Does Unidirectional Buccal Patches Loaded With Lidocaine and Dexmedetomidine Ameliorate Injection Pain in Dental Procedures? A Double-Blinded Randomized Controlled Trial
Abstract
Introduction: Dental anxiety is a critical issue in dentistry. Buccal patches, a noninvasive drug delivery method, can potentially alleviate anxiety and pain during dental procedures. This study aimed to develop a three-layered buccal drug delivery system containing lidocaine or lidocaine–dexmedetomidine for improved pain management in dentistry.
Methods: Three-layered patches were made using ethyl cellulose, eudragit, and Carbopol, incorporating either lidocaine or a combination of lidocaine–dexmedetomidine. Forty participants were included: Both groups received placebo patches on one side of jaw and received their relevant drug patch of lidocaine or lidocaine–dexmedetomidine on the other side. Pain levels were assessed.
Results: The patches were 2-3 mm thick with a pH of 3.5–4.5. Lidocaine release efficiency was 41.69 ± 13.10%, and the combination patches showed 47.17 ± 5.10% for lidocaine and 74.71 ± 6.41% for dexmedetomidine. Release time for lidocaine and the combination patch was 25 and 15 min, respectively, with dexmedetomidine fully released within 3 min. The lidocaine–dexmedetomidine group reported significantly lower pain scores (2.1 ± 0.3) compared to the lidocaine group (4.3 ± 0.4) and placebo (6.8 ± 0.5). The onset and duration of analgesia was faster in the combination group versus the lidocaine group (5.2 ± 0.5 vs. 8.7 ± 0.6 min and 45 ± 5 vs. 30 ± 4 min, respectively).
Conclusion: Buccal patches, especially lidocaine–dexmedetomidine patches, significantly reduce pain and improve patient comfort. These patches offer a promising noninvasive alternative for pain management with enhanced efficacy and patient compliance. Further research is needed to optimize these patches for broader clinical applications.
Trial Registration: Iranian Clinical Trials Registration Center: IRCT20210118050067N2
1. Introduction
Dental caries and the need for dental procedures play a significant role in oral and dental health. It is clear that untreated dental caries and its clinical consequences have a negative impact on the quality of life [1].
Anxiety and subsequent avoidance of dental care create significant problems for patients and dentists. Patients who experience this fear only seek dental care when they experience severe problems, thus increasing the likelihood of going to the dentist with pain, which in turn increases their anxiety. Dental anxiety ranks fifth among the most feared situations. Different cross-sectional studies concluded that this is especially significant in children [2].
The most common method of anesthesia in dentistry is the use of local anesthetic drugs by injection. Lidocaine, usually together with epinephrine injected into the buccal mucosa and interdental septum is commonly used and approved. Fear and anxiety may be caused by the injection of local anesthetics with needles. Injections can cause fear, pain, and injury, leading to patients postponing dental visits especially in children. Therefore, finding a replacement for this type of anesthesia could greatly reduce anxiety [2–6].
Local anesthetics are available as gels, ointments, sprays, and bioadhesive patches. Anesthetic gels and ointments containing articaine and lidocaine and even sprays containing ethyl chloride, which cause local anesthesia by creating cooling effects, are currently approved and are being used routinely. A mucoadhesive local anesthetic patch containing lidocaine base distributed into a bioadhesive matrix was introduced for intraoral use in 1996 in the United States of America. Previous studies have shown that the use of lidocaine patch for local oral anesthesia is efficient, safe, and reliable. Lidocaine patch can be used in children from the age of three [4, 6–9].
There are a few drawbacks to the use of different topical anesthetics. Compounded topical anesthetics may pose additional risks due to their unregulated nature and high concentrations. In contrast, transbuccal patches may provide a more controlled delivery method with potentially fewer complications [10]. Transbuccal drug delivery systems offer benefits such as avoiding risks and discomfort, reducing chances of overdosing or underdosing, allowing for easy administration and termination, and reducing local and systemic adverse effects. Mucosal drug delivery enables controlled release, achieving constant blood levels and reducing side effects. Transbuccal patches are also comfortable and painless, improving patient’s acceptance [7, 8]. On the other hands, Limitations associated with buccal drug delivery include a small surface area for drug absorption and dilution of drugs due to continuous salivary secretion. Accidental ingestion of saliva may affect the bioavailability of drugs. Also, unintentional swallowing of these patches may cause suffocation, especially in children, elderly, and patients with swallowing disorders [7, 8].
Lidocaine is one of the most used anesthetic drugs in dentistry, with negligible adverse effects and acceptable safety [11]. On the other hand, dexmedetomidine is an alpha-2 agonist which acts as a sedative and analgesic drug. It can also reduce the heart rate and blood pressure [12].
Some researchers have shown that injecting lidocaine with dexmedetomidine can increase the pain relief effects. Also, studies showed that adding lidocaine to dexmedetomidine increases the duration of nerve block and decreases the onset time. It is mentionable that lidocaine can only affect the physical pain, and adding dexmedetomidine may also help relax the patient as well. The combination of these two drugs improved the pain after surgery, and vital parameters remained stable after surgery and no complications were observed [12, 13]. Mixing dexmedetomidine with local anesthetics makes the nerve block take longer and also produces an analgesic effect. Also, due to the high hepatic first pass effect, higher bioavailability was reported for buccal (82%), intramuscular (73%), and transbuccal (51%) routes compared to the oral route (16%) [14]. Unlike other existing sedatives, dexmedetomidine has a unique property with minimal effect on breathing, easy and quick control of the level of sedation and alertness, and short recovery time. This makes dexmedetomidine a sedation drug of choice in dental procedures. Studies have shown that the use of dexmedetomidine in maxillofacial surgery reduces bleeding and postoperative pain. Even in high doses, this drug has very little effect on the respiratory condition and is therefore suitable for pediatric use [15]. Lidocaine patch has also been used for skin and mucosal drug delivery in the studies of Clithero et al. [16–18]. Transbuccal patches were also used for other applications such as administration of adipogenic compounds, analgesics, and antihistamines and were proved to be effective [19, 20].
The present project aimed to develop a drug delivery system for local anesthesia in dental procedures. A three-layer transbuccal patch was designed. Lidocaine was incorporated into the patch, and dexmedetomidine was added to enhance anesthesia and induce sedation. The effect of adding dexmedetomidine to lidocaine patches was evaluated in a clinical trial.
2. Materials and Methods
2.1. Preparation of Lidocaine Patch
The prepared lidocaine patches contained three layers, including the backing, middle, and mucoadhesive layers (Figure 1). This patch was prepared according to the previous study on optimization of unidirectional three-layered patches containing lidocaine [21].
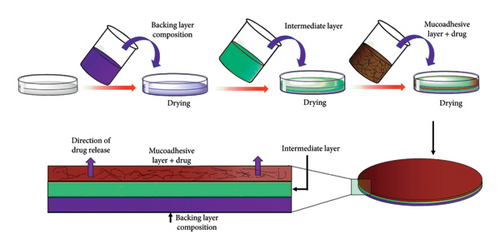
To prepare the backing layer, a 2%–7% ethanolic solution of ethyl cellulose was prepared. This polymeric solution was plasticized with triacetin 30% (w/w) and propylene glycol 5% (weight/weight) based on the weight of the polymer. The solution was well mixed on a stirrer for 1 hour, sonicated for 5 min, then it was refrigerated for 30 min to remove air bubbles. Finally, it was poured into a Teflon mold and placed aside for 24 h to dry. The middle layer was prepared in the same method, using eudragit as the polymeric ingredient at the concentration of 5%–10%. The resulted solution was poured on the previous layer (ethyl cellulose backing layer) and was placed in ambient condition for 24 h to dry. The role of this layer was to create a connection between the support and the mucoadhesive layers in such a way that these two layers do not separate when used by the patient.
To prepare the mucoadhesive layer, first, a 0.5%–2% solution containing Carbopol in water was mixed overnight to complete the hydration of polymeric chains. Then, ethanolic solution containing lidocaine was added. Glycerol at 25% (weight/weight) was used as a plasticizer. The resulted solution was sonicated for 5 min and stirred for 1 h. The solution was poured over the previous two layers.
After 24 h, the dried patches were removed from the mold and divided into discs with a diameter of 1 cm, each one containing 240 mg of lidocaine.
2.2. Preparation of Patches Containing Lidocaine and Dexmedetomidine
The formulation was prepared exactly as previously stated, but 70 micrograms of dexmedetomidine was added to the Carbopol layer during the manufacturing process.
2.3. Improving the Taste of the Drug-Containing Patch
Since lidocaine has a bitter taste which would negatively affect the patients’ compliance, 170 mg of sucralose–acesulfame sweetener mixture (1:1) and 75 mg of orange essential oil were used in the formulation of each patch to mask the bitter taste of lidocaine.
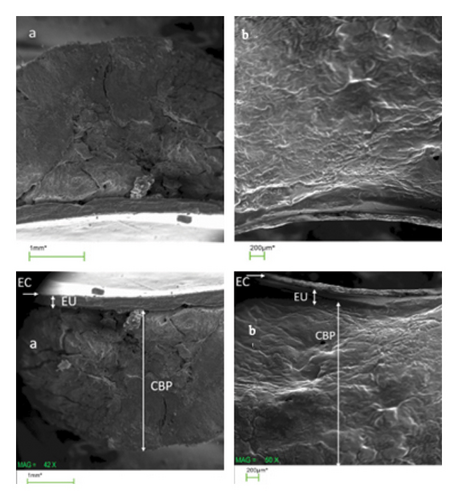
2.4. Examining the Morphology of the Three-Layered Patches Using Scanning Electron Microscope (SEM)
The presence of three layers in the structure of each patch was determined using a scanning electron microscope (SEM). For this purpose, the patch was placed on an aluminum piece, covered by a thin layer of gold and then scanned by an electron microscope (JSM-6010LA; JEOL, Peabody, MA, USA).
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
Infrared spectroscopy was performed in order to investigate the possible interactions between the components of the formulation and the active pharmaceutical ingredients (APIs) and to ensure the absence of chemical interaction that would lead to a change in the structure and hence in the efficacy of the drugs. For this purpose, the infrared spectra (FTIR) of pure lidocaine, pure dexmedetomidine, and the freeze-dried patch containing both APIs and freeze-dried patches lacking the APIs were recorded using a FTIR spectroscope (Shimadzu UV-VIS spectrophotometer mini-1240) in the range of 400–4000 cm−1.
2.6. Clinical Phase
This study was conducted as a randomized and double-blind clinical trial. The study was approved by the ethical committee of the Medical University of Isfahan (Ethical Code: IR.MUI.RESEARCH.REC.1401.130). Patients who visited our clinic for dentistry procedures were evaluated regarding the inclusion and exclusion criteria by the dentist in order to enter the study. Patients, who met the inclusion criteria and were willing to participate, were included in the study after completing the consent form. Before the intervention, the objectives and the method of conducting the clinical trial were explained to the patients. Demographic and clinical information of the study subjects, including age, sex, underlying disease, oral and dental problems, and concurrent medications were recorded.
The criteria for entering the study included age between 20 and 35 years and consent to participate in the study, and the exclusion criteria included suffering from systemic diseases, allergy to lidocaine or dexmedetomidine, history of taking painkillers within the last 4 h, and history of surgery or abscess in the studied area. Also, patients with a phobia of dentistry procedures or injections were excluded after taking a thorough history.
The first group of participants received a patch containing 240 mg of lidocaine + 70 micrograms of dexmedetomidine on one side of the jaw, and a drug-free patch on the other side, and the second group received a patch containing 240 mg of lidocaine on one side of the jaw and a drug-free patch on the other side. The patches were placed in the area of upper teeth four and five on both sides of the jaw.
After 15 min, a supraperiosteal injection of lidocaine 2% was performed by a dentist with a 27-gauge needle in the area of upper teeth four and five on both sides of the jaw. About 0.5 Carbopol or 0.9cc lidocaine was injected for each patient with a epinephrine proportion of 1:80,000. The drugs were provided from Darou Pakhsh company.
These data were added to the manuscript.
After the procedure, the patients were asked to rate the pain they experienced on a VAS scale from 0 to 10.
2.7. Statistical Analysis
Statistical studies were performed using SPSS software (Version 26). Kolmogorov–Smirnov test was used to determine the distribution of data, and based on the results, nonparametric Mann–Whitney test was used. Finally, the results were considered as mean ± SD and values with p value less than 0.05 were considered significant.
To further control for potential confounders and strengthen the statistical robustness, a multivariate analysis was performed using ordinary least squares (OLS) regression models for each outcome variable: VAS pain score, onset time, and duration of analgesia. The treatment group was included as a categorical predictor, and its effect on each outcome was evaluated while controlling for variance.
3. Results
3.1. The Results of Evaluating the Characteristics and Morphology of the Patches
All patches were made up of a three-layer structure, with colorless and transparent layers. The thickness of the patches was measured in the range of 2-3 mm, and their pH was measured in the range of 3.5–4.5.
In SEM photography, three layers of lidocaine and lidocaine–dexmedetomidine patches could be observed (Figure 2).
3.2. In Vitro Release Test
3.2.1. Methods
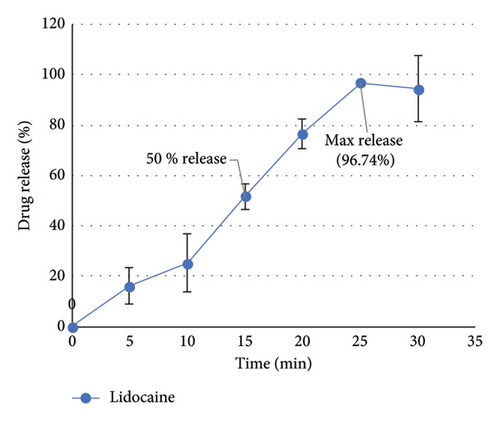
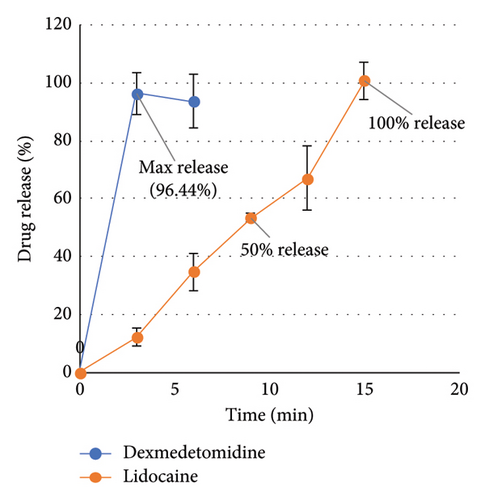
The release efficiency percentage for lidocaine from the patch was 41.69 ± 13.10 and the release efficiency percentage for lidocaine and dexmedetomidine from the patch containing both drugs was calculated as 47.17 ± 5.10 and 74.71 ± 6.41, respectively.
The results showed that 96.74% of lidocaine was released within 25 min from the patches containing only lidocaine. Also, 100% of lidocaine was released within 15 min and 96.44% of dexmedetomidine drug was released within 3 min from the patches containing both drugs (lidocaine–dexmedetomidine).
The results can be seen in Figure 3.
3.3. Structural Characteristics
In the thermal analysis performed for lidocaine hydrochloride powder and the patch containing this API (Figure 4), it was observed that the pure lidocaine had a melting peak at 80°C, while in the patch sample containing it, instead of the previous sharp peak, it had turned into a curve in the spectrum, which could be regarded as evidence of the change in the drug molecular structure from crystalline form to amorphous form.
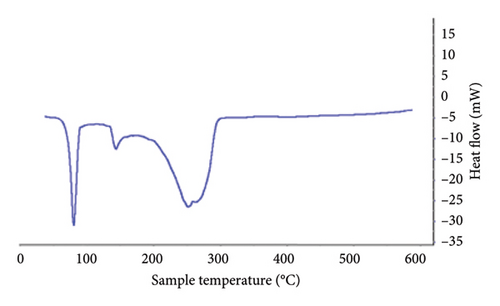
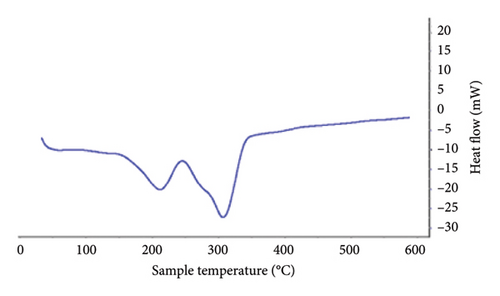
In the thermal diagram of the patches, it could be seen that the melting peak of the drug had shifted slightly to the left in addition to the shape change, which might be due to the overlap of the peak of the drug and the peak of ethyl cellulose in the patch sample. The melting temperature of ethyl cellulose is reported to be 130–133°C [22], but increasing the distance between the ethyl cellulose polymer chains by the plasticizer makes the polymer chains slide more easily on each other and thus melt at lower temperatures, so the melting point was reduced to about 85–115°, which overlaps with the location of the peak caused by the drugs used in the patches prepared in the present formulation.
The results of FTIR analysis confirmed the absence of chemical interaction between formulation components and APIs. The results of examined samples, which included pure lidocaine, pure dexmedetomidine, lidocaine patch, lidocaine–dexmedetomidine patch, and blank patch, are represented in Figure 5.
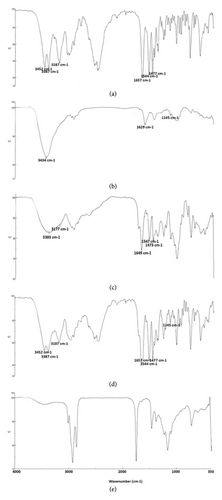
In the spectrum of pure lidocaine, the peak located at 3452 and 3387 cm−1 were due to the N-H bonds, and 3187 cm−1 indicated the C-H bonds in the ring. The C=O stretching bond was observed at 1657 cm−1, and the C=O stretching bond at 1544 cm−1. The peak at 1477 cm−1 showed the C-C bond in the aromatic ring. The set of peaks between 1250 and 1000 cm−1 could be related to the interference between C-N and N-H in the amide [4]. The mentioned peaks were also observed in the patch containing lidocaine.
In the spectrum of pure dexmedetomidine drug, the peak at 3434 cm−1 showed the presence of N-H bond, the peaks at 1542 and 1629 cm−1 showed the presence of C=N stretching bonds, and the peak at 1145 cm−1 showed the presence of C-N bond. Also, the set of peaks between 1487 and 1659 cm−1 could be related to the C=C stretching bond in the aromatic ring [6].
The mentioned peaks for both drugs were also observed in the lidocaine–dexmedetomidine patch, except for the 1629 cm−1 peak and the 3434 cm−1 peak associated with dexmedetomidine, which were masked by lidocaine peaks. (Figure 5).
3.4. Clinical Phase Results
Forty volunteers, who were randomly divided in two groups, participated in this study. The demographic data of participants are shown in Table 1.
| Lidocaine group | Lidocaine–dexmedetomidine group | |
|---|---|---|
| Gender | ||
| Female | 11 (55%) | 8 (40%) |
| Male | 9 (45%) | 12 (60%) |
| Age (year) | 26.57 ± 3.62 | 27.23 ± 2.59 |
| Weight (Kg) | 72.25 ± 23.49 | 73.12 ± 10.44 |
| Underlying disease | ||
| No underlying disease | 18 (90%) | 19 (59%) |
| Thyroid dysfunctions | 2 (10%) | 1 (5%) |
The descriptive result of the clinical phase can be seen in Table 2.
| Treatment group | VAS (mean ± SD) | Onset (mean ± SD) | Duration (mean ± SD) |
|---|---|---|---|
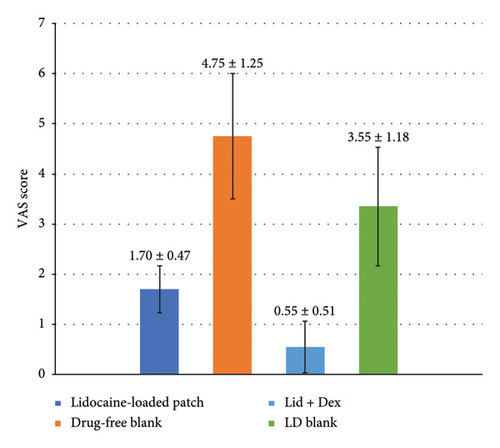
| Blank for lidocaine | 4.75 ± 1.25 | — | 49.25 ± 7.12 |
| Blank for lidocaine + dexmedetomidine | 3.35 ± 1.18 | — | 56.75 ± 7.65 |
| Lidocaine | 1.7 ± 0.47 | 7.05 ± 1.70 | 99.0 ± 25.93 |
| Lidocaine + dexmedetomidine | 0.55 ± 0.51 | 3.3 ± 1.12 | 190.5 ± 17.61 |
The average pain scores in the groups receiving the patch containing lidocaine, the patch containing lidocaine–dexmedetomidine, and the drug-free patch were compared (Figure 6). As illustrated in the graph, the group pretreated with the patch containing lidocaine reported significant lower average pain scores than the group pretreated with the patch without medicine (p < 0.001). The pain scores were also significantly lower in patients pretreated with lidocaine–dexmedetomidine patch compared to both lidocaine patch and blank patch (p < 0.001).
The nonparametric Mann–Whitney test was used to statistically compare the onset of the effect and the duration of the effect of different groups.
According to Figure 7, the onset of the effect of the lidocaine–dexmedetomidine patch was significantly lower than that of the lidocaine patch (p < 0.001).
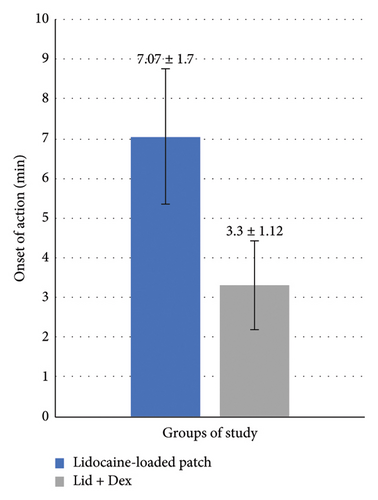
According to Figure 8, the duration of the effect of the lidocaine–dexmedetomidine patch was longer than the duration of the effect of the lidocaine patch due to the synergistic effect of the two drugs (p < 0.001). The duration of the effect of each drug-containing patch was significantly longer than the drug-free patch (p < 0.001).
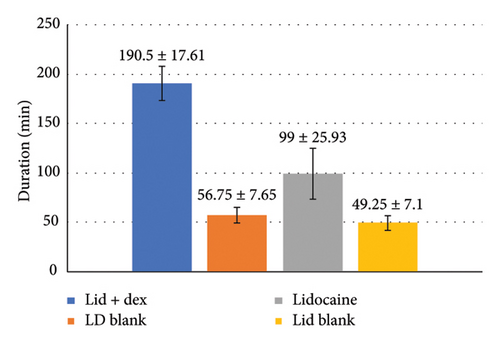
To further control potential confounders and strengthen the statistical robustness, a multivariate analysis was performed using OLS regression models for each outcome variable. The regression analysis showed a significant effect of the treatment group on pain scores, with a p value less than 0.001. The LD group exhibited significantly lower pain scores than both the lidocaine-only (L) and placebo groups. The adjusted R2 value of 0.753 indicates that the treatment group accounts for approximately 75% of the variance in pain scores, reinforcing the strong effect of the LD patch in reducing pain perception.
Similarly, a significant effect of the treatment group on onset time was observed (p < 0.001). The LD group had a significantly shorter onset time compared to the L group and placebo groups, suggesting that the addition of dexmedetomidine accelerates the onset of analgesia. The adjusted R2 value of 0.554 suggests that the treatment group explains about 55% of the variance in onset time, highlighting the enhanced effectiveness of the LD patch in providing rapid pain relief.
Regarding duration of analgesia, the statistical analysis demonstrated a significant effect of the treatment group (p < 0.001), with the LD group exhibiting the longest duration of analgesia. The adjusted R2 value of 0.723 indicates that the treatment group accounts for 72% of the variance in duration. This finding supports the hypothesis that dexmedetomidine synergistically enhances lidocaine’s effects, prolonging the period of pain relief.
4. Discussion
Utilization of anesthetic buccal patches loaded with lidocaine was considered in the current study as a pretreatment step before starting dental procedures. Dexmedetomidine was added to the formulation to enhance the anesthetic effect and also to enhance the patient’s compliance. Mixing dexmedetomidine with local anesthetics makes the nerve block take longer and also produces an analgesic effect. The addition of dexmedetomidine as an alpha-2 agonist would cause vasoconstriction of the anesthesia area, reducing the blood flow, and thus prolongs the effect of the local anesthetic. Such synergy is critical in dental procedures where both rapid onset and extended duration of anesthesia are desirable without increasing systemic toxicity [23, 24]. By increasing the amount of dexmedetomidine, the anesthetic effect of lidocaine also increases. Dexmedetomidine can be used as a safe substitute for adrenaline in combination with local anesthetics such as lidocaine. Dexmedetomidine has sympatholytic, sedative, analgesic, and blood pressure lowering and heart rate lowering effects. Therefore, it can be used as a safe drug in cardiovascular patients. On the other hand, the administered dose used (1 microgram/kg) was not associated with severe adverse effects on the vascular system and would not cause serious complications [25].
When administered via the buccal route, it demonstrates rapid absorption due to the rich vascularization of the oral mucosa. Its buccal bioavailability has been reported to reach approximately 82% in pharmacokinetic studies. Peak plasma concentrations are typically reached within 60 min, and the elimination of half-life is approximately 2 h, making it suitable for short dental procedures [26].
Numerous studies have supported the safety and effectiveness of dexmedetomidine in oral and dental procedures. A recent systematic review by Diniz et al. concluded that dexmedetomidine, when used as an adjunct to local anesthetics, significantly reduced onset time, increased the duration of anesthesia, and improved postoperative pain control without introducing significant hemodynamic instability [27].
Similarly, Naglaa et al. demonstrated in a randomized clinical trial that adding dexmedetomidine to lidocaine significantly increased the success rate of inferior alveolar nerve blocks and decreased patient-reported pain scores during treatment. Importantly, no significant adverse events such as bradycardia or hypotension were observed, supporting its safety profile in outpatient dental settings [28].
Since transbuccal patches are usually well accepted by the patients, this dosage form was selected in the current study. However, some components of the formulation may cause mucosal irritation. This irritation and the fact that the binding of the drug to the mucosa may lead to dose dumping are some of the disadvantages of the buccal patch system [29]. The drug delivery system proposed in the present study was designed to provide an immediate release profile. Thus, the risk of dose dumping would not be an issue compared to some other formulations of buccal patches [30]. But it is worth mentioning that a mild irritation was reported by patients treated with lidocaine–dexmedetomidine patches. This phenomenon was limited to mild erythema in the administration site which was accompanied with minimal irritation reported by the patients. Patients affected by this adverse effect were carefully evaluated and none of them needed any further actions. The irritation was resolved on its own after a maximum of thirty minutes. Reducing the administration time might be useful in reducing the rates of this adverse effect in the future studies.
Research carried out by Adami et al. examined the efficacy of a mucoadhesive patch that includes lidocaine and prilocaine hydrochlorides for providing needle-free local anesthesia during dental treatments. The patch was tested on 58 adult patients and proved to be successful in 90% of cases, eliminating the requirement for additional traditional anesthesia. It induced numbness within 5 min, peaked in effectiveness between 15 and 25 min, and lasted a minimum of 50 min. No discomfort or adverse reactions were reported in the study, emphasizing the patch as a secure and efficient option for dental procedures of medium complexity [31].
Another research study, carried out by Bågesund and Tabrizi, has also explored the impact of lidocaine in the form of buccal patches. The study involved 31 pediatric patients and compared the efficacy of a 20% lidocaine patch (DentiPatch) with a 5% lidocaine gel for topical anesthesia of the oral mucosa. It was a randomized, unblinded, cross-over design. The findings revealed that the patch led to a greater decrease in heart rate during buccal injection, and patients experienced less discomfort with the patch on both buccal and palatal sites. Furthermore, boys exhibited lower heart rates and pain levels compared to girls. In conclusion, both types of anesthesia offered similar pain relief at needle stick and injection sites [32]. DentiPatch has shown to be at least as effective as topical anesthetics even in children but it might need a longer time to take effect [33].
In the clinical phase of our study, the group treated with the patch containing lidocaine–dexmedetomidine reported significantly lower mean pain scores than those treated with the patch containing lidocaine and those treated with a blank patch. This comparison was not investigated before in the form of buccal patches.
According to the results of a study by Alizargar et al., dexmedetomidine besides lidocaine increased the effects of lidocaine and decreased the pain score. It has also shown a shorter oneset of action and longer duration of action in patients immediately after surgery and after 6,12, and 24 h as well [12].
Also, Yaman et al. conducted a study which proved that dexmedetomidine injection increased pain threshold and decreased pain sensation. They observed the highest increase in pain threshold at 10 min after injection. In addition, the increase in pain threshold and, as a result, pain relief, reached its maximum value 20 min after the administration of lidocaine with dexmedetomidine [34].
Different analgesic and anesthetic drugs were also previously investigated as transbuccal patches. Diclofenac was used for the postsurgical pain associated with dental procedures in the form of transbuccal adhesive patches. The transbuccal patch showed a quicker onset, longer duration, and better postoperative pain control compared to oral diclofenac [35]. These results were in accordance with previously published studies, while taking advantage of buccal drug delivery in the current study, compared with injections performed in the previous studies.
All in all, the results of our clinical evaluation showed that adding dexmedetomidine to lidocaine reduces the onset time of the effect and increases the duration of the effect of the patch. However, our study faced certain limitations which could be addressed in the future studies. A larger study population in a multicenter study might help make the results more generalized. Also, making the patch’s flavor better can significantly increase the patients’ compliance especially in children.
5. Conclusion
In this study, a formulation was designed and optimized for the delivery of local anesthetics in buccal form, for the purpose of utilization before dental procedures in order to reduce dental pain and anxiety in patients. The optimal formulation resulted in a good release behavior of the drug because a short-term release was intended to enable the use of the drug within a few minutes before the start of dental procedures. The results of the clinical evaluation showed that adding dexmedetomidine to lidocaine reduces the onset time of the effect and increases the duration of the effect of the patch. Also, due to the synergistic effect of two drugs on local anesthesia, patients reported less pain than the patch containing lidocaine. However, further studies on this issue with larger populations and on different drugs might be beneficial.
Ethics Statement
This manuscript has been approved by the Ethical Committee of Isfahan University of Medical Sciences (Ethical Code: IR.MUI.RESEARCH.REC.1401.130).
Consent
All the participants signed an informed consent form regarding their participation and their approval of data publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
E.G. came up with the presented idea. E.P., M.E.S., and E.G. carried out the experiment. M.E.S. and S.S. helped with the clinical data collection. E.P. and S.S. verified the analytical methods. S.S. wrote the manuscript with support from E.P. and E.G. E.G. and J.V. critically reviewed the final manuscript and discussion. J.V. supervised the project. All authors contributed in the final editing and confirmed the final version of the manuscript.
Funding
This study received no external funding.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.




