd.
2.2. Cu-Modified Fe3O4 Catalyst Synthesis
Three mmol of FeCl3·6H2O (4.915 g) were dissolved in 10 mL of absolute ethanol under magnetic stirring. Then, 0.3 mmol of CuCl2·2H2O (0.307 g) was added to this solution, followed by stirring for 10 min; this solution was labeled solution A. Separately, 9.6 mmol of NaOH (2.375 g) were dissolved in 30 mL of absolute ethanol and vigorously stirred until complete dissolution; this solution was labeled solution B. Solution B was then added to solution A drop by drop, and the mixture was stirred for another 10 min; the resulting solution was labeled solution C. A total of 20 mL of ethylene glycol was added to solution C and mixed; this solution was labeled solution D. Finally, solution D was transferred into a 100 mL reaction kettle and incubated at 200°C for 10 h. The resulting product was cooled to room temperature and washed three times with deionized water and once with ethanol. After drying in an oven at 60°C for 12 h, the final product was ground into a fine powder.
2.3. Cu-Modified Fe3O4 Catalyst Characterization
The crystal structure of the material was studied using XRD (D8 Advance). A transmission electron microscope (JEM-2100) was used to observe the material’s morphology and high-resolution structure at an accelerating voltage of 200 kV. The micromorphology of the composite material was observed using a scanning electron microscope (JSM-7001F). XPS (ESCALAB-250) was used to further confirm the elemental composition of the samples. The free radicals generated during the reaction of the material were measured using an electron paramagnetic resonance spectrometer (EPR, Escan). The concentration of copper ions in aqueous solution was measured by ICP-MS (ELAN-9000).
2.4. Fenton-Like Bactericidal Reaction Performance Testing
E. coli was used for the bactericidal experiments and cultured in LB medium. The dosage of H2O2 used in the Fenton-like reaction sterilization experiment was 5 mM, the concentration of the material used was 1 mg/mL, and the colony-forming unit per mL (CFU/mL) of E. coli was 2 × 109.
A total of 9.9 mL of phosphate buffered saline (PBS) was added into a Petri dish with a 60 mm diameter. Then, 10 mg Cu2+-Fe3O4 nanocomposite was added to the PBS solution and ultrasonically dispersed for 10 min. After ultrasound-based treatment, 5 mM H2O2 and 100 μL of the bacterial suspension were added to the solution and then mixed using a pipette. The initial bacterial concentration was 2 × 107 CFU/mL. At regular intervals, 100 μL of the mixture was collected for serial dilutions and spread onto LB agar plates. Finally, the plates were incubated overnight at 37°C, and the bacterial survival rate was calculated. This experiment was repeated three times.
3. Results and Discussion
3.1. Material Characterization
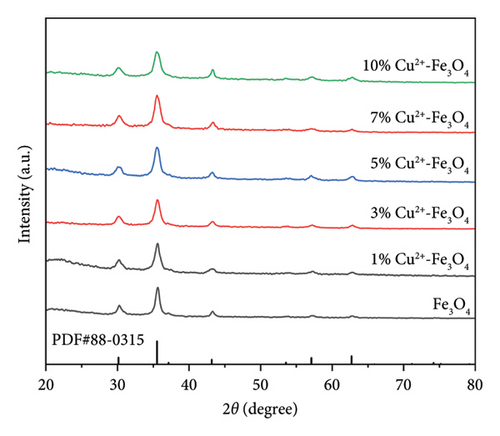
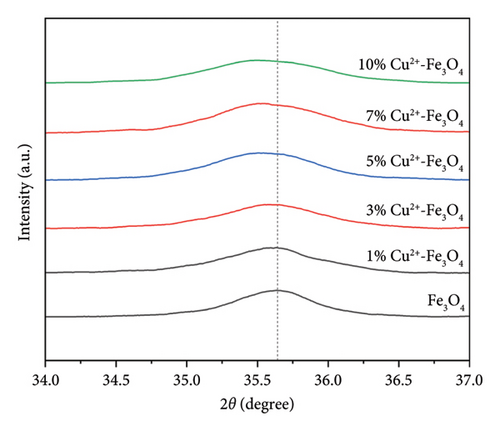
From Figure 1, it can be clearly seen that the 2θ values of Fe3O4 at 30.1°, 35.5°, 37.0°, 43.1°, 47.2°, 53.5°, 57.0°, 62.6°, 65.8°, 70.1°, 74.0°, 75.0°, and 79.0° correspond to the characteristic diffraction peaks (JCPDS number 88–0315) of Fe3O4 [24]. No obvious copper diffraction peaks can be found in Figure 1(a). From Figure 1(b), it can be seen that the position of the main diffraction peak deviates to the left and the linewidth becomes broader as the copper content rises [25], which indicates that copper ions may have replaced some of the iron in the samples.
The morphology and microstructure of the 10% Cu2+-Fe3O4 nanocomposite were observed by transmission electron microscopy (TEM). Figure 2 shows the TEM images of the 10% Cu2+-Fe3O4 nanocomposite. From Figure 2(a), it can be clearly observed that the particles of Cu2+-Fe3O4 nanocomposites are relatively homogeneous, with diameters around 10 nm. The Fe3O4 sample is distributed in the shape of clusters, which is consistent with the findings of Marchianò et al. [26]. Due to the high surface energy and magnetic dipole interactions, the nanomaterials mainly consist of aggregated spherical nanoparticles. In Figure 2(b), a lattice spacing of d = 0.25 nm, corresponding to the characteristic diffraction peaks of the Fe3O4 crystal planes, can be observed.
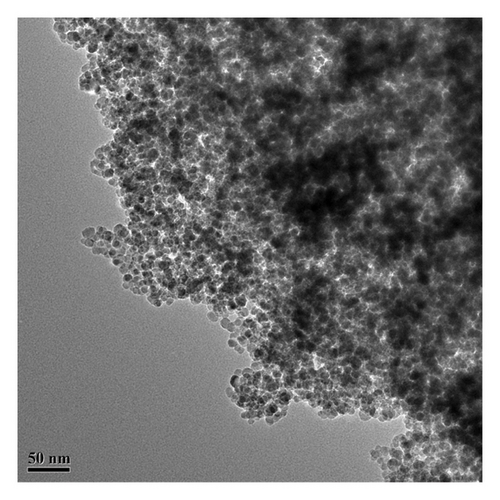
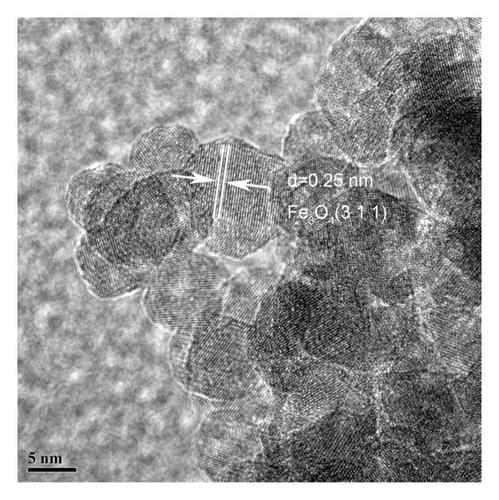
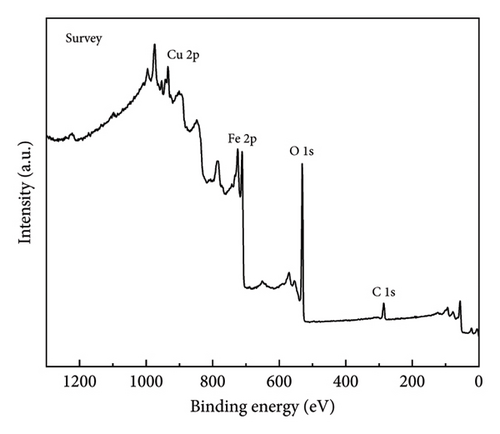
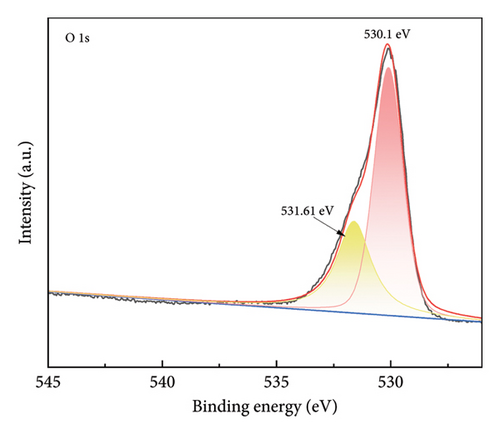
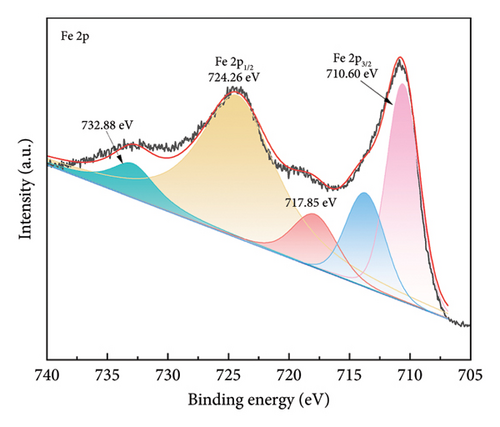
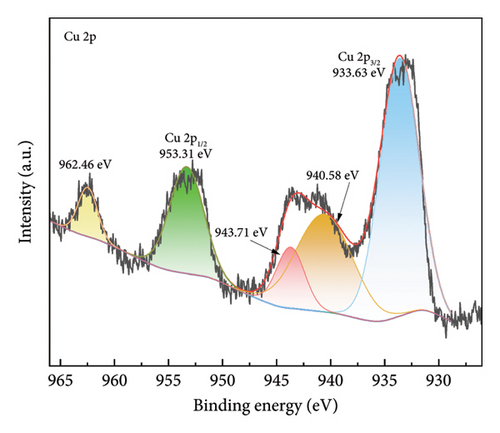
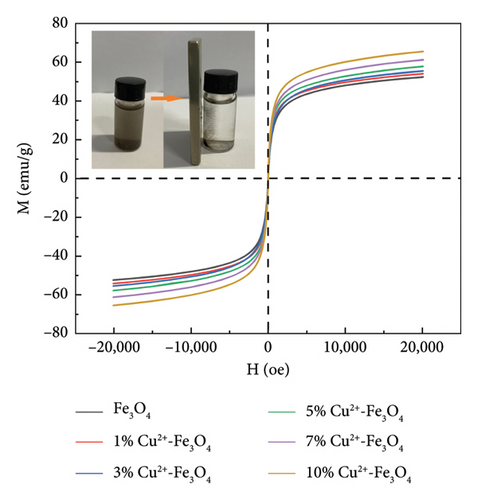
Figure 4 shows the magnetic properties of Cu2+-Fe3O4 nanocomposites with different copper contents. The nanocomposites have similar ferromagnetic properties and the saturation magnetization values gradually increase along with the doping ratio. This may be caused by the cation rearrangement following doping, and the literature shows that Cu2+ can occupy either the tetrahedral or octahedral sites within the spinel structure [33]. According to Tripathy et al. [34], if Cu2+ ions migrate to tetrahedral sites, the number of Fe3+ ions at the octahedral sites increases, while the number of the same ions at the tetrahedral sites decreases, leading to an imbalance in spin distribution on the two sublattices and resulting in enhanced net magnetization. In addition, no remanence and coercivity is observed, indicating that the magnetic particles exhibit superparamagnetic properties [35, 36]. From Figure 4, the maximum saturation magnetization of the nanocomposites reaches 65.5 emu/g. At the same time, when the saturation magnetization reaches 16.3 emu/g, magnetic separation becomes possible with conventional magnets [37]. This indicates that the nanocomposites can be efficiently separated by magnets. As confirmed by the inset in Figure 4, Cu2+-Fe3O4 nanocomposites, uniformly dispersed in water, can be easily separated by magnets in just 1 min. Therefore, the catalyst used in this study can be easily recovered using an external magnetic field after the reaction in aqueous solution, making it ready for reuse. This is an important advantage of Fe3O4 nanocomposites for practical applications.
3.2. Fenton-Like Performance Testing
3.2.1. Bactericidal Performance Test
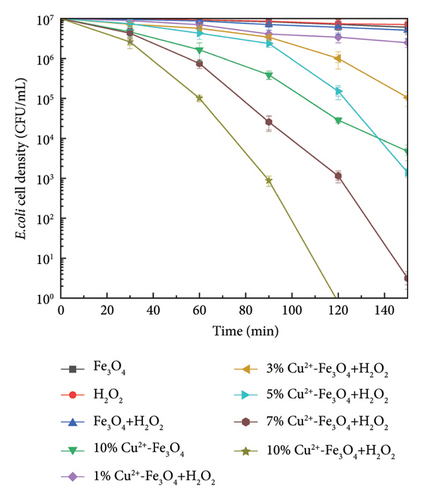
Figure 5(a) presents the results for 2 × 107 CFU/mL of E. coli and the effects of different ratios of Cu2+-Fe3O4 nanocomposite materials after 150 min. The results indicate that the inhibitory effect on E. coli is not significant when the concentration of 5 mM H2O2 or pure Fe3O4 nanomaterials acts on the microorganism separately. The inhibition of E. coli by unmodified Fe3O4 material in the Fenton reaction at the same time with H2O2 was also negligible. Saravanan et al. [38] synthesized magnetic nanocatalysts of Cu@Fe3O4in situ using Moringa oleifera flower extract, demonstrating a significant improvement in the efficiency of degradation of Congo red under visible light. Our modified 10% Cu2+-Fe3O4 nanocomposites successfully eliminated 2 × 107 CFU/mL of E. coli within 120 min without light irradiation, using only the addition of H2O2. Moreover, the bactericidal effect was further enhanced with increasing copper content.
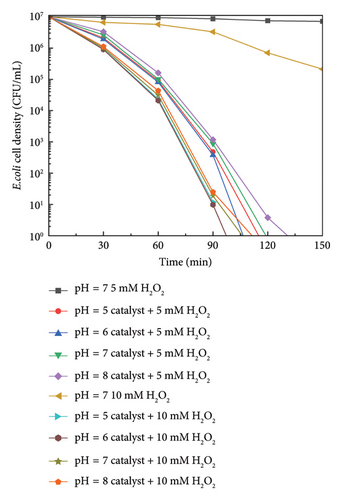
The effect of pH on the survival of E. coli is presented in Figure 5(b). At pH = 7, the bactericidal effect on E. coli was not significant when only H2O2 was added, and even at a concentration of 10 mM, the concentration of the bacterial solution was reduced by only two orders of magnitude. Regardless of pH value (5, 6, 7, or 8) and the H2O2 concentration (5 or 10 mM), the 10% Cu2+-Fe3O4 nanocomposites exhibited a remarkable killing effect on E. coli with a CFU/mL equal to 2 × 107 within 130 min, with the most pronounced bactericidal activity on E. coli observed at pH = 6 and H2O2 concentration of 10 mM. It was also found that E. coli can be more easily adsorbed at lower pH values, which may be due to the negatively charged bacterial membrane [39].
Stability and reusability are important parameters for evaluating the potential applicability of catalysts [40]. Recyclable antimicrobial nanocomposites are environmentally friendly as they improve material utilization [41]. Figure 6 shows the results for the 10% Cu2+-Fe3O4 nanocomposite after three cycles of killing E. coli bacteria at 2 × 107 CFU/mL in the Fenton-like reaction. As shown in Figure 6, the efficiency of the composite material in killing E. coli remains excellent over three cycles. Although the bactericidal effect decreases slightly through third cycles, the E. coli kill ratio in the third Fenton-like reaction still reach to 94.8%. The results demonstrate the reusability of the Cu2+-Fe3O4 nanomaterial [42].

3.2.2. Antibacterial Mechanism Test
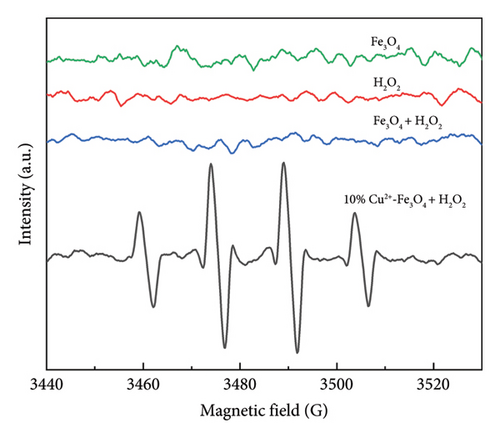
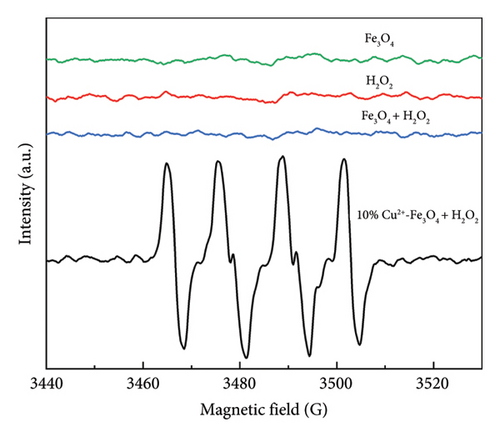
To investigate the antibacterial mechanism of 10% Cu2+-Fe3O4 nanocomposites in Fenton-like reaction, EPR experiments were conducted to investigate the reactive oxygen species produced during the killing of E. coli. In this experiment, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was used as a trapping agent to capture the •OH and •OOH produced by the Fenton-like reaction. Based on the literature, the 4-fold characteristic peak of DMPO-•OH adducts with an intensity ratio of 1:2:2:1 [43].
As shown in Figure 7, •OH and •OOH [44] signal peaks were not detected in the Fe3O4 nanomaterials without the addition of co-catalyst H2O2 or co-catalyst H2O2 was added singly. Even though pure Fe3O4 and H2O2 coexisted, there were still no signal peaks of •OH and •OOH. However, the 10% Cu2+-Fe3O4 nanocomposite showed strong signal peaks of •OH and •OOH after the addition of H2O2, which further fully proves that Cu2+-Fe3O4 nanocomposites produce •OH and •OOH in the Fenton-like reaction. This might be due to the introduction of Cu ions providing more active sites, which improves the carrier separation efficiency and the generation of reactive oxygen signals [45]. Due to the strong oxidizing effect of reactive oxygen radicals, when they come into contact with bacteria, they will destroy the barrier effect of E. coli cell walls and cell membranes, increase the permeability of E. coli cell membranes, and lead to leakage of intracellular proteins so that the bacteria will not be able to maintain normal life activities [46]. In addition, reactive oxygen radicals will pass through the pores on the cell surface and enter the cell interior, further oxidizing and degrading intracellular proteins, destroying intracellular protective enzymes, and ultimately leading to bacterial death [47].
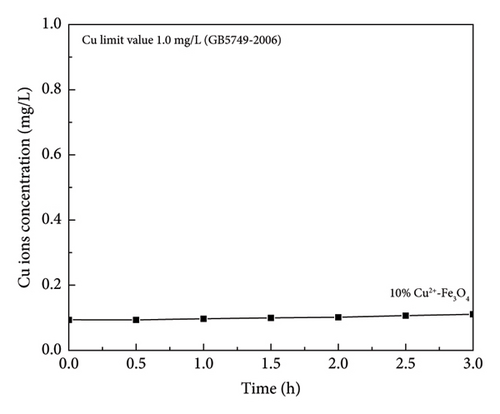
Due to its remarkable toxic domino effect at excess amounts [48], the concentration of Cu2+ dissolved in the aqueous reaction system was measured by ICP-MS. From Figure 8, the solubility of Cu ions was consistently in the range of 0.09–0.11 mg/L, which is much lower than the detection limit of Cu ions in drinking water (1.0 mg/L). This is in full compliance with the “Sanitation Standards for Drinking Water” (GB5749-2006) promulgated by the Ministry of Health [49]. Therefore, for the Cu-modified Fe3O4 nanocomposites synthesized in this work, the dissolved copper ion concentration in water is much lower than the safety threshold, and the impact on the human body and the environment is negligible.
4. Conclusions
Here, we synthesized novel Cu2+-Fe3O4 magnetic nanocomposites by hydrothermal method. Characterization assays confirmed the presence of Cu2+ in the nanocomposites. The innovative synthesized materials exhibited superparamagnetism and could be efficiently separated from aqueous solution with external magnets. Compared with pure Fe3O4, these nanocomposites exhibit superior performance in inhibiting the growth of E. coli within a heterogeneous Fenton system. The results of Fenton antibacterial experiments indicate that optimal antibacterial effect was achieved at pH = 6, a H2O2 concentration of 10 mM, and a copper ion doping ratio of 10%. Furthermore, our research on the mechanism shows that the enhanced catalytic performance of the Fenton reaction can mainly be attributed to the fact that Cu+/Cu2+ act as electron mediation centers to accelerate the oxidation–reduction reaction of Fe2+/Fe3+. Cu2+-Fe3O4 has good biocompatibility as demonstrated by the ICP-MS test. Our findings suggest that this unique Cu2+-Fe3O4 nanocomposite has a potential for water treatment.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was financially supported by the Henan Province International Science and Technology Cooperation Project (number 252102521003), the National Natural Science Foundation of China (number 52102118), the Excellent Youth Foundation of Henan Scientific Committee (number 202300410493), the Training Plan of Young Backbone Teachers in Universities of Henan Province (number 2020GGJS124), and the Key Research Projects of the Science and Technology Department of Henan Province (number 222102230060). Open access publishing was facilitated by University of Genoa, as part of the Wiley - CRUI-CARE agreement.
Acknowledgments
This work was financially supported by the Henan Province International Science and Technology Cooperation Project (number 252102521003), the National Natural Science Foundation of China (number 52102118), the Excellent Youth Foundation of Henan Scientific Committee (number 202300410493), the Training Plan of Young Backbone Teachers in Universities of Henan Province (number 2020GGJS124), and the Key Research Projects of the Science and Technology Department of Henan Province (number 222102230060).
Open Research
Data Availability Statement
The data used to support the findings of this study are available on request from the corresponding authors.




