Antimicrobial Polymeric Nanoparticles in Endodontics: A Systematic Review
Abstract
Background: The efficacy of endodontic treatments, essential for managing infections and injuries in dental pulp, heavily relies on completely eradicating microorganisms from the root canal system. Traditional methods often fail to achieve thorough disinfection, prompting the exploration of polymeric nanoparticles for their potential to enhance cleanliness.
Objectives: This systematic review aims to evaluate the use and effectiveness of polymeric nanoparticles in endodontic treatments, with a specific focus on their antimicrobial properties.
Methods: Studies eligible for inclusion involved inoculated root canals, dentine, or endodontic medicaments containing relevant microbial species. The interventions examined included the application of polymeric nanoparticles that contain antimicrobial agents, sometimes enhanced by ultrasound or photodynamic therapy, compared against control groups without treatment. The primary outcome was the reduction in bacterial load. Systematic searches were conducted in databases such as PubMed, Web of Science, Scopus, and Google Scholar and completed by January 31, 2024. The risk of bias was assessed using a modified Cochrane tool, and results were synthesized descriptively due to heterogeneity in methodologies and reporting across the studies.
Results: Nine studies met the inclusion criteria, primarily focusing on bacterial strains like Enterococcus faecalis and Staphylococcus aureus. Results indicated that nanoparticles exhibited significant antimicrobial activity, especially those made of chitosan and poly (lactic-co-glycolic acid) (PLGA). This suggests a potential to revolutionize root canal disinfection processes. However, clinical translation faces challenges such as ensuring nanoparticle stability, biocompatibility, and cost-effective manufacturing processes.
Discussion: The evidence was limited by variability in study designs and microbial strains, the absence of clinical trials, and inconsistent reporting precluded a meta-analysis. Despite promising in vitro results, several barriers hinder the clinical implementation of nanoparticle-based therapies. Long-term safety data are sparse, and the potential for adverse effects like inflammatory responses and antimicrobial resistance remains a concern. Additionally, achieving optimal delivery within the intricate anatomy of the root canal system requires further innovation. Addressing these challenges is critical for transitioning from experimental to practical applications.
1. Introduction
Endodontic treatment is a routine dental procedure to treat infections and injuries to the dental pulp. The success of endodontic treatment is dependent on the thorough elimination of microorganisms from the root canal system. Traditional disinfection methods, such as chemical irrigation and mechanical debridement, have limitations in reaching all areas of the canal system, leading to incomplete disinfection and potential treatment failure. Hence, alternative approaches to improve root canal disinfection are being explored [1].
Nanotechnology, the science of manipulating materials on a nanoscale level, has gained increasing attention in dentistry for its potential to improve dental treatment outcomes. Among the various types of nanoparticles (NPs), polymeric NPs have attracted significant interest due to their unique physicochemical properties, biocompatibility, and stability. Polymeric NPs are colloidal particles composed of polymers capable of encapsulating and delivering therapeutic agents. The polymer composition of the NPs can be customized to achieve desired physicochemical properties, such as size, surface charge, and biodegradability, making them suitable for various biomedical applications. The release of therapeutic agents from the NPs can be controlled, allowing for sustained and targeted delivery [2].
In endodontics, the use of polymeric NPs has also been explored to enhance root canal disinfection. The unique properties of polymeric NPs, such as their ability to penetrate intricate anatomical structures, present a promising approach to surmount the limitations of traditional disinfection methods. Several studies have shown promising results regarding the effectiveness of polymeric NPs in improving root canal disinfection. For example, NPs composed of chitosan, a biocompatible and biodegradable polymer, have been shown to effectively deliver antimicrobial agents into the root canal system and improve disinfection. Similarly, NPs composed of poly (lactic-co-glycolic acid) (PLGA), a commonly used biodegradable polymer, have shown potential in delivering antibacterial agents and reducing bacterial counts in the root canal system [3]. Despite the potential benefits of using polymeric NPs in endodontics, several challenges still need to be addressed. These challenges include optimizing NP properties for enhanced root canal penetration, developing efficient and safe delivery methods, and evaluating the long-term biocompatibility of these NPs. This systematic review critically evaluates current literature on polymeric NPs in endodontics. Specifically, this review will assess the effectiveness of polymeric NPs in improving root canal disinfection and reducing treatment failure rates.
2. Material and Methods
2.1. Search Strategy
- •
Primary combinations:
- i.
(polymeric nanoparticles) AND (endodontic)
- ii.
(polymeric nanoparticles) AND (root canal treatment)
- iii.
(polymeric nanoparticles) AND (endodontic bacteria)
- i.
-
These combinations represent the core focus of the study, targeting publications directly related to polymeric NPs and their application in endodontics. The Boolean operator AND was used to ensure that both terms within each combination were present in the search results.
- •
Broader combinations: To capture additional relevant studies and account for variations in terminology, broader combinations were applied:
- i.
(polymeric nanoparticles OR nanoparticles) AND (endodontic OR root canal treatment)
- ii.
(polymeric nanoparticles OR nanoparticles) AND (endodontic bacteria OR dental biofilms)
- i.
For the Google Scholar search, the Publish or Perish tool was used, in which the search range of 2000–2023 was set, and the first thousand results of each search were selected using the search above terms. In addition, the reference lists of each paper were scanned to identify additional documents on the issues that had been missed. Only the papers published in English were included. A detailed protocol was developed for this systematic review. The analysis and eligibility criteria were stated and documented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, which are available upon request from the authors. Search criteria were intentionally broad to minimize publication bias and ensure inclusion of all relevant in vitro studies.
2.2. Eligibility Criteria
- 1.
Participants: laboratory-based root canal models.
- 2.
Intervention/exposure: exposure of the samples to polymeric NPs containing antimicrobial agents and aided with ultrasound or photodynamic therapy with antimicrobial activity in infected root canals or dentine.
- 3.
Comparisons: control group (CG) without treatment or alternative biocidal agents to evaluate different disinfection strategies.
- 4.
Outcome: biocidal activity, assessed by the efficacy in reducing or eliminating microorganisms’ load.
- 5.
Study design: all experimental studies performed by valid procedures.
The inclusion criteria were studies on inoculated root canals of extracted teeth or dentine; studies involving the use of polymeric NPs containing antimicrobial agents as an intervention; and studies involving the use of intracanal medicaments or irrigants supplemented with polymeric NPs. Studies can also be included where the intervention is aided with ultrasound or photodynamic therapy with antimicrobial activity in infected root canals or dentine. Studies that have a CG for comparison were included. Studies have shown decreased bacterial load as an outcome. All experimental studies follow valid procedures. The exclusion criteria were studies that do not involve inoculated root canals of extracted teeth, dentine, or endodontic medicaments with relevant microbial species; studies where the intervention does not involve using polymeric NPs containing antimicrobial agents; studies without a CG; studies that do not measure the bacterial load or do not show a decrease in bacterial load as an outcome; nonexperimental studies or studies that do not follow valid procedures; and studies not published in English.
2.3. Selection of Studies
- •
Title and abstract screening: In the first stage, the titles and abstracts of all identified studies were screened by three independent reviewers. To ensure reliability, all reviewers underwent a standardized training session on the inclusion and exclusion criteria prior to the screening process. This training emphasized consistent application of the criteria across all reviewers. The screening process was facilitated by Mendeley software, which streamlined the management and organization of the studies. Discrepancies in the reviewers’ decisions were resolved through discussion, and when consensus could not be reached, a fourth reviewer acted as an adjudicator to finalize the decision. This step aimed to efficiently narrow down the pool of studies while maintaining objectivity and uniformity in the selection process.
- •
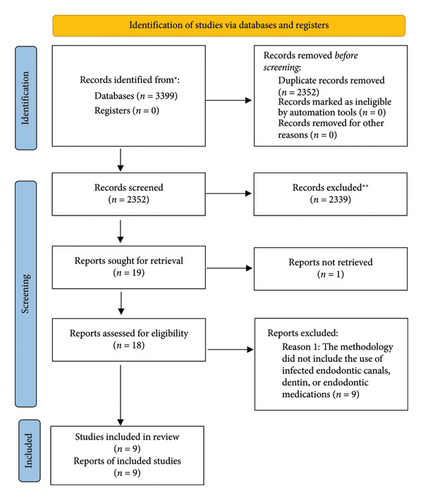
Full-text screening: Studies that passed the title and abstract screening stage proceeded to a full-text review, which followed the same structured and collaborative approach. Three independent reviewers conducted the full-text screening, applying the predefined criteria rigorously. As with the initial screening, disagreements were resolved through discussion, with the fourth reviewer intervening when necessary to achieve consensus. During this stage, the reasons for excluding studies were systematically documented, ensuring a transparent and reproducible account of the decision-making process. This documentation formed the basis of the PRISMA flow diagram, which visually represented the screening and selection process, illustrating the number of studies excluded at each stage and the rationale behind their exclusion (Figure 1).
2.4. Data Collection and Analysis
The selected studies’ data were extracted using a standardized data extraction form. The form was piloted on a few studies and refined as necessary before being used for the entire review. The process involved two independent reviewers who meticulously collected the following details from each study: study design, intervention types, NP characteristics, bacterial strains tested, antimicrobial outcomes, biocompatibility evaluations, and mechanisms of action. Discrepancies in data extraction were resolved through discussions, and a third reviewer was consulted when necessary to achieve consensus. The extracted data were organized and analyzed descriptively due to the high heterogeneity in study designs, interventions, and outcomes reported. This heterogeneity precluded meta-analysis but allowed for a comprehensive synthesis of the findings.
- 1.
Initial quality assessment: Studies initiated with a default quality rating based on the study design. Randomized controlled trials (RCTs) were started as “High” quality, and observational studies began as “Low” quality. Given the focus on in vitro studies in our review, these were automatically rated as “Low” due to inherent design limitations in reflecting clinical scenarios.
- 2.
Criteria for modifying the initial quality:
-
We assessed seven critical domains to adjust the initial quality of evidence:
- i.
Type of evidence: The type of evidence was scored based on the study design, which determined the initial quality rating. RCTs were assigned a “High” rating due to their robust design, which includes randomization and control over confounding variables, providing strong internal validity. Observational studies were rated as “Moderate to Low” because they lack these controls and rely on associations rather than causation. In vitro studies, while valuable for mechanistic insights, were automatically rated as “Low” because they cannot replicate clinical conditions, such as the complexity of root canal systems. No upgrades or downgrades were applied under this domain, as the quality was determined strictly by the inherent strengths and weaknesses of each study design.
- ii.
Risk of bias: The risk of bias was assessed initially by examining the methodological rigor of each study. Studies that demonstrated low risk—through clear randomization, allocation concealment, blinding, consistent application of interventions, and comprehensive data reporting—retained their initial quality rating. However, studies that exhibited flaws such as incomplete data, lack of randomization, unblinded assessments, or poorly defined protocols were downgraded, as these issues could compromise the validity of the findings. If these methodological weaknesses were deemed to substantially affect the credibility of the results, the quality rating decreased by one or more levels, depending on the severity and number of issues identified.
- iii.
Consistency: Consistency was evaluated by analyzing the reproducibility of outcomes across multiple studies. Initially, studies were rated based on whether their results aligned with similar studies addressing the same research question. If outcomes were consistent across studies with minimal variability, the rating could be upgraded to reflect stronger confidence in the reliability of the findings. If minor inconsistencies were observed that did not significantly alter the overall trend, the initial rating was maintained. However, if substantial variability or contradictory findings were identified without adequate explanation, the rating was downgraded. The extent of the downgrade depended on the level of inconsistency and its impact on the overall evidence.
- iv.
Directness: The initial evaluation of directness focused on how closely the study outcomes aligned with the clinical question. Studies that utilized clinically relevant models and outcomes, such as biofilm eradication in root canals with pathogens commonly found in endodontic infections, were rated highly for directness. If a study relied on indirect measures, such as surrogate endpoints or nonrelevant bacterial strains, the quality was downgraded. The degree of downgrade varied depending on the extent to which the indirect measures diverged from clinical relevance. Studies with minimal applicability to clinical practice faced more substantial downgrades.
- v.
Precision: Precision was initially assessed by examining the statistical reliability of the outcomes, focusing on confidence intervals and sample size. Studies with narrow confidence intervals and sufficient sample sizes to ensure low variability were rated higher and, in some cases, could be upgraded. If confidence intervals were broad or spanned clinically irrelevant ranges, or if small sample sizes introduced significant uncertainty, the rating was downgraded. The level of downgrade depended on the extent to which imprecision affected the reliability of the study’s conclusions. High imprecision resulted in more substantial reductions in quality.
- vi.
Magnitude of effect: The initial assessment of the magnitude of effect considered the clinical relevance of the results. Studies demonstrating significant and substantial effects, such as marked bacterial reduction or biofilm disruption, could be upgraded to reflect their importance. Moderate but consistent effects retained their initial rating. If the effects were small, clinically insignificant, or highly variable, the quality was downgraded. The degree of downgrade depended on how marginal the effects were and their relevance to clinical practice. Substantial effects could lead to one or more levels of upgrade if consistently demonstrated across the evidence base.
- vii.
Publication bias: The initial assessment of publication bias considered the transparency of study reporting and the balance of positive and negative results. Studies that demonstrated transparency through preregistration, comprehensive reporting, and balanced representation of findings retained their quality rating. If there was evidence of selective reporting, such as an overrepresentation of positive outcomes or indications of missing negative studies (e.g., through asymmetry in funnel plots), the quality was downgraded. The level of downgrade was determined by the degree to which publication bias was likely to have influenced the evidence base, with more substantial biases leading to greater reductions in quality.
- i.
- 3.
Grading the quality of evidence: The final quality of evidence for each outcome across the included studies was systematically categorized into one of four levels based on the GRADE framework. This grading system reflected the confidence in the accuracy of the estimated effects, considering the influence of factors such as study design, risk of bias, consistency, directness, precision, magnitude of effect, and publication bias. Each level is defined as follows:
- •
High quality: This level indicates very strong confidence that the true effect lies close to the estimated effect. Evidence rated as high typically originates from well-conducted RCT with minimal risk of bias, consistent results across studies, and direct applicability to the clinical question. Precision and publication transparency further reinforce the credibility of the findings at this level.
- •
Moderate quality: Evidence at this level suggests a reasonable degree of confidence in the estimated effect. The true effect is likely close to the estimate, but there is a possibility of it being substantially different. This rating often reflects studies that may have minor limitations, such as moderate risk of bias, some inconsistency among results, or slight indirectness in applicability. While still reliable, moderate-quality evidence necessitates caution when extrapolating findings to broader contexts.
- •
Low quality: This rating indicates limited confidence in the estimated effect. There is a significant chance that the true effect may differ substantially from the estimate. Low-quality evidence is typically associated with in vitro studies, observational research, or experimental designs with notable methodological weaknesses, such as high risk of bias, significant inconsistency, or imprecision in reported outcomes. These findings require further validation before being considered for clinical application.
- •
Very low quality: At this level, there is very little confidence in the estimated effect, and the true effect is likely to differ substantially. Evidence rated as very low often stems from studies with severe methodological limitations, marked inconsistency, indirectness, or substantial imprecision. Such evidence provides minimal support for decision-making and emphasizes the need for further rigorous research to clarify the findings
- •
- 4.
Synthesis of findings:
-
The results from the GRADE assessment were synthesized in a table to provide a clear, evidence-based foundation for conclusions regarding the efficacy and safety of NP-based treatments in endodontics.
3. Results
3.1. Study Selection
Figure 1 presents the flow diagram of the search strategy. Out of 3399 electronic search results, approximately 18 articles were deemed relevant to this research. All abstracts were reviewed for relevance, and only the necessary articles were obtained. The final electronic and manual searches identified approximately 18 research articles for this study. No clinical reports on the application of polymeric antimicrobial NPs in endodontics were found. Thus, the review was restricted to in vitro studies. A descriptive analysis was conducted on nine studies examining the antimicrobial effects of different polymeric NPs on root canal infections. Polymeric NPs containing antimicrobial agents, combined with ultrasound or photodynamic therapy, were central to the relevant studies.
The reviewed studies demonstrate the efficacy of various polymeric NPs in reducing biofilms and bacterial loads in root canal infection models, specifically targeting pathogens such as Enterococcus faecalis, Streptococcus oralis, Prevotella intermedia, Actinomyces naeslundii, and Candida albicans. NPs loaded with agents like chlorhexidine, propolis, and doxycycline showed promising results, evidencing not only a reduction in bacterial viability but also in biofilm volume. These effects were particularly significant against Enterococcus faecalis, a common and challenging pathogen in endodontic infections due to its resistance and ability to form robust biofilms. The efficacy of the NPs is attributed to mechanisms that include controlled release of antimicrobial agents, destabilization of bacterial membranes, and enhanced interaction with bacterial cells due to the physicochemical properties of the NPs. Additionally, biocompatibility was assumed based on the use of FDA-approved materials, although specific studies on biocompatibility were not explicitly conducted in many cases (Table 1).
| Study | Nanoparticle type | Study type | Nanoparticle properties | Antimicrobial analysis methods | Bacterial strain used | Antimicrobial results | Biocompatibility tests conducted | Biocompatibility results | Release kinetics | Antimicrobial mechanism | Light exposure details |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pagonis et al., 2010 [5] | PLGA nanoparticles loaded with methylene blue | In vitro | - Size: 150–200 nm | - Transmission electron microscopy (TEM) for nanoparticle uptake |
|
Approximately 2 log10 reduction in CFUs in planktonic phase and 1 log10 in root canals compared to controls. | Not specifically conducted | Assumed from the use of FDA-approved polymeric materials | Time-dependent release of methylene blue, which regained its phototoxicity upon release. | Methylene blue activated by light produces reactive oxygen species that are cytotoxic to bacterial cells. | Red light at 665 nm used for activation, specific power and fluence details not provided in the extracted data. |
| Shrestha and Kishen 2014 [6] | Rose Bengal–functionalized chitosan (CSRBnps) | In vitro | - Size: 60 ± 20 nm | - Atomic force microscopy (AFM) for cell surface analysis |
|
Significant disruption of multispecies biofilms with notable reduction in biofilm thickness and bacterial viability. | Not specifically conducted | Assumed from the use of biocompatible materials | Not detailed but involves time-dependent interaction with biofilms | The release of singlet oxygen upon light activation enhances bacterial cell membrane disruption. | PDT with 60 J/cm2 using a 540 nm wavelength light source. |
| - Encapsulation efficiency: Not detailed | - Confocal laser scanning microscopy (CLSM) for biofilm penetration | ||||||||||
| Shrestha et al., 2014 [7] | Rose Bengal–functionalized chitosan (CSRBnp) | In vitro | - Size: 60 ± 20 nm | - Confocal laser scanning microscopy for biofilm penetration |
|
CSRBnp completely eliminated Enterococcus faecalis within 24 h, even in the presence of inhibitors like pulp and BSA, which initially impeded antibacterial activity. | Not specifically conducted | Assumed from chitosan’s known biocompatible properties | - Singlet oxygen release measured but not quantitatively detailed | - Singlet oxygen production disrupting bacterial cells | −540 nm wavelength used |
| del Carpio-Perochena and Kishen 2017 [8] | Chitosan nanoparticles and propolis with calcium hydroxide | In vitro and in situ | - CNPs and EPE incorporated into Ca(OH)2 paste | - Confocal microscopy for cell viability in biofilms |
|
Significant reduction of E. faecalis CFU in both single and multispecies biofilms, especially notable with CNPs over 7 and 14 days. | Not specifically conducted | Assumed from chitosan’s known biocompatible properties | Not detailed | Enhanced antibacterial activity due to high surface area and charge density of nanoparticles; interaction with bacterial cell membranes. | Not applicable |
| - Microbiological analysis for CFU reduction | |||||||||||
| Quiram et al., 2018 [9] | Chlorhexidine-loaded trilayered nanoparticles (TNPs) | In vitro | - Size range: 140–295 nm | - Dynamic light Scattering (DLS) for size distribution | Enterococcus faecalis | TNPs showed prolonged inhibition of E. faecalis, maintaining effective bacterial control for up to 21 days. | Not specifically conducted | Assumed from the use of FDA-approved polymeric materials | Biphasic release pattern with initial burst followed by sustained release. | The hydrophilic core encapsulated chlorhexidine, enhancing contact and disruption of bacterial cell membranes. | No |
| - Encapsulation efficiency: 84.5% | - Broth inhibition method for antimicrobial effectiveness | ||||||||||
| Raheem et al., 2019 [10] | Propolis-loaded PLGA nanoparticles (ProE) | In vitro | - Particle size: 200–370 nm | - Direct contact test (DCT) for inhibition zones | Enterococcus faecalis, Streptococcus mutans, Candida albicans | ProE nanoparticles demonstrated significant inhibition of biofilms, with inhibition zones exceeding 32 mm. | MTT assay showed no significant cytotoxicity compared to conventional sealers. | Comparable cytocompatibility to AH Plus, no significant cytotoxicity observed. | Biphasic release: initial burst followed by sustained release up to 72 h. | Enhanced antimicrobial activity due to sustained release and high surface interaction with bacterial cells. | No |
| - Entrapment efficiency: > 80% | - OD600 measurements for microbial growth assessment | ||||||||||
| Abhishek Parolia et al., 2020 [11] | Chitosan–propolis (CPN) | In vitro | Average size 107.74 ± 0.53 nm, zeta potential of 45.2, polydispersity index of 0.225, encapsulation efficiency of 88.8% | Scanning electron Microscopy (SEM) and Confocal laser scanning Microscopy (CLSM) | Enterococcus faecalis (ATCC 29212) | Significant reduction of CFUs, especially with CPN250 | SEM and CLSM | Shows high cell viability and low toxicity | Not specified | Destabilization of bacterial membrane and antimicrobial effect due to size and charge of nanoparticles | No |
| Mona, Hadeel, amd Nagia, 2020 [12] | PLGA-chitosan | In vitro | Size of F2: 202.9 nm, charge: −0.0254 mV; size of F3: 339.6 nm, charge: +28.5 mV; encapsulation efficiency for F2: 64%, F3: 78% | Agar diffusion technique, biofilm inhibition assay | Enterococcus faecalis (E. faecalis) | F1 (ciprofloxacin solution): Inhibition zone of 22 mm; F2 (PLGA nanoparticles): 20 mm; F3 (chitosan-coated nanoparticles): 32 mm | Not specifically conducted | Assumed from the use of FDA-approved polymeric materials | 92.62% release from F2 and 78.3% from F3 over 72 h | Likely involves disruption of bacterial membrane due to positive charge of chitosan; enhanced penetration and sustained release of ciprofloxacin | No |
| Arias-Moliz et al., 2021 [13] | Doxycycline-functionalized polymeric nanoparticles | In vitro | Nanoparticles functionalized with zinc, calcium, or doxycycline | Confocal laser scanning Microscopy (CLSM), field Emission scanning electron Microscopy (FESEM), bacterial culturing | Enterococcus faecalis | Doxycycline-loaded NPs showed significant reduction in biofilm biovolume and high percentage of dead/injured bacterial cells | Not specifically conducted | Assumed from the use of FDA-approved polymeric materials | No | Likely involves disruption of bacterial membrane due to nanoparticle properties; enhanced penetration and sustained release of doxycycline | No |
3.2. Assessment of the Certainty of Evidence According to GRADE
Our systematic review employed the GRADE methodology to assess the quality of evidence across nine in vitro endodontic studies. All included studies were initially categorized as “in vitro” with a default quality rating of “Low” due to their limited applicability to clinical settings. The evaluation of the risk of bias revealed that most studies suffered from a high risk due to their in vitro designs lacking direct control over confounding variables, which resulted in a “Very Low” final quality for most of the studies. However, one study that demonstrated well-defined methodology and better variable control maintained a “Low” final quality rating. In terms of consistency, all studies were assigned a “Low” quality rating. While individual studies showed consistent results, variations across different studies necessitated further investigation and discussion to understand the discrepancies. Directness was uniformly scored as “Very Low” across all studies, reflecting the significant challenges in translating the in vitro results directly to clinical practice.
Precision in most studies was rated as “Very Low” due to wide confidence intervals that impeded definitive conclusions. Nonetheless, three studies that provided more precise estimates and robust statistical analysis were rated slightly higher, with one achieving a “Moderate” quality rating. The magnitude of the observed effects varied across the studies. Those demonstrating substantial effects on critical outcomes like CFU reduction and bacterial damage received a “Moderate” quality rating, enhancing their credibility, while others remained at “Low.” Publication bias was another critical factor, with some studies downgraded to “Very Low” due to potential biases such as selective reporting and the nonpublication of studies with negative outcomes. Conversely, studies that showed no evident biases received higher ratings, positively influencing their overall quality assessment (Table 2). The assessment of evidence certainty using the GRADE methodology highlighted the inherent limitations of in vitro studies in endodontics. While these studies provide valuable insights into mechanisms and efficacy, their default “Low” quality rating underscores their restricted applicability to clinical practice. Most studies were further downgraded to “Very Low” due to high risks of bias, significant indirectness, and imprecision. Although individual studies occasionally demonstrated robust methodologies and substantial effects, variability across studies and challenges in translating findings to clinical settings limited the overall reliability of the evidence.
| Studies | Pagoniset al. 2010 | Shrestha et al. 2014 | Shrestha et al. 2014 | del Carpio-Perochena et al. 2017 | Quiramet al. 2018 | Raheem et al. 2019 | Paroliaet al. 2020 | Arafaet al. 2020 | Arias-Molizet al. 2021 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criterion | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty | Evaluation | Certainty |
| Type of evidence | In vitro study | Low | In vitro study | Low | In vitro study | Low | In vitro and in situ study | Low | In vitro study | Low | In vitro study | Low | In vitro study | Low | In vitro study | Low | In vitro study | Low |
| Risk of bias | High risk due to in vitro design without direct control of confounding variables. | Very low | Well-defined study method, adequate variable control, experiment repeatability. | Low | High risk due to in vitro design without direct control of confounding variables. | Very low | High risk due to in vitro/in situ design and limited direct control of confounding variables. | Very low | High risk due to in vitro design and lack of direct control of confounding variables. | Very low | High risk due to in vitro design and lack of direct control of confounding variables. | Very low | High risk due to in vitro design and lack of direct control of confounding variables. | Very low | High risk due to in vitro design and lack of direct control of confounding variables. | Very low | High risk due to in vitro design and lack of direct control of confounding variables. | Very low |
| Consistency | Consistent results across repeated tests within the study. | Low | Consistent results shown in tests, repetition of experiments shows similar effects. | Low | Consistent results shown in tests, repetition of experiments shows similar effects. | Low | Consistent results across different stages and setups within the study. | Low | Consistent results across different stages and setups within the study. | Low | Consistent results across repeated tests within the study. | Low | Consistent results across repeated tests within the study. | Low | Consistent results across repeated tests within the study. | Low | Consistent results across repeated tests within the study. | Low |
| Directness | Results in vitro with limited direct applicability to clinical practice. | Very low | In vitro results are not directly applicable to clinical practice. | Very low | Results in vitro with limited direct applicability to clinical practice. | Very low | Results in vitro and in situ with limited direct applicability to clinical practice. | Very low | Results in vitro with limited direct applicability to clinical practice. | Very low | Results in vitro with limited direct applicability to clinical practice. | Very low | Results in vitro with limited direct applicability to clinical practice. | Very low | Results in vitro with limited direct applicability to clinical practice. | Very low | Results in vitro with limited direct applicability to clinical practice. | Very low |
| Precision | Wide confidence intervals that do not allow a clear conclusion. | Very low | Precise estimates of biofilm reduction and bacterial damage, robust statistics. | Low | Wide confidence intervals that do not allow a clear conclusion. | Very low | Data provided with clear statistical significance, but sample size and test conditions limit broad applicability. | Very low | Data provided with clear statistical significance, but sample size and test conditions limit broad applicability. | Very low | High precision in measurement of outcomes, detailed statistical analysis. | Low | Wide confidence intervals that do not allow a clear conclusion. | Very low | High precision in measurement of outcomes, detailed statistical analysis. | Low | High precision in measurement of outcomes, detailed statistical analysis. | Low |
| Magnitude of effect | Large effects observed in CFU reduction. | Low | Large effects observed in biofilm reduction and bacterial damage. | Moderate | Large effects observed in CFU reduction. | Low | Large effects observed in reduction of bacterial colonies and viability. | Low | Large effects observed in reduction of bacterial colonies and viability. | Low | Large effects observed in antimicrobial efficacy and nanoparticle characteristics. | Moderate | Large effects observed in CFU reduction. | Low | Large effects observed in antimicrobial efficacy and nanoparticle characteristics. | Moderate | Large effects observed in antimicrobial efficacy and nanoparticle characteristics. | Moderate |
| Publication bias | Lack of publication of studies with negative outcomes. | Very low | Possible publication bias; studies with positive results are more likely to be published. | Very low | Lack of publication of studies with negative outcomes. | Very low | Possible publication bias due to selective reporting in similar studies. | Very low | No evident bias; study design and outcomes clearly described. | Low | No evident bias; study design and outcomes clearly described. | Moderate | Lack of publication of studies with negative outcomes. | Very low | No evident bias; study design and outcomes clearly described. | Moderate | No evident bias; study design and outcomes clearly described. | Moderate |
4. Discussion
Polymeric NPs have primarily been utilized as delivery systems for the controlled release of photosensitizers, antibiotics, and antimicrobial substances compatible with their polymeric composition. Recently, Diogo et al. have described a classification of nanocarriers (NCs) with intrinsic antimicrobial properties. NCs play a pivotal role in developing advanced medical therapies, particularly in the targeted delivery of drugs and therapeutic agents. Our analysis categorizes NCs based on their intrinsic antimicrobial properties and carrier functions, exploring six types: deoxyribonucleic acid nanocarriers (DNA-NCs), apatite NCs, natural NCs, nanobubbles, nanoemulsions, and polymeric NCs. DNA-NCs show promise in gene therapy due to their capacity to safely transport genetic material into cells. Their biocompatibility and degradation into naturally occurring molecules (nucleotides) reduce the risk of adverse immune responses. Future research could focus on improving the transfection efficiency and targeted delivery capabilities of DNA-NCs, potentially revolutionizing treatments for genetic disorders. Apatite NCs are particularly significant in bone regeneration and repair due to their excellent biocompatibility and bioactivity, mimicking the natural mineral components of bone. The challenge remains to harness their full potential in nonskeletal drug delivery systems, where modifications in their surface properties could expand their applicability to broader therapeutic areas. Natural NCs, derived from biological sources, are notable for their eco-friendly synthesis and reduced toxicity. These NCs can be engineered from various natural polymers, lipids, or carbohydrates, which offer inherent biodegradability.
Further research should explore the scalability of production processes and the enhancement of their stability under physiological conditions. Nanobubbles are emerging as a novel tool in medical imaging and drug delivery, enhancing ultrasound and MRI contrast and delivering therapeutic gases or drugs. The stability of nanobubbles and their controlled release mechanisms are critical for future investigation to ensure precise delivery and activation at targeted sites. Nanoemulsions are gaining attention for their ability to improve the solubility and bioavailability of poorly water-soluble drugs. The key to their wider adoption lies in their stability and the ability to control the release kinetics of encapsulated agents. Developing formulations that can withstand physiological variables will enhance clinical efficacy and patient compliance. Polymeric NCs also represent a versatile class of carriers with tunable chemical properties, enabling the encapsulation of diverse therapeutic agents. The ongoing challenge is to develop polymers that are effective in drug delivery and responsive to specific stimuli in the body, thereby providing targeted therapy with minimal side effects [14].
Polymeric NPs offer several advantages over metal and metal oxide NPs in endodontic applications, particularly in terms of disinfection and dental tissue biocompatibility. Firstly, polymeric NPs are generally more biocompatible than their metallic counterparts, which is crucial for applications in sensitive tissues like the periapical tissues. Unlike metallic NPs, which can release potentially toxic ions, polymeric NPs typically present a lower risk of systemic and local toxicity—an essential factor in treatments involving direct contact with living tissue [15]. These NPs can be engineered to release their payloads, such as antibiotics, antimicrobials, or regenerative agents, in a controlled and sustained manner, which improves the efficacy of long-term treatment and reduces the need for multiple applications [16]. Polymers can also be easily modified to perform various functions, such as targeting specific tissues, enhancing tissue penetration, and modifying their degradation to match required treatment times [17]. Lastly, polymeric NPs can offer better chemical and physical stability in the oral environment, which is subject to constant changes in pH and temperature, helping to maintain the effectiveness of the treatment over time [18]. These characteristics make polymeric NPs especially attractive for endodontic applications where treatment efficacy and patient safety are paramount.
In the article by Pagonis et al., PLGA polymeric NPs were employed [19]. PLGA is a compound formed by polyglycolic acid and polylactic acid that is highly biocompatible with the various tissues present in the human body and can also be easily degraded. In addition, the PLGA NPs were loaded with methylene blue, which functions as a photosensitizer [5]. Photosensitizers are compounds or substances that can be activated by photodynamic therapy, which causes said compounds to generate reactive oxygen species that can be used as antimicrobials against Gram-positive and negative bacteria [20]. Pagonis et al. synthesized PLGA NPs loaded with methylene blue. They activated them with a red light at 665 nm and an energy fluence of 60 J/cm2 to test their antimicrobial activity against Enterococcus faecalis in planktonic phase and biofilm in human teeth. Exposure to activated NPs resulted in approximately 40% reduction of planktonic bacteria and 60% reduction of biofilm bacteria. The authors reported that the NPs clustered in the bacterial cell wall, making it permeable to methylene blue. Once the methylene blue was located inside the bacteria and activated by the blue light, it generated reactive oxygen species that damaged the bacteria’s internal structures [5]. The authors also reported that the antimicrobial activity of the photosensitizer can be affected by the presence of proteins. However, using alternative formulations can enhance methylene blue’s photosensitizing activity [21, 22]. Methylene blue is a synthetic dye in the Phenothiazinium subgroup. Along with toluidine blue, it is part of the second generation of photosensitizers initially investigated for cancer treatment (Table 3). Later, methylene blue was also utilized to combat Gram-positive and Gram-negative bacteria in both planktonic and biofilm forms due to its cationic charge [23].
| First generation |
| Hematoporphyrins |
| Second generation |
| Methylene blue |
| Toluidine blue |
| Photosense |
| Foscan |
| 5′-Aminolevulinic acid (ALA) |
| Third generation |
| Drug delivery systems |
| Gene engineering–based technologies |
| Monoclonal antibody receptor combinations |
Alternatively, Shrestha et al., in two separate studies, utilized chitosan NPs functionalized with rose Bengal (RB) against Enterococcus faecalis, Streptococcus oralis, Prevotella intermedia, and Actinomyces naeslundii [6, 7]. RB is a synthetic xanthene dye. It is a dark-red crystalline compound, derived from tetrachlorotetrapropoxyfluorescein, that absorbs visible light in the 500–800 nm range [24]. Likewise, chitosan is the second most abundant biopolymer obtained from chitin. Chitosan has been reported to possess broad-spectrum antimicrobial activity [25]. In photodynamic therapy, the authors activated the RB-functionalized chitosan NPs using 60 J/cm2 in the first study, and 5 and 10 J/cm2 for 1.66 and 3.33 min, respectively, in the second study. In both studies, the authors reported a high antimicrobial activity against biofilms formed by the selected bacteria using polymeric NPs functionalized with RB [6, 7]. Some studies have reported that the penetration of RB into Enterococcus faecalis depends on its concentration and that RB exhibits higher antimicrobial activity than methylene blue. Likewise, the activation of RB with a green light laser enhances its antimicrobial activity [26, 27].
- •
Ideally, it should have an absorption peak between 650 and 800 nm [32].
- •
It should have a high capacity to generate ROS upon irradiation [33].
- •
- •
It should be water-soluble [36].
- •
It should be stable under storage conditions [37].
- •
Possess antimicrobial activity [38].
- •
Exhibit effective absorption, distribution, metabolism, and excretion [36].
- •
Ability to penetrate cellular membranes [39].
- •
Demonstrate low production cost [35].
This systematic review reveals that only two synthetic photosensitizers have been used in conjunction with polymeric NPs to target bacteria in the root canal. However, a wide variety of natural photosensitizers have been described in the literature that could also be used for root canal disinfection. For instance, curcumin, isolated from the plant Curcuma longa, serves as a photosensitizer with extensive antimicrobial activity against Gram-positive and Gram-negative bacteria and fungi. Curcumin, which absorbs blue light at 405–435 nm, has been used to combat periodontitis at concentrations of 5–40 μM [40]. Neelakantan et al. tested the antimicrobial activity of curcumin and sodium hypochlorite when activated with blue light or ultrasound on E. faecalis biofilms. Curcumin, when activated by blue light, demonstrated significant antimicrobial activity against E. faecalis biofilms [41]. Following Curcumin, alkaloids constitute another extensively utilized group of natural photosensitizers. Alkaloids are divided into five categories: quinoline-based alkaloids, pterins, benzylisoquinolines, beta-carbolines, and indigo alkaloids. Photosensitizers from the indigo alkaloid group, extracted from Indigofera tinctoria, have demonstrated broad antimicrobial activity [42]. Anthraquinones, classified as monomeric or dimeric, also form a group of natural photosensitizers. Within this group, the main photosensitizers extracted from plants are Emodin (excitation wavelength: 434 nm), rhein (437 nm), rubiadin (410 nm), physcion (438 nm), carminic acid (494 nm), and purpurin (515 nm) [42]. Perylenequinones form yet another group of natural photosensitizers. Among these, hypericin is one of the most studied. Hypericin, isolated from Hypericum perforatum, demonstrates antimicrobial activity against Gram-positive bacteria and fungi when activated by orange light. However, its antimicrobial activity against Gram-negative bacteria is significantly lower due to poor absorption. Furthermore, hypericin is not soluble in water and requires transport media.
Additionally, its absorption wavelength range is from 590 to 600 nm [43]. Flavins in various organisms also represent a group of natural photosensitizers. Riboflavin is one of the most investigated. It has an absorption peak in the UVA spectrum at 360 nm and an absorption peak in the visible blue light spectrum at around 440 nm. Riboflavin has demonstrated a broad antimicrobial spectrum against Gram-positive and Gram-negative bacteria and fungi [44]. Additional groups of natural photosensitizers encompass porphyrins, chlorins, and bacteriochlorins. Porphyrins absorb at 400 and 630 nm, and, despite their substantial antimicrobial activity, their hydrophobic nature limits their use, necessitating carriers for bactericidal action. ALA, a hydrophilic porphyrin precursor, offers a more practical alternative [45]. The chlorins are derived from chlorophyll and exhibit two absorption peaks, at 650 and 700 nm, respectively. Numerous previous studies have reported their broad antimicrobial activity as a photosensitizer [46]. Contrastingly, present in certain photosynthetic bacteria, Bacteriochlorophylls are tetrahydroporphyrins with two reduced pyrroles located diametrically on the macrocycle. Bacteriochlorophylls a, b, and g are characterized by strong absorption at 772, 794, and 762 nm, respectively. Bacteriochlorophylls have also found application in the photodynamic inactivation of microorganisms [47].
Although using polymeric NPs in combination with photodynamic therapy appears promising, recent studies have reported the possibility that bacteria may generate resistance against photodynamic therapy. Since the primary antimicrobial mechanism of polymeric NPs as carriers for photosensitizers in photodynamic therapy is ROS generation by photosensitizers activated by specific wavelengths of light, bacteria produce enzymes that can neutralize ROS at lethal concentrations. Key enzymes include superoxide dismutase (SOD), which converts O2•− to H2O2 and O2, and glutathione peroxidase, which also neutralizes ROS. Bacteria also have catalases (hydroperoxidase I and II) that can convert H2O2 to H2O and O2 [48]. In addition to the enzymes above that serve as the main defense mechanism against ROS, bacteria also have secondary defense systems such as DNA repair systems and proteolytic and lipolytic enzymes. In terms of DNA repair systems, enzymes such as endonuclease IV (activated in response to oxidative stress) and exonuclease III participate in these [49]. Bacteria also repair covalent bond formations, such as disulfide bonds in proteins, during oxidative stress. Among the main enzymes in this defense mechanism are thioredoxin reductase, glutaredoxin, and protein disulfide isomerase. On the other hand, methionine oxidation to methionine sulfoxide can be repaired by methionine sulfoxide reductase [50].
In addition to the defense mechanisms mentioned above, some bacteria have pigments that can confer resistance to photodynamic therapy. Orlandi et al. reported that the bacterium Pseudomonas aeruginosa, when producing the pigment pyomelanin, exhibited greater resistance to oxidative stress caused by photodynamic therapy. Phenazines and pyoverdine were other pigments produced by Pseudomonas aeruginosa that could counteract the effects of oxidative stress produced by photodynamic therapy [51]. Carotenoids are other bacterial pigments that have protective properties against oxidative stress caused by photodynamic therapy. The bacterium S. aureus produces a carotenoid pigment called staphyloxanthin. Liu et al. showed that a staphyloxanthin-deficient S. aureus was more susceptible to oxidative stress caused by photodynamic therapy [52]. The protective ability of staphyloxanthin against oxidative stress lies in its ability to reduce singlet oxygen entry into the bacteria by lowering membrane permeability [53].
The bacterial extracellular matrix also serves as a defense mechanism against photodynamic therapy. Gad et al. reported that S. aureus can produce slime and reduce the penetration of anionic photosensitizers into bacteria, thereby counteracting their antimicrobial effects. However, this resistance was not observed with cationic photosensitizers [54]. Similarly, bacterial efflux pumps contribute to resistance to photodynamic therapy. This is because some photosensitizers are also substrates for efflux pump activity. Therefore, photosensitizers can be expelled from the bacterial cell by efflux pumps. Tegos and Hamblin first discovered this [55]. Kishen et al. tested the antimicrobial activity of methylene blue and RB, both activated by photodynamic therapy, on E. faecalis biofilms. Their results showed that methylene blue had higher antimicrobial activity against the E. faecalis biofilm than RB.
Similarly, this activity could be improved by using efflux pump inhibitors [56]. Antibiotic-resistant bacteria have also been shown to have greater resistance to photodynamic therapy than bacteria that had not previously developed antibiotic resistance [57, 58]. Finally, St. Denis et al. studied whether the expression of heat shock proteins (GroEL and DnaK) could increase the resistance of E. coli and E. faecalis to photodynamic therapy. The authors concluded that the expression of heat shock proteins contributed to improving the resistance of the bacteria above, so further studies were needed to confirm these findings [59].
This systematic review found three studies in which polymeric NPs are used as carriers for propolis with antimicrobial activity. Propolis is a resinous substance containing 50% plant resins, 30% wax, 10% essential oils, 5% pollen, and 5% other organic compounds [60]. Its general chemical composition varies due to several factors, including plant sources surrounding beehives, honey-bee species, method of collection, geographical and climatic variation, collecting seasons, altitudes, and adequate lighting. The most well-known types of propolis are poplar-type (Eurasian) propolis, Brazilian green and red propolis, and Mediterranean propolis. Currently, around 1000 chemical components of propolis have been found [61]. Some of the chemical compounds found in propolis with antimicrobial activity are described in Table 4.
| Type | Region | Antimicrobial compound | Plant source | Bacteria used |
|---|---|---|---|---|
| Green propolis | Brazil | Apigenin | Baccharis dracunculifolia | Bacillus Subtilis [62] |
| Red propolis | Brazil | Artepellin C | Dalbergia ecastophyllum Clusia sp. | Bacillus subtilis [63] |
| Brown propolis | Cuba | Chlorogenic acids | B. dracunculifolia C. rosea | Enterococcus sp. [64] |
| Mediterranean propolis | Greek | Communic acid | Cupressus sempervirens Pinus species | Enterobacter cloacae [65] |
| Yellow propolis | Cuba | Acetyl triterpenes | Undetermined | Staphylococcus aureus [66] |
| Poplar propolis | Eurasian region | Galangin | Populus sp. | Acinetobacter baumannii [67] |
In the study by Parolia et al., chitosan–propolis NPs with a concentration of 250 μg/mL demonstrated the highest antimicrobial activity compared to the use of propolis at different concentrations (100 and 250 μg/mL) and 2% chlorhexidine gel [11]. Abdel Raheem et al. synthesized propolis-loaded NPs and incorporated them into root canal sealers, comparing their antimicrobial effectiveness to that of the commercial sealer AH Plus. The root canal sealer supplemented with propolis-loaded NPs exhibited acceptable antimicrobial activity against E. faecalis, S. mutans, and C. albicans [10]. According to these studies, polymeric NPs loaded with propolis demonstrated comparable antimicrobial activity to other endodontic methods. Although there are few studies on using polymeric NPs as carriers for propolis, both NPs and propolis have been more extensively studied individually. A recent systematic review and meta-analysis suggested that chlorhexidine has superior antimicrobial activity compared to propolis [68]. Recently, the antimicrobial activity of propolis NPs against E. faecalis biofilms has been investigated compared to 5.25% sodium hypochlorite and Iranian propolis. The results indicated that propolis NPs had superior antimicrobial activity than Iranian propolis but not more than sodium hypochlorite. However, the nanoparticle concentrations were 10 times lower than those used for Iranian propolis [69]. Parolia et al. compared the antimicrobial activity of propolis NPs with different concentrations of propolis alone, 6% sodium hypochlorite, and 2% chlorhexidine. The authors found that after 6 min, propolis NPs (300 μg/mL) showed higher antimicrobial activity than propolis alone but were less effective than sodium hypochlorite and chlorhexidine. However, after 10 min, propolis NPs demonstrated antimicrobial activity equivalent to 6% sodium hypochlorite and 2% chlorhexidine [69].
In this systematic review, three articles were identified in which polymeric NPs loaded with antibiotics were used for root canal disinfection. Polymeric NPs used as antibiotic carriers are classified into two major groups based on composition and structure: nanospheres and nanocapsules. Nanocapsules consist of a polymeric shell that surrounds an oily core in which the antibiotic can be dissolved, although the antibiotic can also be incorporated into the shell’s wall. On the other hand, nanospheres are formed by a polymeric matrix in which the antibiotic is embedded or adsorbed [70]. The use of polymeric NPs to combat endodontic infections has several advantages, such as controlled and constant antibiotic release and a reduction in the dosages required to achieve effective antimicrobial action [71].
Arafa, Mousa, and Afifi studied the antimicrobial activity of polymeric NPs loaded with ciprofloxacin in two different groups and a CG. The CG utilized a ciprofloxacin solution, the first experimental group (G1) was comprised of polymeric NPs loaded with ciprofloxacin, and the second experimental group (G2) included polymeric NPs loaded with ciprofloxacin and chitosan. The second experimental group (G2) showed the lowest antimicrobial activity, followed by the first experimental group (G1) and the CG. However, G2 exhibited the highest biofilm inhibition capacity, followed by G1 and CG. Therefore, G2 was more effective against biofilm compared to the ciprofloxacin solution [12]. These results align with those of Watcharadulyarat et al., where they evaluated the antimicrobial activity of polymeric NPs loaded with ciprofloxacin against E. faecalis. The authors reported that the NPs had increased antimicrobial activity and low cytotoxicity [72].
In the paper by Arias-Moliz, Baca, and Solana, the authors evaluated the antimicrobial activity of nonfunctionalized polymeric NPs and polymeric NPs functionalized with calcium, zinc, and doxycycline. The authors found that nonfunctionalized NPs exhibited the highest antimicrobial activity, followed by doxycycline-functionalized NPs. However, the latter also had the ability to inhibit biofilm formation in addition to their antimicrobial activity. The authors also reported that doxycycline-functionalized NPs exerted their antimicrobial and biofilm formation inhibitory activity through three mechanisms. First, the NPs interact with extracellular polymeric substances, disrupting bacterial biofilm formation and affecting bacterial adhesion to dentin. The second mechanism involves NPs occluding dentinal tubules, preventing bacterial colonization of dentin. Finally, doxycycline-functionalized polymeric NPs were the only ones that demonstrated interaction with the bacterial membrane of E. faecalis, and also altered its structure [13]. Previous studies have reported that polymeric NPs loaded with doxycycline and zinc contribute to dentin remineralization due to their ability to inhibit metalloproteinases [73]. Other studies have also reported that doxycycline-loaded polymeric NPs have broad antimicrobial activity against a wide variety of oral bacteria [74], and are capable of reducing biofilm formation by periodontal [75] and cariogenic [76] bacteria.
Quiram et al. assessed the antimicrobial effect of chlorhexidine-loaded polymeric NPs on E. faecalis. The NPs were evaluated using BHI broth inoculated with E. faecalis and cultured for 4 durations (1, 7, 14, and 21 days). The study results showed that the antimicrobial power of the chlorhexidine-loaded polymeric NPs is time-dependent. The longer the incubation time, the greater the antimicrobial effect, reaching 80% inhibition of bacterial growth at 21 days. In addition, there was a significant difference between the CG and the 21-day group [9].
These results align with those reported by Shirur et al., who evaluated the antimicrobial effect of chlorhexidine-loaded liposomes against Staphylococcus aureus, Fusobacterium nucleatum, and Streptococcus mutans. The authors also reported that the antimicrobial effect of the chlorhexidine-loaded liposomes was superior compared to the use of 2% chlorhexidine alone. Furthermore, the antimicrobial effect was time-dependent, with 50% or greater inhibition of bacterial growth observed at 12 h and 100% inhibition in all tested bacteria at 72 h [77].
The deployment of polymeric NPs in dental applications, particularly in endodontic treatments, brings forward significant therapeutic potential but also underscores concerns over potential side effects and long-term risks. While many of these NPs are constructed from FDA-approved materials, questions linger regarding their biocompatibility, as their degradation products could provoke inflammatory responses or accumulate in the body, leading to toxicity. Their small size allows for deep tissue penetration, which, although beneficial for targeted therapy, may cause tissue irritation or inflammation. Concerns also exist about the long-term stability and accumulation of these NPs in the body, with limited data on how they degrade or if they could lead to chronic health issues over time. There is a risk of allergic reactions, and the antimicrobial properties of the NPs might contribute to the development of antimicrobial resistance if pathogens are not completely eradicated, which has been discussed more extensively previously. Importantly, there is a distinct lack of long-term studies on the side effects of using NPs in endodontics [78]. This oversight might stem from the field’s reliance on highly toxic substances like sodium hypochlorite and calcium hydroxide for disinfection, which are considered the gold standard despite their own risks. This context may have led to complacency toward introducing new materials without extensive long-term safety profiling [79]. On the other hand, in vivo studies demonstrated that incorporating NPs into hydrogels significantly improved their stability by protecting them from premature degradation and burst release, ensuring controlled and sustained drug delivery at the target site. The hydrogels acted as a physical barrier, preventing nanoparticle aggregation and enhancing their mechanical properties, such as strength and elasticity. Furthermore, the formulations exhibited minimal toxicological effects and maintained their physicochemical characteristics over extended periods, even under physiological conditions [80].
This review demonstrates the potential of polymeric NPs as antimicrobial agents in endodontics but highlights several limitations. Most studies were conducted in vitro, which, while valuable, do not fully replicate the complexities of clinical environments. The variability in experimental designs, including differences in bacterial strains, nanoparticle formulations, and outcome assessment methods, limited direct comparability of results and prevented robust subgroup analyses. Additionally, the quality of evidence, as assessed using the GRADE framework, was largely rated as “Low” or “Very Low” due to small sample sizes, nonstandardized protocols, and potential publication bias. Another significant limitation was the lack of long-term evaluations. None of the included studies assessed nanoparticle stability, degradation products, or cytotoxicity over time, raising concerns about safety in clinical applications. Furthermore, while the mechanisms of action of NPs were discussed, few studies explored the potential for bacterial resistance, a critical factor that could undermine their long-term efficacy. Finally, the clinical applicability of polymeric NPs remains uncertain. Key issues such as optimizing delivery methods, ensuring compatibility with existing endodontic materials, and addressing cost-effectiveness have yet to be adequately studied. To bridge these gaps, future research should focus on conducting preclinical and clinical trials, standardizing methodologies, and evaluating long-term safety and effectiveness, paving the way for NPs to become a transformative tool in endodontics.
5. Conclusion
This systematic review evaluated the antimicrobial efficacy of polymeric NPs in endodontic applications, with a focus on their potential to enhance root canal disinfection. The findings demonstrated that NPs, particularly those composed of chitosan and PLGA, significantly reduced bacterial loads and biofilm formation in laboratory-based models. Despite these promising results, the quality of evidence was limited, as most studies were rated “Very Low” or “Low” according to the GRADE framework. These limitations were primarily attributed to the in vitro nature of the studies, methodological variability, and challenges in translating findings to clinical practice.
While the reviewed studies highlight the potential of polymeric NPs as innovative antimicrobial agents, several barriers remain for their clinical implementation. Key limitations include the lack of standardized protocols, insufficient evaluation of long-term safety, and limited data on the risk of bacterial resistance. Furthermore, the variability in nanoparticle formulations and the absence of clinical trials emphasize the need for further research. Future studies should prioritize addressing these gaps by focusing on optimizing nanoparticle properties, conducting in vivo and clinical trials, and assessing cost-effectiveness and delivery systems to facilitate integration into clinical practice. By overcoming these challenges, polymeric NPs may become a viable tool for improving outcomes in endodontic disinfection.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Acknowledgments
No funding was received for this review, registered in the PROSPERO database under CRD42023450832.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




