Influence of Rifampicin on the Pharmacokinetics of the Glucokinase Activator Globalagliatin: A Single-Center, Open-Label, Fixed-Sequence Investigation in Healthy Chinese Volunteers
Abstract
Objective: The orally bioavailable glucokinase activator, globalagliatin, is used to improve glucose homeostasis. Its metabolism is primarily dependent on cytochrome P450 (CYP) 3A4. Here, the influence of rifampicin, a potent inducer of CYP3A4 and CYP2C19 inducer, moderate inducer of CYP1A2, CYP2B6, CYP2C8, and CYP2C9, and inhibitor of P-gp, on the pharmacokinetics of globalagliatin, were investigated in healthy Chinese subjects.
Methods: This single-center, open-label, one-sequence investigation was performed over 22 days in 24 healthy Chinese volunteers. The volunteers were given single oral doses of 80 mg of globalagliatin on Days 1 and 15 on an empty stomach, while rifampicin 600 mg was given orally once a day from Days 8–21 before breakfast. Blood samples were collected at 0 h (1 h before globalagliatin administration on Days 1 and 15) and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, 72, 96, 120, and 168 h after dosing to monitor the globalagliatin pharmacokinetic parameters. Blood samples on Day 8 were collected before rifampicin administration. The plasma levels of globalagliatin were assessed using LC-MS/MS. Pharmacokinetic parameters were calculated using Phoenix WinNonlin Version 8.3.3 and analyzed with SAS Version 9.4. Continuous monitoring was performed to assess drug safety and tolerance.
Results: Analysis of the effect of rifampicin on globalagliatin pharmacokinetics in 24 healthy participants showed that with rifampicin, the Cmax of globalagliatin decreased by 88.9%, while the AUC0–t and AUC0–∞ values were reduced by 97.0% and 96.4%, respectively. The geometric mean ratios of globalagliatin Cmax, AUC0–t, and AUC0–∞ and their 90% CI values were 11.09% (90% CI:9.40–13.10%), 2.96% (90% CI:2.59–3.39%), and 3.60% (90% CI:3.20–4.04%), respectively. The mean elimination half-life was reduced by 27.91 h, while Tmax was prolonged by 3.52 h. Six treatment-related adverse events were reported by five subjects (20.8%), with all being of Grade 1 severity.
Conclusions: Cotreatment with rifampicin significantly reduces the plasma levels of globalagliatin. The safety and tolerability of globalagliatin, both as monotherapy and in combination with rifampicin, were good in healthy Chinese volunteers.
Trial Registration: Chinese Clinical Trial Register: CTR20210959
1. Introduction
Type 2 diabetes mellitus (T2DM) results from metabolic dysregulation and is typically associated with hyperglycemia, insulin resistance, diminished insulin secretion, and abnormally elevated hepatic glucose production. T2DM represents a major health problem due to its high prevalence and morbidity. Although it is traditionally considered a disease of middle and older age, significant increases in T2DM incidence have been seen since the 2000s in younger demographics, including adults < 40 years, adolescents, and even children [1, 2]. Studies have reported associations between T2DM and both microvascular and macrovascular dysfunction, including cardiovascular diseases (CVDs) [3], diabetic kidney disease (DKD) [4], retinopathy [5], peripheral neuropathy [6], and even neurodegenerative conditions such as Alzheimer’s [7] and Parkinson’s [8] diseases, all of which present significant challenges for clinicians and healthcare systems.
Glucokinase (GK) phosphorylates glucose to glucose-6-phosphate. GK is expressed predominantly in the pancreas and liver, the two primary organs involved in glucose metabolism [9]. In pancreatic β-cells, activation of GK enhances insulin secretion sensitivity and regulates the glucose-stimulated insulin secretion (GSIS) threshold, while in the liver, increases in GK promote both glucose production and glycogen synthesis. Consequently, allosteric activators of GK have attracted attention for treating T2DM [10]. According to the specificity of the GK targets, glucokinase activators (GKAs) can be categorized as either hepatoselective or dual activators. GKAs can also be divided into full or partial activators, depending on their effects on enzyme kinetics [11]. Several new types of GKA have been reported recently, including HMS5552 (dorzagliatin) [12–15], SY-004 (globalagliatin) [16–18], TTP399 [15, 19], PB-201(PF-04937319) [20, 21], and others [22, 23].
Globalagliatin, a selective dual-acting full GKA, targets both pancreatic β-cells and hepatocytes. With a code name of SY-004 and formerly known as LY2608204, it was developed by Yabao Pharmaceutical Group Co Ltd. (China). Six Phase I trials, including one in healthy individuals and another in patients with T2DM, both conducted in China, indicated favorable safety and tolerability profiles at doses of 2–120 mg and 20–400 mg, respectively, with marked hypoglycemic effects [16, 17]. Globalagliatin has a molecular weight of 596.26 Da, and a molecular formula of C28H38ClN3O3S3. Its IUPAC nomenclature is (1R,2S)-2-cyclohexyl-1-(4-(cyclopropylsulfonyl) phenyl)-N-(5-((2-(pyrrolidine-1-yl) ethyl)sulfanyl)-2-thiazolyl)-cyclopropylcarboxamide hydrochloride. It demonstrates high levels of protein binding in human plasma, exceeding 99.4%. The metabolites of globalagliatin are primarily oxidized products resulting from oxidation reactions (mono-oxidation and oxidative deamination), which primarily include M13 (oxidation/dehydroxypyrrolidine), M14 to M18 (these five metabolites are produced by monooxygenation of cyclopropane on cyclohexylcyclopropane or monooxygenation of cyclopropane on cyclohexane), and M19 (S-oxidation of sulfoxide), and no Phase II metabolites have been found. Metabolites with low globalagliatin content include M1 (oxidative dealkylation of alcohols to pyrrolidone), M20 to M22 and M24 (cyclopropane or cyclohexane cyclopropanopyrrolidone/dehydrooxygenation), M25 and M30 (biological oxidation of cyclohexane), and M26a (cyclopropane or cyclohexane cyclopropanopyrrolidone/biological oxidation with sulfoxide formation of S-oxidation) (unpublished data).
Preliminary studies on the effects of globalagliatin on cytochrome P450 (CYP450) enzymes have shown that while globalagliatin does not induce CYP2B6 and CYP1A2 activities, it has a bidirectional effect on CYP3A, acting as both an inducer and an inhibitor. Additionally, globalagliatin has been shown to inhibit CYP2D6, CYP2C19, CYP2C9, and CYP2C8 to varying degrees, acting as a mixed inhibitor (both competitive and noncompetitive). No significant inhibition of CYP2B6 and CYP1A2 has been observed. Globalagliatin may also act as a weak substrate for efflux transporters, for instance, acting as a substrate for P-gp at a concentration of 1 μM. The enzymes responsible for the breakdown of globalagliatin were identified using chemical inhibitors in human liver microsomes as well as recombinant cytochrome P450 (CYP450) enzymes. The results indicated that globalagliatin undergoes mono-oxidation and oxidative deamination in liver microsomes with NADPH. It was found that CYP3A4 was primarily responsible for these reactions, while CYP2C8, CYP2D6, and CYP3A5 were also involved, albeit to a lesser extent (unpublished). This indicates potential drug–drug interactions (DDIs) between globalagliatin and CYP3A4 inhibitors or inducers. The effects of the potent CYP3A inhibitor ketoconazole on globalagliatin pharmacokinetics (PKs) were investigated in a clinical trial on healthy subjects. It was found that coadministration of globalagliatin and 400 mg ketoconazole increased the values of PK parameters, seen in 3.15-fold and 4.86-fold increases in the geometric mean maximum plasma concentration (Cmax) and the plasma concentration–time curve from time 0 to infinity (AUC0–∞), respectively, indicating that CYP3A-mediated metabolism is the primary elimination route of globalagliatin in humans (unpublished). To date, however, there have been no investigations into the influence of CYP3A4 inducers on globalagliatin PKs. Rifampicin, commonly used as a model compound to evaluate induction effects on CYP3A4 and CYP2C19 (strong induction), CYP1A2, CYP2B6, CYP2C8, and CYP2C9 (moderate induction), and inhibition of P-gp, was selected for the present study.
Here, the effects of concomitant rifampicin administration on globalagliatin PKs were investigated in a single-center, open-label, fixed-sequence trial in healthy individuals in China.
2. Materials and Methods
The study protocol was approved by the Drug and Medical Device Branch of Wannan Medical College Yijishan Hospital Institutional Research Ethics Board and was conducted in the Phase I clinical trial ward of Yijishan Hospital of Wannan Medical College, Wuhu, China. It followed the principles of Good Clinical Practice (GCP) and the Declaration of Helsinki guidelines, ensuring the protection of the rights, safety, and welfare of human participants in clinical investigations, according to current regulatory requirements. Written informed consent was provided by all participants.
2.1. Participants
According to the FDA guidelines on DDI studies, healthy volunteers were used. The inclusion criteria were as follows: The subject was able to communicate effectively with the investigator, comprehend the requirements, and provide informed consent; healthy volunteers, male or female, aged 18 years or older; body mass index (BMI) of 19.0–26.0 kg/m2, with body weights of ≥ 50 kg for males and ≥ 45 kg for females; fasting blood glucose levels ≥ 3.9 and < 6.1 mmol/L; volunteers and their spouses were required to refrain from reproductive or sperm/egg donation and agree to use effective contraception for a minimum of one month before providing written informed consent to three months after the last drug administration.
The exclusion criteria included the following: Participation in clinical drug trials or other medical trial activities within the past three months; history of severe systemic disease, hypoglycemia, postural hypotension, or any condition deemed by the investigator to significantly alter drug metabolism, or increased risk associated with the drug under investigation; allergies to food, the drug under investigation, or similar drugs, or history of allergies to rifampicin or rifamycin antibiotics; abuse or use of drugs during the previous five years, or positive results in urine drug tests; blood loss ≥ 400 mL or blood donation within the previous four weeks, or receipt of blood/blood components during the same period, or plans for blood donation within three months after study completion; needle phobia or difficulty tolerating venipuncture and blood collection; severe infections, trauma, or major surgeries within four weeks before the study or planned surgery during the study period; taking of prescription/over-the-counter medications, herbal products, or supplements during the two weeks prior to the study; smoking ≥ 5 cigarettes/day or drinking alcohol ≥ 14 units/week (1 unit = 45 mL of liquor with high alcohol content, or 150 mL of liquor with moderate alcohol content, or 360 mL of beer) during the three months prior to the study, with smoking and alcohol prohibited during the trial, monitored by alcohol breath tests; consumption of certain foods or drinks (such as chocolate, caffeine-containing foods, pitaya, grapefruit, mango, or orange juice) within 48 h before or during the trial; pregnant or lactating women, including positive pregnancy tests; positivity for hepatitis B surface antigen or antibodies against hepatitis C, HIV, or syphilis; liver function abnormalities: ALT > 1.5 × upper limit of normal (ULN), AST > 1.5 × ULN, or total bilirubin (TBIL) > 1.5 × ULN; abnormal findings on 12-lead ECG (QT interval [QTcB] > 450 ms [male] and > 470 ms [female]), or significantly abnormal physical, vital signs, laboratory, radiographic, or abdominal ultrasound (liver, gallbladder, spleen, pancreas, kidney) findings.
2.2. Study Drugs
The study used the following medications: Globalagliatin (80 mg per capsule, batch No. 821050021; Beijing Yabao Biological Pharmaceutical Co., Ltd., Beijing, China); Rifampicin (0.15 g per capsule, batch No. E2521091; Shenyang Hongqi Pharmaceutical Co Ltd, Shenyang, China). Both drugs were provided by Suzhou Yabao Pharmaceutical R&D Co., Ltd.
2.3. Study Design and Blood Sampling
The design of the study is shown in Figure 1. Screening of volunteers was conducted from Days −30 to −1, during which participants were recruited, registered, and examined, and physical and chemical indices were determined. Volunteers who met the criteria for inclusion underwent admission to the Phase I clinical trial ward on the day before the study (Day −1) where they remained until Day 22, after completing the relevant examinations.
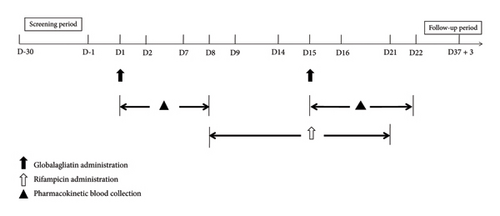
All volunteers fasted overnight (≥ 10 h) before taking the drugs, which were consumed with warm water (240 mL). On Day 1, volunteers took globalagliatin 80 mg on an empty stomach, fasting for 4 h postadministration with no drinking of water for 1 h before and after the medication. From Days 8–14, the participants received rifampicin 600 mg at the same time each day while fasting before breakfast and were instructed to fast for 1 h after taking the drug with no water consumption, as above. On Day 15, the participants received a combination of rifampicin 600 mg and globalagliatin 80 mg on an empty stomach, followed by 4 h of fasting, and water restriction for 1 h before and after medication. On Days 16–21, volunteers continued with the same regimen of one dose of rifampicin 600 mg in the morning while fasting, with water restriction as above. The participants could only leave the hospital after completing safety checks on Day 22. Follow-up telephone calls were made on Day 37 (+3 days) to record adverse events (AEs) and any concomitant medications taken during the follow-up period.
During their stay in the Phase I trial ward, the volunteers were provided with a standard diet and were prohibited from consuming other foods. They were also instructed to avoid eating grapefruit, pitaya, mango, xanthine-containing foods, chocolate, caffeinated foods or beverages, and alcohol for 48 h before and during the study. Smoking and alcohol consumption were not permitted from the time of admission to discharge, and intense physical activity was prohibited.
Blood samples were taken from the participants at 0 h (1 h before drug dosing on Days 1 and 15) and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, 72, 96, 120, and 168 h after dosing for monitoring of globalagliatin PK parameters. On Day 8, blood was collected before rifampicin administration. Three milliliters of venous blood were drawn into tubes with EDTA-K2. After centrifugation (1700 g, 10 min, 2°C–8°C), the plasma was transferred to cryotubes (1.5 mL) which were stored temporarily at −20°C followed by long-term storage at −80°C. The storage time of the plasma did not exceed 2 h from centrifugation to placement in the −20°C freezer and did not exceed 12 h from centrifugation to transfer to the −80°C freezer. The samples were subsequently sent to Shanghai Xihua Testing Technology Service Co Ltd, where the concentrations of globalagliatin were assayed.
2.4. Analysis of Plasma Drug Concentrations
The plasma globalagliatin levels were determined by Shanghai Xihua Testing Technology Service Co Ltd, using a validated, sensitive, and specific LC-MS/MS method. Chromatographic separation was performed on a Venusil XBP C18 column (3 μm, 100A, 2.1 × 50 mm) attached to an LC-20AD high-performance liquid chromatography (HPLC) system (Shimadzu, Japan). The column temperature was maintained at 40°C, and chromatographic separation was performed at a flow rate of 0.4 mL/min. The mobile phase consisted of water and acetonitrile containing 5 mM ammonium acetate and 1.0% formic acid, with a specified gradient (initially, B = 40%; 0.50 min, B = 40%; 2.00 min, B = 50%; 2.10 min, B = 90%; 3.10 min, B = 90%; 3.20 min, B = 40%; stopping at 4.20 min). Quantification was performed on AB Sciex Triple Quad 6500 mass spectrometer in positive ion mode for MS/MS detection, employing multiple reaction monitoring (MRM) scans via an electrospray ionization (ESI) source.
2.5. Sample Size Estimation
A previous study showed that the coefficients of variation (CV%) for the PK exposure parameters, the peak plasma concentration (Cmax), and the area under the plasma concentration–time curve from time zero to infinity (AUC0–∞) were 35% and 36%, respectively. In the domestic Stage Ia 80 mg group [17], the CV% values for Cmax, the area under the plasma concentration–time curve from time zero to the time of the last measurable concentration (AUC0–t), and AUC0–∞ were 20.3%, 39.1%, and 40.8%, respectively. Assuming an intraindividual CV of 20% and a 90% confidence interval (CI), a sample of 20 would result in an accuracy of 10.89%. Considering potential sample attrition, a sample size of 24 was used.
2.6. Analysis of PKs
The PK parameters of globalagliatin under different administration conditions (combined and single) were determined using Phoenix WinNonlin Version 8.3.3 with noncompartmental analysis. The calculated PK parameters included Cmax, AUC0–t, AUC0–∞, the time to reach maximum plasma concentration (Tmax), the terminal elimination half-life (t1/2z), the elimination rate constant (λz), the apparent volume of distribution (Vz/F) obtained using the formula Vz/F = dose/AUC0–∞/λz, the apparent oral clearance (CLz/F) calculated using the equation CLz/F = dose/AUC0–∞, and the residual area percentage (AUC_%Extrap) determined using the formula AUC_%Extrap = ([AUC0–∞ − AUC0–t]/AUC0–∞) ∗ 100%. The plasma globalagliatin concentrations below the quantitative limit (BQL) were set to 0 for analysis.
2.7. Drug Safety and Tolerance
The safety and tolerance of globalagliatin were evaluated in all participants who had received ≥ 1 dose. The indicators assessed included AEs, serious AEs (SAEs), changes in vital signs and physical parameters, laboratory abnormalities, ECG changes, and hypoglycemic events. The severity of AEs was assessed according to CTCAE Version 5.0, and the relationships between the AEs and the experimental drug were determined.
2.8. Statistical Analysis
The number of cases, the arithmetic mean and standard deviation, and minimum and maximum values were determined for continuous variables. Additionally, the geometric mean and geometric CV were calculated for the PK parameters. Categorical data are shown as frequencies and percentages. For AUC0–t, AUC0–∞, and Cmax, natural log transformation was applied, and a linear mixed-effects model was utilized to calculate the ratio of geometric means and the 90% CIs. The administration status (combined vs. single) represented a fixed effect, while the subject was considered a random effect. For Tmax, a nonparametric Wilcoxon paired test was utilized to examine differences between subjects under different administration conditions. All analyses were performed using SAS Version 9.4.
3. Results
3.1. Participants
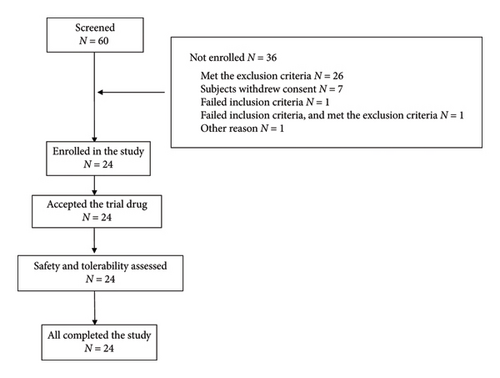
The study was conducted between April 2021 and March 2022. Of the original 60 individuals screened, 36 failed to meet the screening criteria. Consequently, 24 subjects were enrolled and randomly assigned to receive drug treatment. There were no dropouts during the study. Figure 2 illustrates the enrollment process. All 24 participants were of Han ethnic origin, the predominant ethnic group in China, and included 17 males and 7 females. The average age (±SD) was 30.7 ± 12.14 years, with a mean height of 166.2 ± 8.37 cm and a mean weight of 61.1 ± 10.21 kg. The mean BMI was 22.01 ± 2.124 kg/m2. Demographic details are provided in Table 1.
| Characteristic | Age (y) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| Mean ± SD | 30.7 ± 12.14 | 166.2 ± 8.37 | 61.1 ± 10.21 | 22.01 ± 2.124 |
| Median | 25.0 | 166.0 | 57.5 | 21.60 |
| Min∼max | 18∼53 | 152∼182 | 50∼81 | 19.2∼25.7 |
- Abbreviations: BMI, body mass index; SD, standard deviation.
3.2. Safety and Tolerance
The safety dataset included all 24 volunteers. Overall, six treatment-emergent AEs (TEAEs) were documented in five subjects (20.8%), all of which were Grade I in severity. Of these, one volunteer experienced a relapse, and five subjects recovered. No AEs or SAEs of Grade II or higher occurred, and no participants left due to TEAEs. No hypoglycemic events were reported. The observed AEs included reductions in the white blood cell (WBC) count (3 cases), ECG QT prolongation (2 cases), and elevated levels of creatine phosphokinase (CPK) (1 case). Specifically, one case of ECG QT prolongation occurred during globalagliatin monotherapy (between Days 1 and 8), while during the combination treatment with rifampicin (Days 8–22), three cases of reduced WBC counts, one case of ECG QT prolongation, and one case of elevated CPK were reported. Details of the AEs are shown in Table 2.
| SOC | Statistical result | |
|---|---|---|
| PT | Volunteer (%) | Case |
| Total TEAEs n (%) | 5(20.8) | 6 |
| Decreased WBC count n (%) | 3(12.5) | 3 |
| Prolonged ECG QT interval n (%) | 1(4.2) | 2 |
| Elevated creatine phosphokinase n (%) | 1(4.2) | 1 |
- Note: Volunteer (%): One volunteer had up to one count in the same term (SOC or PT). Case: A volunteer was counted by the actual number of occurrences in the same term (SOC or PT). %: Number of adverse events/reactions as a percentage of total subjects in each group.
3.3. PK Properties
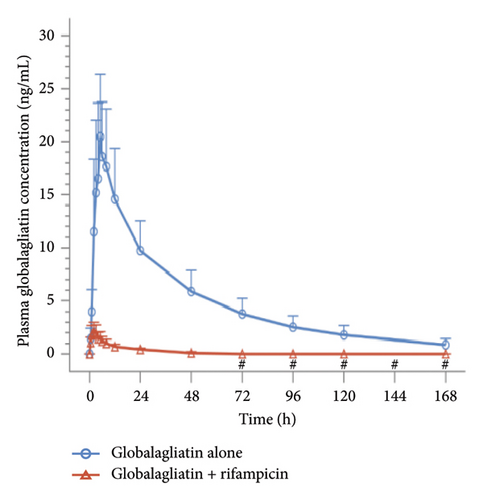
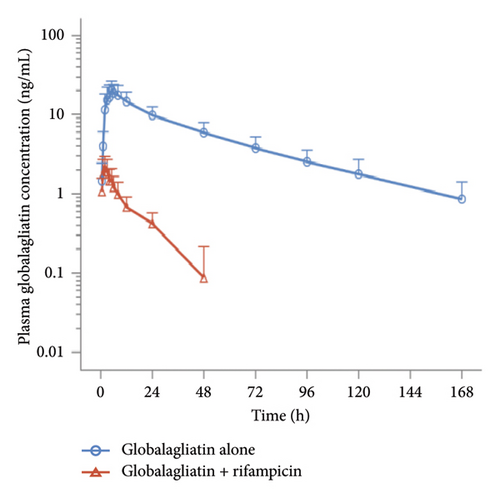
Plasma concentrations were determined for all subjects. Prior to drug administration on Days 1 (D1) and 15 (D15), the plasma concentrations of globalagliatin were below the limit of quantification (BQL, < 0.200 ng/mL), with no plasma concentrations exceeding the upper limit of quantification (200 ng/mL). When globalagliatin was coadministered with rifampicin, the geometric means of the Cmax, AUC0–t, and AUC0–∞ parameters were markedly reduced. Specifically, Cmax decreased by 88.9% from 20.87 ng/mL to 2.32 ng/mL, AUC0–t decreased by 97.0% from 770.97 h∗ng/mL to 22.85 h∗ng/mL, and AUC0–∞ decreased by 96.4% from 823.85 h∗ng/mL to 29.63 h∗ng/mL. The median Tmax for globalagliatin was prolonged by 3 h with rifampicin coadministration, specifically, from 4.98 to 1.98 h. The mean elimination half-life T1/2z was shortened by 27.91 h, from 43.18 ± 10.69 to 15.27 ± 4.26 h. Additionally, the mean clearance rate (CLz/F) increased by 2754.05 L/h, from 104.18 ± 42.73 to 2858.23 ± 956.05 L/h. The geometric mean ratios of Cmax, AUC0–t, and AUC0–∞, along with their 90% CIs, for globalagliatin coadministered with rifampicin versus administration of globalagliatin alone were 11.09 (9.40, 13.10), 2.96 (2.59, 3.39), and 3.60 (3.20, 4.04), respectively. The PK parameters of globalagliatin during the two treatment periods are detailed in Tables 3, 4, 5, and 6, and the plasma concentrations of globalagliatin (single dose of 80 mg) given alone or together with rifampicin (600 mg once per day) as a function of time are illustrated in Figure 3.
| PK parameter | Globalagliatin alone | Globalagliatin + rifampicin |
|---|---|---|
| Cmax (ng/mL) | ||
| Mean ± SD | 21.94 ± 6.54 | 2.48 ± 0.86 |
| CV% | 29.8 | 34.8 |
| Median | 21.70 | 2.36 |
| Min∼max | 8.10∼33.1 | 0.948∼3.78 |
| GM | 20.87 | 2.32 |
| GM-CV% | 35.1 | 40.6 |
| AUC0–t (h∗ng/mL) | ||
| Mean ± SD | 816.48 ± 270.06 | 24.98 ± 10.95 |
| CV% | 33.1 | 43.8 |
| Median | 787.46 | 22.62 |
| Min∼max | 354.50∼1424.99 | 11.11∼50.27 |
| GM | 770.97 | 22.85 |
| GM-CV% | 36.9 | 45.2 |
| AUC0–∞ (h∗ng/mL) | ||
| Mean ± SD | 877.52 ± 307.16 | 31.49 ± 11.60 |
| CV% | 35.0 | 36.8 |
| Median | 851.64 | 27.85 |
| Min∼max | 363.94∼1647.55 | 16.67∼59.43 |
| GM | 823.85 | 29.63 |
| GM-CV% | 38.7 | 36.3 |
| Tmax (h) | ||
| Mean ± SD | 5.69 ± 2.42 | 2.17 ± 1.37 |
| CV% | 42.6 | 63.3 |
| Median | 4.98 | 1.98 |
| Min∼max | 1.98∼11.98 | 0.48∼5.98 |
| GM | 5.26 | 1.83 |
| GM-CV% | 41.9 | 64.9 |
| t1/2z (h) | ||
| Mean ± SD | 43.18 ± 10.69 | 15.27 ± 4.26 |
| CV% | 24.7 | 27.9 |
| Median | 43.20 | 15.70 |
| Min∼max | 23.45∼67.95 | 9.13∼23.77 |
| GM | 41.87 | 14.68 |
| GM-CV% | 26.3 | 29.7 |
| Vz/F (L) | ||
| Mean ± SD | 6108.31 ± 1846.25 | 60686.77 ± 22996.38 |
| CV% | 30.2 | 37.9 |
| Median | 5749.07 | 51631.21 |
| Min∼max | 3855.62∼11255.13 | 36959.35∼125551.81 |
| GM | 5866.14 | 57188.11 |
| GM-CV% | 29.2 | 35.1 |
| CLz/F (L/h) | ||
| Mean ± SD | 104.18 ± 42.73 | 2858.23 ± 956.05 |
| CV% | 41.0 | 33.4 |
| Median | 93.99 | 2874.52 |
| Min∼max | 48.56∼219.82 | 1346.18∼4799.59 |
| GM | 97.10 | 2699.91 |
| GM-CV% | 38.7 | 36.3 |
| λz (1/h) | ||
| Mean ± SD | 0.02 ± 0.00 | 0.05 ± 0.01 |
| CV% | 27.7 | 29.9 |
| Median | 0.02 | 0.04 |
| Min∼max | 0.01∼0.03 | 0.03∼0.08 |
| GM | 0.02 | 0.05 |
| GM-CV% | 26.3 | 29.7 |
| AUC_% Extrap (%) | ||
| Mean ± SD | 6.36 ± 3.29 | 22.53 ± 7.52 |
| CV% | 51.7 | 33.4 |
| Median | 5.58 | 20.97 |
| Min∼max | 1.92∼13.51 | 12.46∼42.06 |
| GM | 5.58 | 21.41 |
| GM-CV% | 57.6 | 33.2 |
| Parameter | Variation | F value | p value |
|---|---|---|---|
| Cmax | Administration status | 513.526 | < 0.001 |
| AUC0–t | Administration status | 2003.819 | < 0.001 |
| AUC0–∞ | Administration status | 2435.113 | < 0.001 |
| Parameter | GM LS mean | Ratio (%) | 90% CI (%) | |
|---|---|---|---|---|
| Globalagliatin alone | Globalagliatin + rifampicin | |||
| Cmax (ng/mL) | 20.87 | 2.32 | 11.09 | 9.40–13.10 |
| AUC0–t (h∗ng/mL) | 770.97 | 22.85 | 2.96 | 2.59–3.39 |
| AUC0–∞ (h∗ng/mL) | 823.85 | 29.63 | 3.60 | 3.20–4.04 |
| Parameter | Statistics | p-value | Statistical method |
|---|---|---|---|
| Tmax | 137.000 | < 0.001 | Paired Wilcoxon test |
4. Discussion
This single-center, open-label, one-sequence study investigation conducted on healthy Chinese volunteers assessed the influence of the CYP3A4 inducer, rifampicin, on the PK profiles of globalagliatin. Earlier domestic Phase Ia studies showed that, in healthy individuals, PK parameters of globalagliatin, including Cmax, AUC0–t, and AUC0–∞, showed linear dynamics in the 20–120 mg range [17]. Based on these results, an 80 mg dose was selected for this trial, which was considered both safe and well tolerated in healthy individuals. The participants received rifampicin, a potent CYP3A4 and CYP2C19 inducer, moderate inducer of CYP1A2, CYP2B6, CYP2C8, and CYP2C9, and inhibitor of P-gp, daily at doses of 600 mg per day from days 8–21. Rifampicin is commonly used to assess PK effects on CYP3A substrates and is generally well tolerated [24, 25]. The PK profile of globalagliatin, given alone at a dose of 80 mg, was consistent with the findings of an earlier report [17, 18].
The clinical development of certain GKA candidates has been hindered due to safety concerns or unsatisfactory clinical effects [22]. For example, piragliatin, a second-generation GKA developed by Roche, was withdrawn in Phase II trials due to metabolite accumulation and concerns over liver safety, including elevated transaminase levels, particularly with long-term use in treating chronic diseases such as T2DM [26]. Similarly, although MK-0941, developed by Merck, demonstrated initial efficacy in a Phase III trial with markedly improved HbA1c and postprandial glucose levels in T2DM patients, the hypoglycemic effect diminished after 30 weeks, accompanied by hypoglycemia and hyperlipidemia, leading to its termination [27]. Other GKA candidates, such as AZD1656 and AMG151 (ARRY-403), also showed initial improvements in blood glucose levels but induced AEs such as elevated triglycerides or hypoglycemia, resulting in their discontinuation [28–30].
In this study, one subject experienced two episodes of QT prolongation (QTcB values > 450 ms), one before rifampicin administration on Day 8, and the other after administration on Day 15. Both instances were self-limiting, and the QTcB values normalized without intervention. QT prolongation has also been observed in previous studies with globalagliatin [16, 17], and a similar effect on the QT interval was also observed during treatment with the GKA piragliatin [31]. Importantly, no hypoglycemic events were reported in the present study, nor were there any AEs or SAEs greater than Grade I severity. No participants withdrew due to AEs. It was thus concluded that globalagliatin (80 mg) was safe and well tolerated when administered alone or in combination with rifampicin (600 mg daily). Furthermore, previous Phase Ia and Phase Ib studies, along with a study on food effects, have demonstrated the good safety and tolerability of globalagliatin in Chinese volunteers [16–18].
4.1. PKs of Globalagliatin in the Presence of Rifampicin
Coadministration of rifampin with globalagliatin in healthy Chinese volunteers led to a significant decrease in the plasma globalagliatin concentration. Specifically, the Cmax of globalagliatin decreased by 88.9%, AUC0–t by 97.0%, and AUC0–∞ by 96.4%. The geometric mean ratios of globalagliatin Cmax, AUC0–t, and AUC0–∞ and their 90% CI values were 11.09% (90% CI: 9.40–13.10%), 2.96% (90% CI: 2.59–3.39%), and 3.60% (90% CI: 3.20–4.04%), respectively. Additionally, the Tmax was prolonged by 3.52 h, the mean elimination half-life T1/2z was reduced by 27.91 h, and the elevated clearance rate increased by 2754.05 L/h. These findings indicate that rifampicin significantly promotes globalagliatin metabolism, leading to reduced plasma concentrations.
4.2. Clinical Implications
The study findings underscore the importance of avoiding concomitant administration of globalagliatin with rifampicin, as this can significantly lower the plasma concentration of the drug, potentially compromising its efficacy, and likely the explanation for the comprehensive impact of rifampicin on the previously described enzymes. When coadministration with rifampicin is unavoidable, adjustment of the globalagliatin dose is recommended to maintain its therapeutic effect.
4.3. Limitations
The study has several limitations. Firstly, the limited sample size and population inadequacies of the DDI study, conducted on healthy volunteers with a small sample, may result in difficulties in predicting rare disease or population-specific interaction effects, such as those in the elderly or individuals with impaired liver or kidney function. Secondly, there are limitations associated with surrogate endpoints in the study, as only the plasma concentrations and PK parameters of globalagliatin were measured without the assessment of glycemic control indicators such as HbA1c and fasting blood glucose, despite the absence of hypoglycemic events occurred during the trial. Finally, the limitations of the polypharmacy scenario are noted. Treatment plans for diabetic patients typically include the prescription of metformin and SGLT2 inhibitors, among others. Rifampicin may produce cumulative effects by its ability to induce multiple enzymes and transporters; however, this study only assessed the interaction between two drugs. Further investigation is thus required.
5. Conclusions
In conclusion, globalagliatin showed both safety and good tolerance in healthy Chinese volunteers, both alone or in combination with rifampicin. The study found that rifampicin, acting as a strong inducer of CYP3A4 and CYP2C19, moderate inducer of CYP1A2, CYP2B6, CYP2C8, and CYP2C9, and inhibitor of P-gp, significantly enhanced globalagliatin metabolism, leading to a substantial reduction in its plasma concentrations. Therefore, when globalagliatin is coadministered with rifampicin, appropriate dose adjustment is necessary to maintain its pharmacological efficacy.
Ethics Statement
The protocol of this study was approved and authorized by the Drug and Medical Device Branch of Wannan Medical College Yijishan Hospital Institutional Research Ethics Board, Wuhu, China (Ethics Approval ID: 2021-LSY-09).
Consent
All volunteers agreed to participate and provided written informed consent prior to inclusion.
Conflicts of Interest
Weijin Liu, Cuilian Jiang, and Lin Tan are employees of Yabao Pharmaceutical R&D Co., Ltd. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Author Contributions
This study was designed by Weijin Liu, Cuilian Jiang, Lin Tan, Suoshuan Liu, Hua Sun, and Haitang Xie, while Yaqin Wang, Ya Liu, Maodi Xu, Minhui Wang, and Xiaohu Wang performed the research. All authors read and approved the manuscript.
Yaqin Wang and Ya Liu contributed equally to this work.
Funding
This study was funded by the ‘Climbing Peak’ Training Program for Innovative Technology team of Yijishan Hospital, Wannan Medical College (KPF2019016), the Research Fund of Wannan Medical College (WK2020F23), and Yabao Pharmaceutical R&D Co., Ltd, China, that provided study drugs and participated in the study design, data analysis, interpretation, manuscript review, and publication decision (Service contract number of Yijishan Hospital, Wannan Medical College: 703202109011). The authors would like to thank the support of Suzhou Yabao Pharmaceutical R&D Co., Ltd.
Acknowledgments
The authors thank all volunteers who participated in the trial, as well as the field researchers (from the Drug Evaluation Center, Yijishan Hospital of Wannan Medical College). The authors would like to thank the support of Suzhou Yabao Pharmaceutical R&D Co., Ltd.
Open Research
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author Haitang Xie upon reasonable request.




