Efficacy of Biological or Targeted Synthetic Disease-Modifying Anti-Rheumatic Drugs in Active Psoriatic Arthritis: A Network Meta-Analysis of Randomized Controlled Trials
Abstract
Objective: Many biological or targeted synthetic disease-modifying anti-rheumatic drugs (b/ts DMARDs) are used in the treatment of psoriatic arthritis (PsA) during recent years, but there are few head-to-head studies that directly compare these drugs to evaluate and compare the relative efficacy of these treatments at week 24. The aim of this study is to conduct a comprehensive comparison of all clinically used bDMARDs or tsDMARDs for PSA using a network meta-analysis to evaluate relative efficacy of these drugs, which is evaluated by ACR20, ACR50, ACR70, PASI75, and PASI90.
Methods: All randomized controlled trials of these treatments are searched in PubMed, Web of Science, and Embase, and data are extracted from the included articles, and network meta-analysis is performed using the Stata 13 software.
Results: secukinumab 300 mg is the top-ranked treatment for ACR20 and PASI90, infliximab 5 mg/kg is the top-ranked treatment for ACR50, and adalimumab 40 mg is the top-ranked treatment for ACR70 and PASI75.
Conclusions: Tumor necrosis factor-α inhibitors and interleukin 17A inhibitors are the top-ranked treatments for arthritis and skin responses of active PsA.
1. Introduction
Psoriatic arthritis (PsA) is a common subtype of spondyloarthritis, and its clinical manifestations include peripheral arthritis, spondylitis, dactylitis, enthesitis, psoriasis, and nail disease, as well as extra-articular features such as uveitis, cardiovascular disease, and inflammatory bowel disease. Other disorders like osteoporosis and metabolic diseases are also linked to PsA. All of these bring enormous burdens to patients and their families. PsA has five subtypes: oligoarticular subtype, polyarticular subtype, distal subtype, arthritis mutilans, and axial or spondyloarthritis subtype [1–4]. The prevalence of PsA is 0.1%–1% and 14%–30% in the general population and in psoriasis patients, respectively [5, 6]. Deformities and joint damages develop in a large number of PsA patients, influencing the physical functions and qualities of life and increasing financial burdens of patients and their family [7, 8].
Before the year 2000, the treatment options for PsA are essentially limited to nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate, sulfasalazine, and cyclosporine. The effects of these drugs are limited. Since the year 2000, biological synthetic disease-modifying anti-rheumatic drugs (bDMARDs) and targeted synthetic disease-modifying anti-rheumatic drugs (tsDMARDs), such as tumor necrosis factor-α (TNF-α) inhibitors (adalimumab, certolizumab pegol, infliximab, etanercept, golimumab), interleukin 17A (IL-17A) inhibitors (secukinumab, ixekizumab), IL-17 receptor inhibitor (brodalumab), JAK inhibitors (upadacitinib, tofacitinib, baricitinib), IL-12/23 inhibitor (ustekinumab), IL-23 inhibitors (guselkumab, tildrakizumab, risankizumab), phosphodiesterase 4 inhibitor (apremilast), and T-cell modulator (abatacept), are used to treat PsA, improving the prognosis of the disease [4, 7]. But there are few head-to-head studies that directly compare all these drugs to evaluate and compare the relative efficacy of these treatments, and few meta-analyses are about long-term efficacy of these drugs [9–12].
In our study, we conduct a comprehensive comparison of all clinically used bDMARDs or tsDMARDs in the treatment of active PsA using a network meta-analysis to evaluate the relative efficacy of these drugs at week 24, helping us find which drugs are more effective in the treatment of joint/skin involvement of PsA, improving the prognosis of the disease and the life qualities of PsA patients.
2. Methods
2.1. Study Design
The protocols of this study are designed in accordance with the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISNMA) guidelines [13]. The research question is “which biological or targeted synthetic disease-modifying anti-rheumatic drug is more effective in active psoriatic arthritis”?
2.2. Search Strategies
Literature search is conducted to identify all randomized controlled trials (RCTs) that compared the efficacy of bDMARDs or tsDMARDs in PsA. Studies lists in PubMed, Web of Science, and Embase published up to December 2024 are identified. English search terms included (“Arthritis, Psoriatic” OR “Psoriasis, Arthritic” OR “Arthritic Psoriasis” OR “Psoriatic Arthritis” OR “Psoriasis Arthropathica” OR “Psoriatic Arthropathy” OR “Arthropathies, Psoriatic” OR “Arthropathy, Psoriatic” OR “Psoriatic Arthropathies”) AND (“adalimumab” OR “infliximab” OR “certolizumab pegol” OR “etanercept” OR “golimumab” OR “secukinumab” OR “ixekizumab” OR “brodalumab” OR “ustekinumab” OR “guselkumab” OR “tildrakizumab” OR “risankizumab” OR “apremilast” OR “abatacept” OR “upadacitinib”).
2.3. Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are developed and used to screen all identified studies. The inclusion criteria are as follows: age ≥ 18 years; fulfilled the Classification Criteria for Psoriatic Arthritis (CASPAR) [14]; with active disease (≥ 3 swollen joints and ≥ 3 tender joints); active plaque psoriasis (at least one plaque of at least 2 cm diameter) or nail changes consistent with psoriasis or documented history of plaque psoriasis; NSAIDs, prednisone, methotrexate, sulfasalazine, and leflunomide are allowed to use before or now; the drugs are all approved for clinical use and used according to the instructions, which include adalimumab, infliximab, certolizumab pegol, etanercept, golimumab, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, apremilast, abatacept, and upadacitinib [15–48]; all the studies are published full-text RCTs; the end time of the RCT is 24 weeks. The exclusion criteria are as follows: combined with other active skin conditions that would interfere with study evaluations; combined with other autoimmune diseases; recent serious infection; tuberculosis or hepatitis infection; other types of studies other than RCT; the end time of the RCT in not week 24.
2.4. Screening and Selection
After the automatic removal of duplicates in EndNote X8, two authors independently screen the titles and abstracts, the full text of every potentially relevant study is then obtained to determine its eligibility for inclusion. Any discrepancies are resolved by discussing to reach a consensus.
2.5. Critical Appraisal
Two authors independently assessed the quality of the included studies. RCT Critical Appraisal Sheet (https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools) is used for the critical appraisal. Discrepancies are resolved through discussion.
2.6. Data Extraction
The data of the included studies are the study number, sample size, patients characteristics, age (years), gender (male/female), disease duration, drugs (glucocorticoid, NSAIDs, and methotrexate) used before or now, study drug (bDMARDs or tsDMARDs) and how it used, study time, ACR20 (at least 20% improvement in American College of Rheumatology score), ACR50 (at least 50% improvement in American College of Rheumatology score), ACR70 (at least 70% improvement in American College of Rheumatology score), PASI75 (≥ 75% reduction in Psoriasis Area and Severity Index), and PASI90 (≥ 90% reduction in Psoriasis Area and Severity Index). Among them, ACR20, ACR50, ACR70, PASI75, and PSAI90 are extracted as the outcomes. Data are presented as mean ± standard deviation (SD) or percent.
2.7. Statistical Analysis
Network meta-analysis is performed using the Stata 13 software, and the exact commands of Stata are shown in supporting information 1. Publication bias is observed through funnel plots.
3. Results
3.1. Characteristics and Qualities of Included Studies
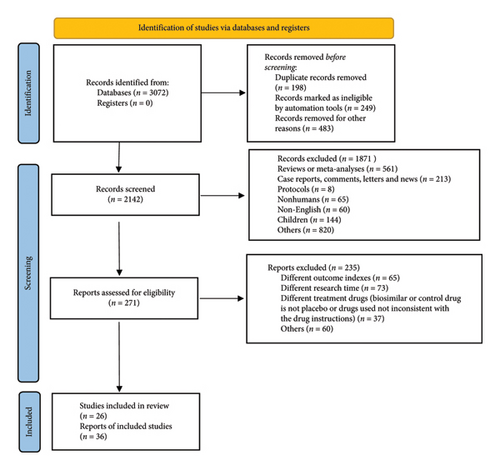
The search strategies yield a total of 3072 publications; a total of 25 unique RCTs enrolled a total of 11,375 patients are included in the final analysis after the screening and selection process by two authors independently. The exact selection process is outlined in the PRISMA flow diagram (Figure 1). The summary of included studies and patients’ baseline characteristics are presented in supporting Table 1, and its legend is presented in supporting information 2. The mean ages of patients range from 44.2 to 54.1 years, and the mean disease durations range from 3.4 to 11.0 years. The proportion of male patients ranges from 39.0% to 61.0%; 56.0%–88.0% patients are using or have used NSAIDs, 5.0%–26.3% patients are using or have used glucocorticoids, 34.0%–69.8% patients are using or have used methotrexate. The methodological quality of the included studies is summarized in supporting Figures 1 and 2, and the funnel plots for ACR20, ACR50, ACR70, PASI75, and PASI90 are almost symmetric, indicating little publication bias (supporting Figure 3).
3.2. Network Meta-Analysis Results
A network meta-analysis is conducted for each outcome of interest: attainment of ACR20 response, ACR50 response, ACR70 response, PASI 75 response, and PASI 90 response. In the network diagram, each intervention is represented by a node, and randomized comparisons are shown as links between the nodes. Cumulative ranking plot and surface under the cumulative ranking curve (SUCRA) show the best treatment choice.
3.3. Arthritis Response Scores
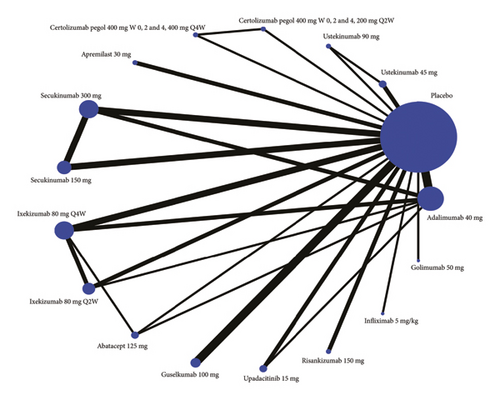
Twenty-four studies are included in the comparison of ACR20 at week 24, the network diagram for ACR20 is provided in Figure 2(a), and the results of pairwise comparisons for all treatments in the ACR20 are shown in Tables 1. Among all the drugs, secukinumab 300 mg is obviously better than other drugs except golimumab 50 mg and infliximab 5 mg/kg. The rankings based on SUCRA values are highest for secukinumab 300 mg (97.0%), followed by golimumab 50 mg (86.4%) and adalimumab 40 mg (86.1%) (Table 2).
| (a) Results of pairwise comparisons for all treatments in the ACR20 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 3.19 (1.81, 5.61) | 1.48 (0.66, 3.32) | 2.43 (1.21, 4.88) | 4.05 (3.07, 5.36) | 10.51 (5.78, 19.14) | 0.52 (0.27, 1.01) | 3.35 (2.06, 5.44) | 4.19 (2.48, 7.06) | 5.72 (3.38, 9.69) | 3.32 (2.17, 5.08) | 3.07 (1.65, 5.68) | 0.81 (0.38, 1.72) | 7.61 (4.43, 13.06) | 0.05 (0.03, 0.10) | 1.18 (0.60, 2.34) | 0.41 (0.19, 0.90) |
| 0.31 (0.18, 0.55) | Golimumab 50 mg | 0.80 (0.32, 2.03) | 0.36 (0.18, 0.71) | 0.18 (0.09, 0.36) | 0.49 (0.25, 0.99) | 0.30 (0.14, 0.64) | 0.49 (0.21, 1.17) | 0.41 (0.18, 0.98) | 0.53 (0.26, 1.07) | 1.37 (0.57, 3.31) | 0.44 (0.19, 0.98) | 0.55 (0.24, 1.25) | 0.75 (0.32, 1.72) | 0.43 (0.20, 0.94) | 0.40 (0.16, 0.98) | 0.99 (0.43, 2.30) |
| 0.68 (0.30, 1.52) | 1.25 (0.49, 3.15) | Infliximab 5 mg/kg | 0.45 (0.22, 0.90) | 0.22 (0.11, 0.45) | 0.61 (0.30, 1.25) | 0.37 (0.17, 0.81) | 0.62 (0.26, 1.48) | 0.52 (0.22, 1.24) | 0.66 (0.32, 1.35) | 1.71 (0.70, 4.17) | 0.54 (0.24, 1.24) | 0.68 (0.29, 1.58) | 0.93 (0.40, 2.17) | 0.54 (0.24, 1.18) | 0.50 (0.20, 1.23) | 1.23 (0.52, 2.90) |
| 0.41 (0.20, 0.83) | 2.79 (1.41, 5.53) | 2.24 (1.12, 4.51) | Risankizumab 150 mg | 0.49 (0.34, 0.71) | 1.37 (0.98, 1.93) | 0.83 (0.51, 1.33) | 1.38 (0.75, 2.56) | 1.16 (0.63, 2.13) | 1.48 (1.04, 2.10) | 3.83 (2.02, 7.24) | 1.22 (0.71, 2.08) | 1.52 (0.87, 2.68) | 2.08 (1.18, 3.68) | 1.21 (0.75, 1.95) | 1.12 (0.58, 2.15) | 2.77 (1.55, 4.96) |
| 0.25 (0.19, 0.33) | 5.68 (2.79, 11.56) | 4.56 (2.20, 9.42) | 2.03 (1.41, 2.93) | Upadacitinib 15 mg | 2.79 (1.89, 4.13) | 1.68 (1.00, 2.82) | 2.81 (1.47, 5.35) | 2.36 (1.25, 4.46) | 3.00 (2.00, 4.50) | 7.77 (3.99, 15.16) | 2.47 (1.40, 4.37) | 3.10 (1.70, 5.64) | 4.23 (2.31, 7.74) | 2.45 (1.46, 4.12) | 2.27 (1.14, 4.49) | 5.63 (3.04, 10.41) |
| 0.10 (0.05, 0.17) | 2.03 (1.01, 4.08) | 1.63 (0.80, 3.33) | 0.73 (0.52, 1.02) | 0.36 (0.24, 0.53) | Guselkumab 100 mg | 0.60 (0.37, 0.99) | 1.00 (0.54, 1.89) | 0.84 (0.45, 1.57) | 1.07 (0.73, 1.57) | 2.78 (1.45, 5.34) | 0.89 (0.51, 1.54) | 1.11 (0.62, 1.98) | 1.51 (0.84, 2.72) | 0.88 (0.53, 1.44) | 0.81 (0.42, 1.58) | 2.01 (1.11, 3.66) |
| 1.91 (0.99, 3.70) | 3.38 (1.56, 7.33) | 2.71 (1.23, 5.97) | 1.21 (0.75, 1.95) | 0.60 (0.35, 1.00) | 1.66 (1.01, 2.73) | Abatacept 125 mg | 1.67 (0.82, 3.41) | 1.40 (0.69, 2.84) | 1.78 (1.07, 2.97) | 4.63 (2.22, 9.64) | 1.47 (0.77, 2.81) | 1.84 (0.94, 3.61) | 2.52 (1.28, 4.95) | 1.46 (0.80, 2.66) | 1.35 (0.64, 2.86) | 3.35 (1.68, 6.66) |
| 0.30 (0.18, 0.49) | 2.02 (0.85, 4.81) | 1.62 (0.67, 3.91) | 0.72 (0.39, 1.34) | 0.36 (0.19, 0.68) | 1.00 (0.53, 1.87) | 0.60 (0.29, 1.22) | Ixekizumab 80 mg Q2W | 0.84 (0.48, 1.46) | 1.07 (0.56, 2.02) | 2.77 (1.21, 6.35) | 0.88 (0.42, 1.87) | 1.10 (0.51, 2.40) | 1.51 (0.69, 3.29) | 0.87 (0.43, 1.79) | 0.81 (0.35, 1.88) | 2.00 (0.91, 4.41) |
| 0.24 (0.14, 0.40) | 2.41 (1.02, 5.69) | 1.93 (0.81, 4.62) | 0.86 (0.47, 1.58) | 0.42 (0.22, 0.80) | 1.19 (0.64, 2.21) | 0.71 (0.35, 1.45) | 1.19 (0.69, 2.07) | Ixekizumab 80 mg Q4W | 1.27 (0.68, 2.39) | 3.30 (1.45, 7.52) | 1.05 (0.50, 2.21) | 1.31 (0.61, 2.84) | 1.80 (0.83, 3.89) | 1.04 (0.51, 2.11) | 0.96 (0.42, 2.22) | 2.39 (1.09, 5.22) |
| 0.17 (0.10, 0.30) | 1.89 (0.94, 3.83) | 1.52 (0.74, 3.12) | 0.68 (0.48, 0.97) | 0.33 (0.22, 0.50) | 0.93 (0.64, 1.36) | 0.56 (0.34, 0.93) | 0.94 (0.49, 1.77) | 0.79 (0.42, 1.48) | Secukinumab 150 mg | 2.59 (1.34, 5.02) | 0.83 (0.47, 1.45) | 1.03 (0.57, 1.87) | 1.41 (0.78, 2.56) | 0.82 (0.49, 1.36) | 0.76 (0.38, 1.49) | 1.88 (1.02, 3.45) |
| 0.30 (0.20, 0.46) | 0.73 (0.30, 1.76) | 0.59 (0.24, 1.43) | 0.26 (0.14, 0.49) | 0.13 (0.07, 0.25) | 0.36 (0.19, 0.69) | 0.22 (0.10, 0.45) | 0.36 (0.16, 0.83) | 0.30 (0.13, 0.69) | 0.39 (0.20, 0.75) | Secukinumab 300 mg | 0.32 (0.15, 0.69) | 0.40 (0.18, 0.88) | 0.54 (0.25, 1.21) | 0.32 (0.15, 0.66) | 0.29 (0.12, 0.69) | 0.72 (0.56, 0.94) |
| 0.33 (0.18, 0.60) | 2.29 (1.02, 5.16) | 1.84 (0.81, 4.19) | 0.82 (0.48, 1.40) | 0.40 (0.23, 0.71) | 1.13 (0.65, 1.96) | 0.68 (0.36, 1.30) | 1.13 (0.53, 2.41) | 0.95 (0.45, 2.01) | 1.21 (0.69, 2.12) | 3.14 (1.45, 6.80) | Apremilast 30 mg | 1.25 (0.61, 2.55) | 1.71 (0.83, 3.50) | 0.99 (0.52, 1.89) | 0.92 (0.42, 2.01) | 2.27 (1.10, 4.70) |
| 1.23 (0.58, 2.60) | 1.83 (0.80, 4.21) | 1.47 (0.63, 3.43) | 0.66 (0.37, 1.16) | 0.32 (0.18, 0.59) | 0.90 (0.50, 1.61) | 0.54 (0.28, 1.06) | 0.91 (0.42, 1.97) | 0.76 (0.35, 1.64) | 0.97 (0.54, 1.75) | 2.51 (1.13, 5.56) | 0.80 (0.39, 1.63) | Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 1.37 (0.84, 2.22) | 0.79 (0.40, 1.55) | 0.73 (0.33, 1.64) | 1.82 (0.86, 3.85) |
| 0.13 (0.08, 0.23) | 1.34 (0.58, 3.09) | 1.08 (0.46, 2.51) | 0.48 (0.27, 0.85) | 0.24 (0.13, 0.43) | 0.66 (0.37, 1.19) | 0.40 (0.20, 0.78) | 0.66 (0.30, 1.45) | 0.56 (0.26, 1.21) | 0.71 (0.39, 1.29) | 1.84 (0.83, 4.08) | 0.58 (0.29, 1.20) | 0.73 (0.45, 1.19) | Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 0.58 (0.29, 1.14) | 0.54 (0.24, 1.21) | 1.33 (0.63, 2.83) |
| 18.83 (10.26, 34.56) | 2.31 (1.07, 5.02) | 1.86 (0.84, 4.09) | 0.83 (0.51, 1.34) | 0.41 (0.24, 0.68) | 1.14 (0.69, 1.87) | 0.68 (0.38, 1.25) | 1.14 (0.56, 2.34) | 0.96 (0.47, 1.95) | 1.22 (0.73, 2.03) | 3.17 (1.52, 6.61) | 1.01 (0.53, 1.93) | 1.26 (0.64, 2.48) | 1.72 (0.88, 3.39) | Ustekinumab 90 mg | 0.92 (0.44, 1.96) | 2.29 (1.15, 4.56) |
| 0.84 (0.43, 1.67) | 2.50 (1.02, 6.12) | 2.01 (0.81, 4.97) | 0.90 (0.47, 1.72) | 0.44 (0.22, 0.87) | 1.23 (0.63, 2.40) | 0.74 (0.35, 1.57) | 1.24 (0.53, 2.88) | 1.04 (0.45, 2.40) | 1.32 (0.67, 2.60) | 3.43 (1.45, 8.10) | 1.09 (0.50, 2.39) | 1.37 (0.61, 3.06) | 1.87 (0.83, 4.20) | 1.08 (0.51, 2.29) | Ustekinumab 45 mg | 2.48 (1.09, 5.63) |
| 2.44 (1.11, 5.36) | 1.01 (0.43, 2.34) | 0.81 (0.34, 1.91) | 0.36 (0.20, 0.65) | 0.18 (0.10, 0.33) | 0.50 (0.27, 0.90) | 0.30 (0.15, 0.59) | 0.50 (0.23, 1.10) | 0.42 (0.19, 0.92) | 0.53 (0.29, 0.98) | 1.38 (1.07, 1.79) | 0.44 (0.21, 0.91) | 0.55 (0.26, 1.17) | 0.75 (0.35, 1.60) | 0.44 (0.22, 0.87) | 0.40 (0.18, 0.92) | Adalimumab 40 mg |
| (b) Results of pairwise comparisons for all treatments in the ACR50 | ||||||||||||||||
| Placebo | 2.69 (0.86, 8.44) | 1.92 (0.82, 4.49) | 5.29 (3.58, 7.82) | 11.54 (5.20, 25.64) | 0.62 (0.26, 1.50) | 5.60 (2.35, 13.35) | 4.67 (2.46, 8.85) | 5.55 (2.94, 10.47) | 4.05 (2.22, 7.38) | 2.93 (1.15, 7.50) | 1.18 (0.39, 3.60) | 9.75 (4.65, 20.45) | 0.03 (0.01, 0.07) | 0.64 (0.26, 1.56) | 0.36 (0.13, 1.00) | |
| 0.37 (0.12, 1.17) | Infliximab 5 mg/kg | 0.23 (0.07, 0.72) | 0.08 (0.03, 0.26) | 0.21 (0.07, 0.66) | 0.10 (0.03, 0.35) | 0.29 (0.08, 1.07) | 0.23 (0.06, 0.82) | 0.32 (0.10, 1.01) | 0.69 (0.18, 2.68) | 0.34 (0.08, 1.36) | 0.28 (0.08, 0.99) | 0.33 (0.09, 1.18) | 0.24 (0.07, 0.84) | 0.18 (0.04, 0.74) | 0.58 (0.16, 2.19) | |
| 0.52 (0.22, 1.22) | 4.33 (1.39, 13.51) | Risankizumab 150 mg | 0.36 (0.23, 0.57) | 0.90 (0.54, 1.48) | 0.44 (0.23, 0.84) | 1.27 (0.60, 2.71) | 0.98 (0.46, 2.09) | 1.37 (0.83, 2.26) | 3.00 (1.26, 7.11) | 1.45 (0.58, 3.67) | 1.21 (0.59, 2.48) | 1.44 (0.71, 2.94) | 1.05 (0.53, 2.08) | 0.76 (0.28, 2.05) | 2.53 (1.13, 5.68) | |
| 0.19 (0.13, 0.28) | 11.98 (3.84, 37.42) | 2.77 (1.75, 4.37) | Upadacitinib 15 mg | 2.49 (1.52, 4.08) | 1.22 (0.64, 2.33) | 3.53 (1.66, 7.51) | 2.72 (1.28, 5.78) | 3.80 (2.28, 6.32) | 8.29 (3.50, 19.63) | 4.02 (1.59, 10.17) | 3.35 (1.64, 6.87) | 3.98 (1.95, 8.13) | 2.91 (1.47, 5.76) | 2.11 (0.78, 5.69) | 7.00 (3.12, 15.72) | |
| 0.09 (0.04, 0.19) | 4.82 (1.52, 15.28) | 1.11 (0.67, 1.84) | 0.40 (0.25, 0.66) | Guselkumab 100 mg | 0.49 (0.25, 0.96) | 1.42 (0.65, 3.09) | 1.09 (0.50, 2.38) | 1.53 (0.87, 2.69) | 3.34 (1.40, 7.96) | 1.62 (0.63, 4.17) | 1.35 (0.64, 2.83) | 1.60 (0.77, 3.35) | 1.17 (0.58, 2.37) | 0.85 (0.31, 2.33) | 2.82 (1.23, 6.46) | |
| 1.61 (0.66, 3.92) | 9.83 (2.88, 33.59) | 2.27 (1.19, 4.34) | 0.82 (0.43, 1.57) | 2.04 (1.04, 4.01) | Abatacept 125 mg | 2.89 (1.20, 7.01) | 2.23 (0.92, 5.40) | 3.12 (1.57, 6.19) | 6.81 (2.56, 18.08) | 3.30 (1.17, 9.30) | 2.75 (1.17, 6.45) | 3.27 (1.40, 7.64) | 2.39 (1.05, 5.44) | 1.73 (0.58, 5.17) | 5.75 (2.27, 14.57) | |
| 0.18 (0.07, 0.43) | 3.40 (0.94, 12.31) | 0.78 (0.37, 1.67) | 0.28 (0.13, 0.60) | 0.70 (0.32, 1.53) | 0.35 (0.14, 0.84) | Ixekizumab 80 mg Q2W | 0.77 (0.43, 1.37) | 1.08 (0.49, 2.36) | 2.35 (0.82, 6.72) | 1.14 (0.38, 3.44) | 0.95 (0.37, 2.42) | 1.13 (0.44, 2.87) | 0.82 (0.33, 2.05) | 0.60 (0.19, 1.91) | 1.99 (0.73, 5.44) | |
| 0.21 (0.11, 0.41) | 4.41 (1.22, 15.98) | 1.02 (0.48, 2.17) | 0.37 (0.17, 0.78) | 0.92 (0.42, 1.99) | 0.45 (0.19, 1.09) | 1.30 (0.73, 2.31) | Ixekizumab 80 mg Q4W | 1.40 (0.64, 3.07) | 3.05 (1.07, 8.73) | 1.48 (0.49, 4.47) | 1.23 (0.48, 3.15) | 1.47 (0.58, 3.73) | 1.07 (0.43, 2.66) | 0.78 (0.24, 2.48) | 2.58 (0.94, 7.06) | |
| 0.18 (0.10, 0.34) | 3.15 (0.99, 10.06) | 0.73 (0.44, 1.20) | 0.26 (0.16, 0.44) | 0.65 (0.37, 1.15) | 0.32 (0.16, 0.64) | 0.93 (0.42, 2.04) | 0.71 (0.33, 1.57) | Secukinumab 150 mg | 2.18 (0.89, 5.36) | 1.06 (0.41, 2.75) | 0.88 (0.42, 1.87) | 1.05 (0.50, 2.21) | 0.77 (0.37, 1.57) | 0.55 (0.20, 1.53) | 1.84 (0.80, 4.26) | |
| 0.25 (0.14, 0.45) | 1.44 (0.37, 5.59) | 0.33 (0.14, 0.79) | 0.12 (0.05, 0.29) | 0.30 (0.13, 0.72) | 0.15 (0.06, 0.39) | 0.43 (0.15, 1.22) | 0.33 (0.11, 0.94) | 0.46 (0.19, 1.13) | Secukinumab 300 mg | 0.49 (0.15, 1.58) | 0.40 (0.15, 1.12) | 0.48 (0.17, 1.33) | 0.35 (0.13, 0.95) | 0.25 (0.07, 0.87) | 0.84 (0.63, 1.14) | |
| 0.34 (0.13, 0.87) | 2.98 (0.74, 12.02) | 0.69 (0.27, 1.74) | 0.25 (0.10, 0.63) | 0.62 (0.24, 1.59) | 0.30 (0.11, 0.85) | 0.88 (0.29, 2.65) | 0.67 (0.22, 2.04) | 0.94 (0.36, 2.45) | 2.06 (0.63, 6.71) | Apremilast 30 mg | 0.83 (0.28, 2.45) | 0.99 (0.34, 2.91) | 0.72 (0.25, 2.08) | 0.52 (0.15, 1.88) | 1.74 (0.56, 5.45) | |
| 0.85 (0.28, 2.59) | 3.57 (1.01, 12.67) | 0.83 (0.40, 1.69) | 0.30 (0.15, 0.61) | 0.74 (0.35, 1.56) | 0.36 (0.15, 0.85) | 1.05 (0.41, 2.68) | 0.81 (0.32, 2.06) | 1.13 (0.54, 2.40) | 2.47 (0.89, 6.88) | 1.20 (0.41, 3.53) | Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 1.19 (0.71, 1.99) | 0.87 (0.36, 2.09) | 0.63 (0.20, 1.96) | 2.09 (0.78, 5.56) | |
| 0.10 (0.05, 0.22) | 3.01 (0.85, 10.64) | 0.69 (0.34, 1.42) | 0.25 (0.12, 0.51) | 0.62 (0.30, 1.30) | 0.31 (0.13, 0.71) | 0.89 (0.35, 2.25) | 0.68 (0.27, 1.73) | 0.95 (0.45, 2.01) | 2.08 (0.75, 5.77) | 1.01 (0.34, 2.96) | 0.84 (0.50, 1.41) | Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 0.73 (0.30, 1.75) | 0.53 (0.17, 1.64) | 1.76 (0.66, 4.66) | |
| 33.18 (14.57, 75.54) | 4.12 (1.18, 14.31) | 0.95 (0.48, 1.88) | 0.34 (0.17, 0.68) | 0.85 (0.42, 1.73) | 0.42 (0.18, 0.95) | 1.21 (0.49, 3.01) | 0.93 (0.38, 2.31) | 1.31 (0.64, 2.67) | 2.85 (1.05, 7.74) | 1.38 (0.48, 3.98) | 1.15 (0.48, 2.77) | 1.37 (0.57, 3.28) | Ustekinumab 90 mg | 0.72 (0.24, 2.21) | 2.41 (0.93, 6.24) | |
| 1.57 (0.64, 3.84) | 5.68 (1.35, 23.97) | 1.31 (0.49, 3.54) | 0.47 (0.18, 1.28) | 1.18 (0.43, 3.24) | 0.58 (0.19, 1.73) | 1.67 (0.52, 5.33) | 1.29 (0.40, 4.11) | 1.80 (0.65, 4.98) | 3.93 (1.15, 13.48) | 1.91 (0.53, 6.85) | 1.59 (0.51, 4.95) | 1.89 (0.61, 5.87) | 1.38 (0.45, 4.20) | Ustekinumab 45 mg | 3.32 (1.01, 10.98) | |
| 2.75 (1.00, 7.58) | 1.71 (0.46, 6.40) | 0.40 (0.18, 0.89) | 0.14 (0.06, 0.32) | 0.36 (0.15, 0.81) | 0.17 (0.07, 0.44) | 0.50 (0.18, 1.38) | 0.39 (0.14, 1.06) | 0.54 (0.23, 1.25) | 1.18 (0.88, 1.59) | 0.57 (0.18, 1.80) | 0.48 (0.18, 1.27) | 0.57 (0.21, 1.51) | 0.42 (0.16, 1.08) | 0.30 (0.09, 0.99) | Adalimumab 40 mg | |
| (c) Results of pairwise comparisons for all treatments in the ACR70 | ||||||||||||||||
| Placebo | 5.08 (1.81, 14.28) | 13.43 (0.65,276.60) | 1.29 (0.36, 4.60) | 7.79 (3.61, 16.80) | 8.48 (3.94, 18.23) | 0.98 (0.02, 50.67) | 6.73 (2.46, 18.44) | 8.54 (3.15, 23.15) | 6.66 (2.30, 19.29) | 2.45 (0.58, 10.44) | 2.27 (0.38, 13.78) | 24.14 (5.34,109.11) | 0.01 (0.00, 0.04) | 0.20 (0.04, 1.09) | 0.24 (0.04, 1.49) | |
| 0.20 (0.07, 0.55) | Infliximab 5 mg/kg | 0.16 (0.03, 0.82) | 0.08 (0.01, 0.38) | 0.24 (0.05, 1.23) | 0.09 (0.02, 0.51) | 0.47 (0.08, 2.97) | 0.28 (0.04, 1.78) | 0.43 (0.08, 2.38) | 0.47 (0.08, 2.59) | 0.05 (0.00, 3.72) | 0.37 (0.06, 2.32) | 0.47 (0.08, 2.93) | 0.37 (0.06, 2.37) | 0.14 (0.02, 1.11) | 1.33 (0.16, 11.42) | |
| 0.07 (0.00, 1.53) | 6.13 (1.22, 30.91) | Risankizumab 150 mg | 0.46 (0.22, 0.98) | 1.46 (0.66, 3.22) | 0.55 (0.21, 1.45) | 2.90 (0.92, 9.10) | 1.72 (0.54, 5.47) | 2.63 (1.04, 6.68) | 2.87 (1.13, 7.25) | 0.33 (0.01, 17.74) | 2.28 (0.73, 7.09) | 2.89 (0.94, 8.91) | 2.25 (0.69, 7.37) | 0.83 (0.18, 3.87) | 8.16 (1.65, 40.31) | |
| 0.77 (0.22, 2.76) | 13.24 (2.62, 66.93) | 2.16 (1.02, 4.56) | Upadacitinib 15 mg | 3.15 (1.42, 6.98) | 1.18 (0.44, 3.15) | 6.27 (1.99, 19.74) | 3.71 (1.16, 11.87) | 5.69 (2.23, 14.49) | 6.19 (2.44, 15.73) | 0.71 (0.01, 38.35) | 4.92 (1.57, 15.37) | 6.23 (2.01, 19.32) | 4.87 (1.48, 15.98) | 1.79 (0.38, 8.38) | 17.63 (3.56, 87.30) | |
| 0.13 (0.06, 0.28) | 4.21 (0.81, 21.72) | 0.69 (0.31, 1.52) | 0.32 (0.14, 0.71) | Guselkumab 100 mg | 0.38 (0.14, 1.04) | 1.99 (0.61, 6.46) | 1.18 (0.36, 3.88) | 1.81 (0.68, 4.77) | 1.97 (0.75, 5.18) | 0.23 (0.00, 12.29) | 1.56 (0.49, 5.03) | 1.98 (0.62, 6.32) | 1.55 (0.46, 5.22) | 0.57 (0.12, 2.72) | 5.60 (1.11, 28.34) | |
| 0.12 (0.05, 0.25) | 11.18 (1.97, 63.56) | 1.82 (0.69, 4.84) | 0.84 (0.32, 2.25) | 2.66 (0.96, 7.33) | Abatacept 125 mg | 5.29 (1.43, 19.57) | 3.14 (0.84, 11.74) | 4.80 (1.56, 14.81) | 5.23 (1.70, 16.09) | 0.60 (0.01, 34.03) | 4.15 (1.13, 15.25) | 5.27 (1.45, 19.19) | 4.11 (1.07, 15.76) | 1.51 (0.29, 8.01) | 14.89 (2.67, 83.03) | |
| 1.02 (0.02, 53.21) | 2.11 (0.34, 13.27) | 0.34 (0.11, 1.08) | 0.16 (0.05, 0.50) | 0.50 (0.15, 1.63) | 0.19 (0.05, 0.70) | Ixekizumab 80 mg Q2W | 0.59 (0.28, 1.25) | 0.91 (0.25, 3.25) | 0.99 (0.28, 3.53) | 0.11 (0.00, 6.72) | 0.78 (0.19, 3.28) | 0.99 (0.24, 4.13) | 0.78 (0.18, 3.38) | 0.29 (0.05, 1.68) | 2.81 (0.46, 17.35) | |
| 0.15 (0.05, 0.41) | 3.57 (0.56, 22.61) | 0.58 (0.18, 1.85) | 0.27 (0.08, 0.86) | 0.85 (0.26, 2.79) | 0.32 (0.09, 1.19) | 1.69 (0.80, 3.56) | Ixekizumab 80 mg Q4W | 1.53 (0.42, 5.56) | 1.67 (0.46, 6.04) | 0.19 (0.00, 11.39) | 1.32 (0.31, 5.61) | 1.68 (0.40, 7.06) | 1.31 (0.30, 5.77) | 0.48 (0.08, 2.86) | 4.75 (0.76, 29.57) | |
| 0.12 (0.04, 0.32) | 2.33 (0.42, 12.90) | 0.38 (0.15, 0.96) | 0.18 (0.07, 0.45) | 0.55 (0.21, 1.46) | 0.21 (0.07, 0.64) | 1.10 (0.31, 3.94) | 0.65 (0.18, 2.37) | Secukinumab 150 mg | 1.09 (0.59, 2.01) | 0.13 (0.00, 7.01) | 0.86 (0.24, 3.07) | 1.10 (0.31, 3.86) | 0.86 (0.23, 3.18) | 0.32 (0.06, 1.62) | 3.10 (0.57, 16.85) | |
| 0.15 (0.05, 0.43) | 2.14 (0.39, 11.84) | 0.35 (0.14, 0.88) | 0.16 (0.06, 0.41) | 0.51 (0.19, 1.34) | 0.19 (0.06, 0.59) | 1.01 (0.28, 3.61) | 0.60 (0.17, 2.17) | 0.92 (0.50, 1.69) | Secukinumab 300 mg | 0.12 (0.00, 6.43) | 0.79 (0.22, 2.82) | 1.01 (0.29, 3.54) | 0.79 (0.21, 2.91) | 0.29 (0.06, 1.49) | 2.85 (0.52, 15.46) | |
| 0.41 (0.10, 1.73) | 18.57 (0.27,1283.94) | 3.03 (0.06,162.91) | 1.40 (0.03, 75.51) | 4.42 (0.08,239.64) | 1.66 (0.03, 93.87) | 8.79 (0.15,519.15) | 5.21 (0.09,308.80) | 7.98 (0.14,446.28) | 8.69 (0.16,485.49) | Apremilast 30 mg | 6.90 (0.12,406.48) | 8.75 (0.15,514.15) | 6.83 (0.11,407.97) | 2.52 (0.04,168.89) | 24.74 (0.36,1696.52) | |
| 0.44 (0.07, 2.66) | 2.69 (0.43, 16.83) | 0.44 (0.14, 1.37) | 0.20 (0.07, 0.64) | 0.64 (0.20, 2.06) | 0.24 (0.07, 0.88) | 1.27 (0.30, 5.33) | 0.75 (0.18, 3.20) | 1.16 (0.33, 4.11) | 1.26 (0.36, 4.46) | 0.14 (0.00, 8.54) | Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 1.27 (0.63, 2.54) | 0.99 (0.23, 4.28) | 0.36 (0.06, 2.13) | 3.59 (0.58, 22.00) | |
| 0.04 (0.01, 0.19) | 2.12 (0.34, 13.20) | 0.35 (0.11, 1.07) | 0.16 (0.05, 0.50) | 0.50 (0.16, 1.61) | 0.19 (0.05, 0.69) | 1.01 (0.24, 4.18) | 0.60 (0.14, 2.50) | 0.91 (0.26, 3.22) | 0.99 (0.28, 3.49) | 0.11 (0.00, 6.72) | 0.79 (0.39, 1.58) | Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 0.78 (0.18, 3.35) | 0.29 (0.05, 1.67) | 2.83 (0.46, 17.26) | |
| 131.10 (25.35,677.91) | 2.72 (0.42, 17.54) | 0.44 (0.14, 1.45) | 0.21 (0.06, 0.67) | 0.65 (0.19, 2.18) | 0.24 (0.06, 0.93) | 1.29 (0.30, 5.60) | 0.76 (0.17, 3.36) | 1.17 (0.31, 4.34) | 1.27 (0.34, 4.72) | 0.15 (0.00, 8.75) | 1.01 (0.23, 4.37) | 1.28 (0.30, 5.50) | Ustekinumab 90 mg | 0.37 (0.06, 2.22) | 3.62 (0.57, 22.94) | |
| 4.93 (0.92, 26.53) | 7.38 (0.90, 60.70) | 1.20 (0.26, 5.62) | 0.56 (0.12, 2.61) | 1.76 (0.37, 8.39) | 0.66 (0.12, 3.49) | 3.49 (0.60, 20.49) | 2.07 (0.35, 12.25) | 3.17 (0.62, 16.34) | 3.45 (0.67, 17.76) | 0.40 (0.01, 26.69) | 2.74 (0.47, 16.00) | 3.48 (0.60, 20.17) | 2.71 (0.45, 16.35) | Ustekinumab 45 mg | 9.83 (1.22, 79.55) | |
| 4.18 (0.67, 25.99) | 0.75 (0.09, 6.44) | 0.12 (0.02, 0.61) | 0.06 (0.01, 0.28) | 0.18 (0.04, 0.90) | 0.07 (0.01, 0.37) | 0.36 (0.06, 2.19) | 0.21 (0.03, 1.31) | 0.32 (0.06, 1.75) | 0.35 (0.06, 1.91) | 0.04 (0.00, 2.77) | 0.28 (0.05, 1.71) | 0.35 (0.06, 2.16) | 0.28 (0.04, 1.75) | 0.10 (0.01, 0.82) | Adalimumab 40 mg | |
| Treatment | ACR20 | ACR50 | ACR70 | |||
|---|---|---|---|---|---|---|
| SUCRA | Mean rank | SUCRA | Mean rank | SUCRA | Mean rank | |
| Placebo | 0.1 | 17 | 0.5 | 15.9 | 5.8 | 15.1 |
| Adalimumab 40 mg | 86.1 | 3.2 | 84.7 | 3.3 | 92.1 | 2.2 |
| Ustekinumab 45 mg | 36.6 | 11.2 | 33.3 | 11 | 29.8 | 11.5 |
| Ustekinumab 90 mg | 41.1 | 10.4 | 46.5 | 9 | 61.9 | 6.7 |
| Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 76 | 4.8 | 64.9 | 6.3 | 70.6 | 5.4 |
| Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 57 | 7.9 | 54.3 | 7.9 | 60.8 | 6.9 |
| Apremilast 30 mg | 41.8 | 10.3 | 64 | 6.4 | 23.8 | 12.4 |
| Secukinumab 300 mg | 97 | 1.5 | 92 | 2.2 | 70.9 | 5.4 |
| Secukinumab 150 mg | 56.8 | 7.9 | 63.6 | 6.5 | 67.4 | 5.9 |
| Ixekizumab 80 mg Q4W | 38.7 | 10.8 | 41.9 | 9.7 | 50.4 | 8.4 |
| Ixekizumab 80 mg Q2W | 51.4 | 8.8 | 57.9 | 7.3 | 71.2 | 5.3 |
| Abatacept 125 mg | 18.6 | 14 | 13.2 | 14 | 17.8 | 13.3 |
| Guselkumab 100 mg | 50.8 | 8.9 | 36.4 | 10.5 | 44.7 | 9.3 |
| Upadacitinib 15 mg | 6.4 | 16 | 8.8 | 14.7 | 13.9 | 13.9 |
| Risankizumab 150 mg | 26.2 | 12.8 | 42.8 | 9.6 | 31.9 | 11.2 |
| Infliximab 5 mg/kg | 78.5 | 4.4 | 95.2 | 1.7 | 87.1 | 2.9 |
| Etanercept 25 mg | / | / | / | / | / | / |
| Golimumab 50 mg | 86.9 | 3.1 | / | / | / | / |
Twenty-two studies are included in the comparison of ACR50, the network diagram for ACR50 is provided in Figure 2(b), and the results of pairwise comparisons for all treatments in the ACR50 are shown in Tables 1 and 2. Among all the drugs, infliximab 5 mg/kg is obviously better than other drugs except ixekizumab 80 mg Q2W, secukinumab 150 mg, secukinumab 300 mg, apremilast 30 mg, certolizumab pegol 400 mg (W0, 2 and 4, 200 mg Q2W), and adalimumab 40 mg. The rankings based on SUCRA values are highest for infliximab 5 mg/kg (95.2%), followed by secukinumab 300 mg (92.0%) and adalimumab 40 mg (84.7%) (Table 2).
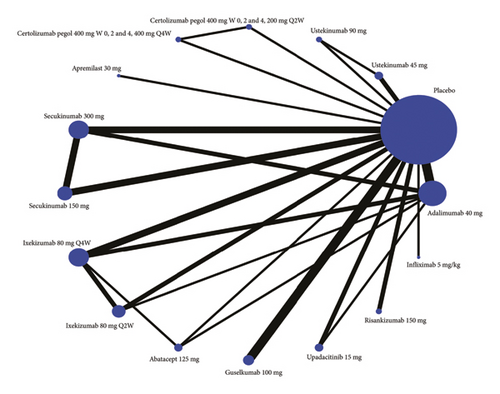
Twenty studies are included in the comparison of ACR70, the network diagram for ACR70 is provided in Figure 2(b), and the results of pairwise comparisons for all treatments in the ACR70 are shown in Tables 1, 2, 3. The rankings based on SUCRA values are highest for adalimumab 40 mg (92.1%), followed by infliximab 5 mg/kg (87.1%) and ixekizumab 80 mg Q2W (71.2%) (Table 2).
| (a) Results of pairwise comparisons for all treatments in the PASI75 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 33.57 (12.26, 91.93) | 0.26 (0.07, 0.95) | 21.22 (8.35, 53.93) | 0.34 (0.10, 1.18) | 1.73 (0.59, 5.07) | 6.58 (3.33, 13.00) | 7.91 (3.90, 16.05) | 5.49 (1.53, 19.67) | 8.47 (4.75, 15.12) | 9.06 (5.08, 16.15) | 13.49 (7.29, 24.98) | 19.97 (6.67, 59.82) | 0.54 (0.15, 1.91) | 114.84 (27.43, 480.71) | 0.17 (0.04, 0.80) | 0.09 (0.02, 0.49) |
| 0.03 (0.01, 0.08) | Golimumab 50 mg | 0.11 (0.01, 1.16) | 1.43 (0.08, 24.72) | 0.05 (0.01, 0.44) | 0.17 (0.02, 1.29) | 0.02 (0.00, 0.16) | 0.37 (0.04, 3.49) | 0.23 (0.03, 2.14) | 0.07 (0.01, 0.60) | 0.09 (0.01, 0.73) | 0.06 (0.01, 0.65) | 0.09 (0.01, 0.75) | 0.10 (0.01, 0.81) | 0.15 (0.02, 1.21) | 0.22 (0.02, 2.17) | 1.26 (0.11, 14.88) |
| 3.88 (1.05, 14.30) | 9.19 (0.87, 97.56) | Etanercept 25 mg | 13.13 (1.23, 140.33) | 0.50 (0.13, 1.94) | 1.54 (0.42, 5.60) | 0.18 (0.05, 0.70) | 3.38 (0.69, 16.69) | 2.14 (0.45, 10.08) | 0.66 (0.16, 2.72) | 0.80 (0.19, 3.32) | 0.55 (0.09, 3.27) | 0.85 (0.22, 3.35) | 0.91 (0.23, 3.58) | 1.36 (0.34, 5.42) | 2.01 (0.38, 10.53) | 11.57 (1.74, 76.83) |
| 0.05 (0.02, 0.12) | 0.70 (0.04, 12.11) | 0.08 (0.01, 0.81) | Infliximab 5 mg/kg | 0.04 (0.00, 0.31) | 0.12 (0.02, 0.91) | 0.01 (0.00, 0.11) | 0.26 (0.03, 2.46) | 0.16 (0.02, 1.51) | 0.05 (0.01, 0.43) | 0.06 (0.01, 0.52) | 0.04 (0.00, 0.46) | 0.07 (0.01, 0.53) | 0.07 (0.01, 0.57) | 0.10 (0.01, 0.86) | 0.15 (0.02, 1.53) | 0.88 (0.07, 10.48) |
| 2.94 (0.85, 10.21) | 18.44 (2.29, 148.64) | 2.01 (0.52, 7.80) | 26.34 (3.24, 213.99) | Upadacitinib 15 mg | 3.09 (1.59, 6.00) | 0.36 (0.16, 0.80) | 6.79 (2.15, 21.44) | 4.29 (1.45, 12.70) | 1.33 (0.55, 3.20) | 1.60 (0.65, 3.93) | 1.11 (0.28, 4.46) | 1.71 (0.77, 3.82) | 1.83 (0.82, 4.08) | 2.73 (1.19, 6.25) | 4.04 (1.18, 13.81) | 23.22 (5.00, 107.84) |
| 0.58 (0.20, 1.70) | 5.97 (0.77, 46.08) | 0.65 (0.18, 2.36) | 8.52 (1.09, 66.35) | 0.32 (0.17, 0.63) | Guselkumab 100 mg | 0.12 (0.06, 0.23) | 2.20 (0.75, 6.41) | 1.39 (0.51, 3.78) | 0.43 (0.20, 0.93) | 0.52 (0.23, 1.15) | 0.36 (0.10, 1.35) | 0.55 (0.28, 1.10) | 0.59 (0.30, 1.17) | 0.88 (0.43, 1.80) | 1.31 (0.41, 4.15) | 7.51 (1.71, 32.90) |
| 0.15 (0.08, 0.30) | 51.26 (6.32, 415.85) | 5.58 (1.42, 21.90) | 73.24 (8.96, 598.67) | 2.78 (1.25, 6.20) | 8.59 (4.34, 17.01) | Abatacept 125 mg | 18.87 (5.91, 60.29) | 11.93 (3.98, 35.74) | 3.70 (1.51, 9.03) | 4.45 (1.78, 11.09) | 3.09 (0.76, 12.53) | 4.76 (2.10, 10.80) | 5.09 (2.25, 11.54) | 7.58 (3.26, 17.66) | 11.23 (3.25, 38.80) | 64.55 (13.78, 302.35) |
| 0.13 (0.06, 0.26) | 2.72 (0.29, 25.77) | 0.30 (0.06, 1.46) | 3.88 (0.41, 37.08) | 0.15 (0.05, 0.47) | 0.46 (0.16, 1.33) | 0.05 (0.02, 0.17) | Ixekizumab 80 mg Q2W | 0.63 (0.28, 1.42) | 0.20 (0.06, 0.66) | 0.24 (0.07, 0.81) | 0.16 (0.03, 0.83) | 0.25 (0.08, 0.81) | 0.27 (0.08, 0.86) | 0.40 (0.12, 1.31) | 0.59 (0.13, 2.64) | 3.42 (0.59, 19.70) |
| 0.18 (0.05, 0.65) | 4.30 (0.47, 39.46) | 0.47 (0.10, 2.21) | 6.14 (0.66, 56.79) | 0.23 (0.08, 0.69) | 0.72 (0.26, 1.96) | 0.08 (0.03, 0.25) | 1.58 (0.70, 3.56) | Ixekizumab 80 mg Q4W | 0.31 (0.10, 0.98) | 0.37 (0.12, 1.20) | 0.26 (0.05, 1.26) | 0.40 (0.13, 1.20) | 0.43 (0.14, 1.28) | 0.64 (0.21, 1.94) | 0.94 (0.22, 3.97) | 5.41 (0.98, 29.87) |
| 0.12 (0.07, 0.21) | 13.87 (1.66, 116.03) | 1.51 (0.37, 6.21) | 19.82 (2.35, 167.02) | 0.75 (0.31, 1.81) | 2.32 (1.07, 5.03) | 0.27 (0.11, 0.66) | 5.11 (1.51, 17.23) | 3.23 (1.02, 10.24) | Secukinumab 150 mg | 1.20 (0.71, 2.04) | 0.83 (0.20, 3.55) | 1.29 (0.53, 3.15) | 1.38 (0.56, 3.37) | 2.05 (0.82, 5.14) | 3.04 (0.84, 11.05) | 17.46 (3.58, 85.27) |
| 0.11 (0.06, 0.20) | 11.52 (1.37, 97.22) | 1.25 (0.30, 5.22) | 16.47 (1.94, 139.94) | 0.63 (0.25, 1.54) | 1.93 (0.87, 4.28) | 0.22 (0.09, 0.56) | 4.24 (1.24, 14.52) | 2.68 (0.83, 8.64) | 0.83 (0.49, 1.41) | Secukinumab 300 mg | 0.69 (0.16, 2.98) | 1.07 (0.43, 2.67) | 1.14 (0.46, 2.85) | 1.71 (0.67, 4.36) | 2.52 (0.68, 9.31) | 14.51 (2.94, 71.65) |
| 0.07 (0.04, 0.14) | 16.61 (1.53, 179.94) | 1.81 (0.31, 10.71) | 23.74 (2.18, 258.81) | 0.90 (0.22, 3.62) | 2.78 (0.74, 10.50) | 0.32 (0.08, 1.32) | 6.12 (1.20, 31.08) | 3.87 (0.80, 18.78) | 1.20 (0.28, 5.09) | 1.44 (0.34, 6.20) | Apremilast 30 mg | 1.54 (0.38, 6.27) | 1.65 (0.41, 6.70) | 2.46 (0.60, 10.14) | 3.64 (0.68, 19.58) | 20.92 (3.07, 142.39) |
| 0.05 (0.02, 0.15) | 10.76 (1.33, 87.34) | 1.17 (0.30, 4.60) | 15.38 (1.88, 125.74) | 0.58 (0.26, 1.30) | 1.80 (0.91, 3.58) | 0.21 (0.09, 0.48) | 3.96 (1.24, 12.67) | 2.51 (0.84, 7.51) | 0.78 (0.32, 1.90) | 0.93 (0.37, 2.33) | 0.65 (0.16, 2.63) | Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 1.07 (0.66, 1.74) | 1.59 (0.68, 3.71) | 2.36 (0.68, 8.15) | 13.55 (2.89, 63.51) |
| 1.84 (0.52, 6.46) | 10.07 (1.24, 81.69) | 1.10 (0.28, 4.30) | 14.39 (1.76, 117.61) | 0.55 (0.24, 1.22) | 1.69 (0.85, 3.34) | 0.20 (0.09, 0.45) | 3.71 (1.16, 11.84) | 2.34 (0.78, 7.02) | 0.73 (0.30, 1.78) | 0.87 (0.35, 2.18) | 0.61 (0.15, 2.46) | 0.94 (0.57, 1.52) | Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 1.49 (0.64, 3.47) | 2.21 (0.64, 7.62) | 12.68 (2.71, 59.40) |
| 0.01 (0.00, 0.04) | 6.76 (0.82, 55.42) | 0.74 (0.18, 2.93) | 9.66 (1.17, 79.78) | 0.37 (0.16, 0.84) | 1.13 (0.55, 2.32) | 0.13 (0.06, 0.31) | 2.49 (0.76, 8.10) | 1.57 (0.51, 4.81) | 0.49 (0.19, 1.22) | 0.59 (0.23, 1.50) | 0.41 (0.10, 1.68) | 0.63 (0.27, 1.46) | 0.67 (0.29, 1.56) | Ustekinumab 90 mg | 1.48 (0.42, 5.21) | 8.51 (1.79, 40.45) |
| 5.73 (1.25, 26.24) | 4.57 (0.46, 45.15) | 0.50 (0.10, 2.60) | 6.52 (0.66, 64.96) | 0.25 (0.07, 0.85) | 0.77 (0.24, 2.43) | 0.09 (0.03, 0.31) | 1.68 (0.38, 7.45) | 1.06 (0.25, 4.48) | 0.33 (0.09, 1.20) | 0.40 (0.11, 1.46) | 0.27 (0.05, 1.48) | 0.42 (0.12, 1.47) | 0.45 (0.13, 1.57) | 0.68 (0.19, 2.38) | Ustekinumab 45 mg | 5.75 (0.95, 34.91) |
| 11.23 (2.05, 61.45) | 0.79 (0.07, 9.38) | 0.09 (0.01, 0.57) | 1.13 (0.10, 13.49) | 0.04 (0.01, 0.20) | 0.13 (0.03, 0.58) | 0.02 (0.00, 0.07) | 0.29 (0.05, 1.68) | 0.18 (0.03, 1.02) | 0.06 (0.01, 0.28) | 0.07 (0.01, 0.34) | 0.05 (0.01, 0.33) | 0.07 (0.02, 0.35) | 0.08 (0.02, 0.37) | 0.12 (0.02, 0.56) | 0.17 (0.03, 1.06) | Adalimumab 40 mg |
| (b) Results of pairwise comparisons for all treatments in the PASI90 | ||||||||||||||||
| Placebo | 33.16 (10.28, 106.98) | 0.22 (0.05, 0.98) | 20.18 (6.49, 62.76) | 0.29 (0.07, 1.24) | 23.12 (1.09, 489.64) | 6.28 (2.75, 14.37) | 594.69 (35.23, 10,039.04) | 0.01 (0.00, 0.25) | 8.83 (3.85, 20.22) | 13.84 (6.09, 31.46) | 232.41 (14.09, 3834.53) | 0.04 (0.00, 0.81) | ||||
| 0.03 (0.01, 0.10) | Golimumab 50 mg | 1.56 (0.03, 84.35) | 0.10 (0.01, 1.76) | 0.11 (0.01, 2.03) | 0.22 (0.01, 3.76) | 0.47 (0.02, 9.93) | 0.28 (0.01, 5.96) | 0.09 (0.00, 1.68) | 8.39 (0.15, 455.09) | 0.12 (0.01, 2.36) | 0.20 (0.01, 3.69) | 3.28 (0.06, 175.00) | ||||
| 4.50 (1.02, 19.79) | 0.64 (0.01, 34.72) | Infliximab 5 mg/kg | 0.07 (0.00, 1.13) | 0.07 (0.00, 1.31) | 0.14 (0.01, 2.42) | 0.30 (0.01, 6.38) | 0.18 (0.01, 3.83) | 0.06 (0.00, 1.08) | 5.38 (0.10, 292.52) | 0.08 (0.00, 1.52) | 0.13 (0.01, 2.37) | 2.10 (0.04, 112.49) | ||||
| 0.05 (0.02, 0.15) | 9.81 (0.57, 169.51) | 15.30 (0.88, 264.87) | Risankizumab 150 mg | 1.06 (0.43, 2.60) | 2.13 (1.18, 3.84) | 4.59 (1.33, 15.83) | 2.79 (0.84, 9.31) | 0.87 (0.35, 2.18) | 82.36 (4.75, 1428.69) | 1.22 (0.49, 3.07) | 1.92 (0.77, 4.78) | 32.19 (1.90, 546.37) | ||||
| 3.47 (0.81, 14.84) | 9.23 (0.49, 173.21) | 14.39 (0.76, 270.64) | 0.94 (0.38, 2.30) | Upadacitinib 15 mg | 2.00 (0.80, 4.97) | 4.32 (1.05, 17.84) | 2.63 (0.66, 10.53) | 0.82 (0.26, 2.59) | 77.47 (4.11, 1461.14) | 1.15 (0.36, 3.64) | 1.80 (0.57, 5.67) | 30.28 (1.64, 558.59) | ||||
| 0.04 (0.00, 0.92) | 4.62 (0.27, 80.16) | 7.20 (0.41, 125.25) | 0.47 (0.26, 0.85) | 0.50 (0.20, 1.24) | Guselkumab 100 mg | 2.16 (0.62, 7.54) | 1.31 (0.39, 4.43) | 0.41 (0.16, 1.04) | 38.75 (2.21, 678.93) | 0.58 (0.23, 1.47) | 0.90 (0.36, 2.28) | 15.14 (0.89, 258.38) | ||||
| 0.16 (0.07, 0.36) | 2.14 (0.10, 45.32) | 3.33 (0.16, 70.81) | 0.22 (0.06, 0.75) | 0.23 (0.06, 0.96) | 0.46 (0.13, 1.61) | Ixekizumab 80 mg Q2W | 0.61 (0.29, 1.29) | 0.19 (0.05, 0.79) | 17.94 (0.84, 382.25) | 0.27 (0.06, 1.12) | 0.42 (0.10, 1.74) | 7.01 (0.34, 146.26) | ||||
| 0.00 (0.00, 0.03) | 3.51 (0.17, 73.45) | 5.47 (0.26, 114.75) | 0.36 (0.11, 1.19) | 0.38 (0.09, 1.52) | 0.76 (0.23, 2.56) | 1.64 (0.78, 3.48) | Ixekizumab 80 mg Q4W | 0.31 (0.08, 1.27) | 29.47 (1.40, 619.46) | 0.44 (0.11, 1.78) | 0.69 (0.17, 2.78) | 11.52 (0.56, 237.00) | ||||
| 75.61 (3.97, 1441.11) | 11.28 (0.60, 213.21) | 17.57 (0.93, 333.13) | 1.15 (0.46, 2.88) | 1.22 (0.39, 3.86) | 2.44 (0.96, 6.21) | 5.28 (1.26, 22.13) | 3.21 (0.79, 13.07) | Secukinumab 150 mg | 94.64 (4.97, 1800.32) | 1.40 (0.44, 4.53) | 2.20 (0.69, 7.06) | 36.99 (1.99, 687.60) | ||||
| 0.11 (0.05, 0.26) | 0.12 (0.00, 6.46) | 0.19 (0.00, 10.09) | 0.01 (0.00, 0.21) | 0.01 (0.00, 0.24) | 0.03 (0.00, 0.45) | 0.06 (0.00, 1.19) | 0.03 (0.00, 0.71) | 0.01 (0.00, 0.20) | Secukinumab 300 mg | 0.01 (0.00, 0.28) | 0.02 (0.00, 0.44) | 0.39 (0.27, 0.56) | ||||
| 0.07 (0.03, 0.16) | 8.03 (0.42, 151.85) | 12.51 (0.66, 237.26) | 0.82 (0.33, 2.05) | 0.87 (0.27, 2.75) | 1.74 (0.68, 4.43) | 3.76 (0.89, 15.77) | 2.29 (0.56, 9.32) | 0.71 (0.22, 2.30) | 67.37 (3.54, 1280.96) | Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 1.57 (0.92, 2.68) | 26.33 (1.42, 489.73) | ||||
| 0.00 (0.00, 0.07) | 5.12 (0.27, 96.67) | 7.98 (0.42, 151.04) | 0.52 (0.21, 1.30) | 0.55 (0.18, 1.74) | 1.11 (0.44, 2.81) | 2.40 (0.57, 10.02) | 1.46 (0.36, 5.92) | 0.45 (0.14, 1.46) | 42.98 (2.26, 815.47) | 0.64 (0.37, 1.09) | Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 16.80 (0.90, 311.76) | ||||
| 25.38 (1.23, 524.82) | 0.30 (0.01, 16.27) | 0.48 (0.01, 25.40) | 0.03 (0.00, 0.53) | 0.03 (0.00, 0.61) | 0.07 (0.00, 1.13) | 0.14 (0.01, 2.98) | 0.09 (0.00, 1.79) | 0.03 (0.00, 0.50) | 2.56 (1.79, 3.67) | 0.04 (0.00, 0.71) | 0.06 (0.00, 1.11) | Adalimumab 40 mg | ||||
3.4. Skin Response Scores
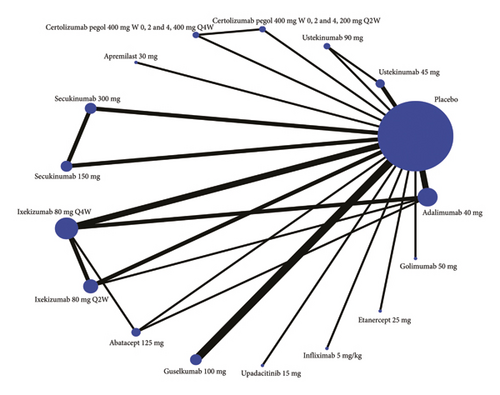
Eighteen studies are included in the comparison of PASI75, its network diagram is shown in Figure 3(a), and the results of pairwise comparisons for all treatments in the PASI75 are provided in Tables 1, 2, 3. The rankings based on SUCRA values are highest for adalimumab 40 mg (92.9%), followed by infliximab 5 mg/kg (92.7%) and golimumab 50 mg (89.3%) (Table 4).
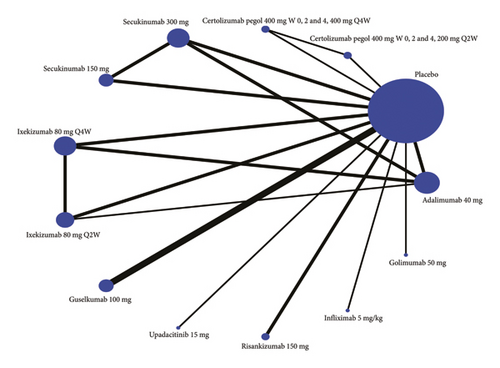
| Treatment | PASI75 | PASI90 | ||
|---|---|---|---|---|
| SUCRA | Mean rank | SUCRA | Mean rank | |
| Placebo | 0.2 | 17 | 0 | 13 |
| Adalimumab 40 mg | 92.9 | 2.1 | 83.9 | 2.9 |
| Ustekinumab 45 mg | 66.8 | 6.3 | / | / |
| Ustekinumab 90 mg | 56.1 | 8 | / | / |
| Certolizumab pegol 400 mg W 0, 2 and 4, 200 mg Q2W | 41 | 10.4 | 47.7 | 7.3 |
| Certolizumab pegol 400 mg W 0, 2 and 4, 400 mg Q4W | 38.1 | 10.9 | 28.7 | 9.6 |
| Apremilast 30 mg | 26.7 | 12.7 | / | / |
| Secukinumab 300 mg | 36.6 | 11.1 | 96.5 | 1.4 |
| Secukinumab 150 mg | 28.3 | 12.5 | 19.3 | 10.7 |
| Ixekizumab 80 mg Q4W | 68.2 | 6.1 | 54.8 | 6.4 |
| Ixekizumab 80 mg Q2W | 79.8 | 4.2 | 69.2 | 4.7 |
| Abatacept 125 mg | 6.6 | 15.9 | / | / |
| Guselkumab 100 mg | 61.7 | 7.1 | 50.4 | 7 |
| Upadacitinib 15 mg | 19.9 | 13.8 | 25.4 | 10 |
| Risankizumab 150 mg | / | / | 21.9 | 10.4 |
| Infliximab 5 mg/kg | 92.7 | 2.2 | 78.6 | 3.6 |
| Etanercept 25 mg | 45 | 9.8 | / | / |
| Golimumab 50 mg | 89.3 | 2.7 | 73.6 | 4.2 |
Seventeen studies are included in the comparison of PASI90, the network diagram for PASI90 is provided in Figure 3(b), and the results of pairwise comparisons for all treatments in the PASI90 are shown in Tables 2 and 3. The rankings based on SUCRA values are highest for secukinumab 300 mg (96.5%), followed by adalimumab 40 mg (83.9%) and infliximab 5 mg/kg (78.0%) (Table 4).
4. Discussion
This network meta-analysis compares the efficacy of all clinically used bDMARDs or tsDMARDs for PsA by assessing ACR20, ACR50, and ACR70 for arthritis, and PASI75 and PASI90 for psoriasis at week 24. Results of the analysis show that secukinumab 300 mg is the top-ranked treatment for ACR20 and PASI90, infliximab 5 mg/kg is the top-ranked treatment for ACR50, and adalimumab 40 mg is the top-ranked treatment for ACR70 and PASI75.
Compared with other network meta-analysis of RCTs to evaluate treatments for PsA, our study included more different drugs or longer efficacy [10–12, 49–51]; in particular, our analysis systematically evaluates all the clinically used drugs for ACR and PASI responses at week 24, rather than ranged from week 14 to week 16. And all the included drugs are approved for clinical use by FDA, so the results of our study are more significant for the guidance of treatments selection in clinics.
In accordance with other studies, our network meta-analysis proves that adalimumab 40 mg, infliximab 5 mg/kg, and secukinumab 300 mg are the most effective agents for ACR (joint symptoms) and PASI responses (skin symptoms) [49]. Adalimumab and infliximab are both anti-TNF monoclonal antibodies that have been shown to have efficacy in patients with rheumatoid arthritis and ankylosing spondylitis [52–57]; they are also used in PsA [58, 59]. Some papers showed that anti-TNF-α therapy may aggravate psoriasis, while some authors thought that anti-TNF-α therapy–induced palmoplantar lesions were not really psoriasis and these drugs were mostly de novo lesions rather than an exacerbation of previous eruptions [60, 61]. In our study, anti-TNF-α therapy also shows good therapeutic effect for skin symptoms of PsA rather than exacerbation, which may help prove the exact efficacy of anti-TNF-α therapy in the treatment of skin lesions in PsA, but this needs more studies and data to verify.
In recent years, with the progressing of researches on the pathogenesis of PsA, interleukin-17 pathway has been proposed to play important roles in the pathogenesis of PsA [62]; secukinumab (a fully human IgG1κ monoclonal antibody that binds to IL-17A), ixekizumab (a recombinant IgG4κ monoclonal antibody that binds to IL-17A), and brodalumab (a recombinant IgG2 fully human monoclonal antibody that binds to the IL-17 receptor A (IL-17RA)) are found effective in the PsA treatment [17, 22, 35, 63]; apremilast, an oral phosphodiesterase 4 inhibitor, down-regulating the inflammation through decreasing inflammatory cytokines expression and increasing anti-inflammatory cytokines expression [64] and showing efficacy in the treatment of PsA [20, 65]; upadacitinib (janus kinase inhibitor (JAKi) with selectivity for JAK1 over JAK2, JAK3, and tyrosine kinase 2), ustekinumab (a fully human IgG 1κ monoclonal antibody that binds to the common p40 subunit shared by interleukins 12 and 23), guselkumab, tildrakizumab, and risankizumab (a human monoclonal antibody targeting the IL-23p19-subunit), and abatacept (a T-cell modulator) are all showing efficacy in the treatment of PsA by RCTs [17, 23, 34, 36, 39, 41], but there are few studies to compare the effects of these drugs in one study. We compare them together using network meta-analysis, the results show that secukinumab 300 mg has better efficacy for arthritis and skin responses than brodalumab, apremilast, guselkumab, tildrakizumab, risankizumab, and abatacept. These are not completely consistent with previous results [49, 66, 67], which may be related with the different pre-treatment drugs, different races, enrollment bias, different early escape rates in the RCTs, etc.
There are of course some limitations associated with our network meta-analysis. First, few studies provided direct evidence from head-to-head trials. Second, publication bias is not assessed. Third, the difference of pre-treatment drugs (different conventional synthetic disease-modifying anti-rheumatic drugs, bDMARDs naïve, or bDMARD experienced) and races of the included patients are not assessed. Fourth, the time of assessment is limited to week 24, lacking long-term outcomes. We believe more valuable results will be found in the future as the number of studies increases and the time extends.
Even though our study has these limitations, it provides the most recent and comprehensive summary of the bDMARDs or tsDMARDs used in clinical practice in the PsA treatments, which help us make a better decision about which medication to choose in the treatment of active PsA. Further meta-analysis or further head-to-head studies or long-time studies may provide stronger evidences to prove which drug is better.
5. Conclusion
Our network meta-analysis proves that tumor necrosis factor-α inhibitors and interleukin 17A inhibitors are the top-ranked treatments for arthritis and skin responses of active PsA than other drugs, which may help us make better treatment decisions in clinics.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81901668) and Beijing Jishuitan Hospital Nova Program (XKXX202112).
Acknowledgments
The authors have nothing to report.
Supporting Information
Supporting Information 1: The exact commands of Stata used in this study.
Supporting Information 2: Table legend of supporting table 1.
Supporting Figure 1: Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Supporting Figure 2: Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Supporting Figure 3: Funnel plot of primary outcomes analyzed in network meta-analysis.
Supporting table 1: The baseline characteristics of included studies and patients.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the references.




