Real-Practice Analysis of Potential Antibiotic Interactions in Patients Treated With Immune Checkpoint Inhibitors: An Observational Study From an Italian Referral Cancer Center
Abstract
Purpose: Inappropriate use of antibiotics contributes to the increase in antimicrobial resistance with negative health safety implications. Global health organizations have promoted projects to improve proper and rational use of antibiotics. Recent studies show how antibiotics can also influence the efficacy of immune checkpoint inhibitors (ICIs) treatment in cancer patients. This work analyzes the impact of concomitant antibiotic therapy in patients affected by skin, lung, and kidney cancer, who started immunotherapy in 2020-2021 at Veneto Institute of Oncology IRCCS. The aim of this study is to evaluate clinical outcomes, treatment efficacy, discontinuation causes, and occurrence of adverse drug reactions in cancer patients treated with ICIs.
Methods: Data from the real-world retrospective study were extracted from Territorial Pharmaceutical Care databases, medical records, and the AIFA registry. A descriptive analysis of the study population was performed, as well as of patient outcomes in terms of progression-free survival (PFS) and overall survival (OS).
Results: A total of 239 subjects affected by kidney (8%), lung (48%), and skin (44%) cancer were enrolled; of these, 50%, 32%, 9%, and 9% were treated with single-agent nivolumab, pembrolizumab, atezolizumab, and cemiplimab, respectively. A total of 119 patients received concomitant antibiotic therapy. Median OS was 8.8 months (95% CI: 5.02–11.8) and 31.05 months (95% CI: 24.81–NA) in subjects with high and low antibiotic exposure, respectively. Multivariate subgroup analysis confirmed that high antibiotic exposure is an unfavorable factor also for median PFS.
Conclusion: A prolonged exposure to antibiotics correlates with unfavorable outcomes in cancer patients undergoing concomitant immunotherapy. Thus, managing antibiotic exposure is fundamental for optimizing ICI treatment.
1. Background
Antimicrobial resistance (AMR) poses a significant threat to public health due to its epidemiological and economic impacts, primarily resulting from the overuse and misuse of antibiotics [1]. AMR leads to increased morbidity and mortality from antibiotic-resistant bacterial infections, with an estimated 50,000 annual deaths in Europe and the United States. The economic consequences include lost working days, increased mortality, and higher healthcare costs due to prolonged hospitalizations, greater reliance on diagnostic procedures, and more expensive antibiotics. In Italy, the National Plan to Contrast Antimicrobial Resistance addresses this issue on national and regional levels [2].
Understanding the interactions between antibiotics and immune checkpoint inhibitors (ICIs) is crucial, especially given the rising use of ICIs in oncology and the widespread use of antibiotics. Nivolumab, pembrolizumab, and cemiplimab target Programmed Death 1 (PD-1) receptors on T lymphocytes, while avelumab, atezolizumab, and durvalumab target Programmed Death Ligand 1 (PD-L1). Ipilimumab inhibits the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) checkpoint. These drugs are increasingly used due to their long-lasting efficacy [3]. However, immune-mediated adverse reactions can sometimes necessitate discontinuing therapy. Recent observations suggest that steroids and immunosuppressants may impact the efficacy of ICIs [4].
Furthermore, clinical studies indicate that antibiotics can significantly reduce ICI efficacy due to their adverse effects on the gut microbiome, which is crucial for antitumor immune activation [5, 6]. The negative impact on efficacy appears to correlate with the number and duration of antibiotic use, though evidence remains inconsistent [7]. An institutional survey at Veneto Institute of Oncology IRCCS revealed a 45.6% increase in antibiotic use from 2016 to 2021, highlighting the need to improve antibiotic prescribing practices, particularly in patients receiving ICIs.
This study aims to assess the impact of antibiotic use on overall survival (OS) and progression-free survival (PFS) in cancer patients undergoing ICI therapy, focusing on cutaneous, nonsmall cell lung (NSCLC), and renal carcinomas. The study compares patients who received antibiotics within 30 days before and in the 30 days after immunotherapy completion with those who did not. Secondary objectives include evaluating ICI treatment efficacy in real-world settings, reasons for treatment discontinuation, prescribed antibiotic classes, and adverse drug reactions (ADRs).
2. Materials and Methods
2.1. Study Design
The retrospective study, approved by the local Ethical Committee, is included in an AIFA-funded project performed and coordinated by the Veneto Oncology Institute’s Hospital Pharmacy, in collaboration with Territorial Pharmaceutical Services (ULSS6 and ULSS2).
2.2. Patient Population
The study population included patients aged ≥ 18 years with skin cancer, NSCLC, and renal carcinoma who initiated one of the following ICI treatments between 2020 and 2021: nivolumab, cemiplimab, pembrolizumab, and atezolizumab. Patients included in interventional trials and those receiving ICIs in combination with other drugs were excluded.
2.3. Resources and Data Collection
Patients’ data were collected from electronic medical records, AIFA registry, and administrative databases. Antibiotic dispensation data were obtained from territorial pharmacies. Only Anatomical Therapeutic Chemical (ATC) classification system J01 antibiotics dispensed within 30 days before the immunotherapy initiation to 30 days after its conclusion were considered. Patients’ enrollment period ranges from January 1st, 2020, to December 31st, 2021, and data collection extended to September 15th, 2023. REDCap software was used to create an electronic case report form for data collection. Antibiotic exposure level was calculated, as a percentage of “days of antibiotic therapy/days of ICI treatment”. The “days of antibiotic therapy” were calculated based on defined daily dose (DDD) of antibiotics dispensed for each patient, providing insights into the potential relationship between antibiotic exposure and immunotherapy outcomes. The efficacy of ICIs treatment in terms of OS and PFS and reasons for treatment suspension were also evaluated, as well as ADRs frequency within the study population. Finally, the study analyzes antibiotics management in oncology patients undergoing ICIs treatment, stratifying them by the ATC level IV.
2.4. Statistical Analysis
A descriptive analysis was performed to assess baseline patients’ demographic and clinical characteristics.
PFS was calculated from ICI treatment initiation to radiological PD, all-cause death or censorship, while OS from immunotherapy initiation to all-cause death or censoring, and both represented by Kaplan–Meier curves. Subgroups survival analysis compared Kaplan–Meier curves using the log-rank test. Univariate and multivariate Cox proportional hazards regression models assessed the association between survival and antibiotic intake and the degree of antibiotic exposure. Covariates in multivariate models included gender, age, ECOG-PS at first ICI cycle, presence of metastases, ADR exposure, stage, treatment line and ICI classes. R statistical software, Version 4.3.1, setting the significance level at 5%, was used for all statistical analyses.
3. Results
3.1. Patients’ Characteristics
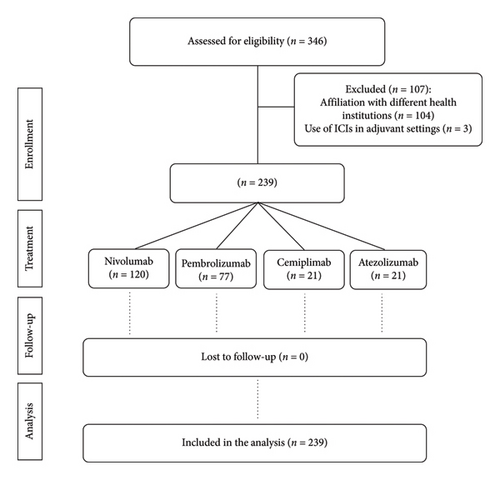
The study included 239 subjects who underwent immunotherapy within 2020 and 2021 at Veneto Institute of Oncology, an Italian referral cancer center. A total of 346 patients were extracted, of which 104 were excluded due to a different healthcare affiliation and 3 to adjuvant immunotherapy setting (Figure 1). Study population includes 84 females and 155 males, ranging in age from 30 to 99, with a median age of 73 years and a more representative age range of 70–79 (Table 1). Neoplastic disease was distributed as follows: 35% having melanoma, 9% skin squamous cell carcinoma, 48% NSCLC, and 8% renal carcinoma. A total of 120 patients received nivolumab, 21 cemiplimab, 77 pembrolizumab, and 21 atezolizumab. Melanoma patients were mainly treated with nivolumab (92%) and pembrolizumab (8%), while NSCLC patients received atezolizumab (18%), nivolumab (21%), and pembrolizumab (61%). Renal carcinoma patients received nivolumab, while skin squamous cell carcinoma patients received cemiplimab. Most subjects (74%) suffer from metastatic disease, while 26% suffer from locally advanced disease. ECOG-PS was 0 in 46% of the cases at the beginning of immunotherapy, 1 in 46%, and 2 in 8.0%. A total of 158 patients initiated immunotherapy as first-line therapy, 68 as second-line therapy, and 12 as third-line therapy and one patient was considered for immunotherapy beyond the fourth line.
| Characteristics | Patients’ number (%) |
|---|---|
| Gender | |
| Male | 155 (65%) |
| Female | 84 (35%) |
| Age | |
| < 65 years | 63 (26%) |
| ≥ 65 years | 176 (74%) |
| Cancer type | |
| Melanoma | 84 (35%) |
| Cutaneous squamous cell carcinoma | 21 (9%) |
| NSCLC | 115 (48%) |
| Renal carcinoma | 19 (8%) |
| Setting | |
| Locally advanced | 62 (26%) |
| Metastatic | 177 (74%) |
| Stage | |
| II | 3 (1%) |
| III | 45 (19%) |
| IV | 183 (77%) |
| Undefined | 8 (3%) |
| ECOG PS | |
| 0 | 110 (46%) |
| 1 | 109 (46%) |
| 2 | 20 (8%) |
| Line of treatment for advanced/metastatic disease | |
| I | 158 (66%) |
| II | 68 (28%) |
| > II | 13 (6%) |
| ICIs | |
| Nivolumab | 120 (50%) |
| Pembrolizumab | 77 (32%) |
| Cemiplimab | 21 (9%) |
| Atezolizumab | 21 (9%) |
3.2. Outcomes Analysis Resulting From Antibiotics Administration in Patients Undergoing ICIs Treatment
3.2.1. Effects of Antibiotics Administration on ICIs Efficacy
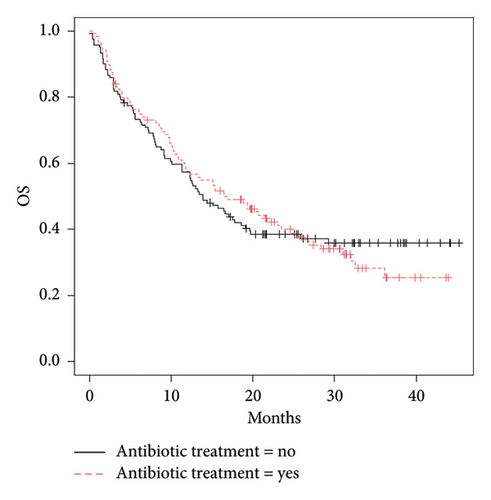
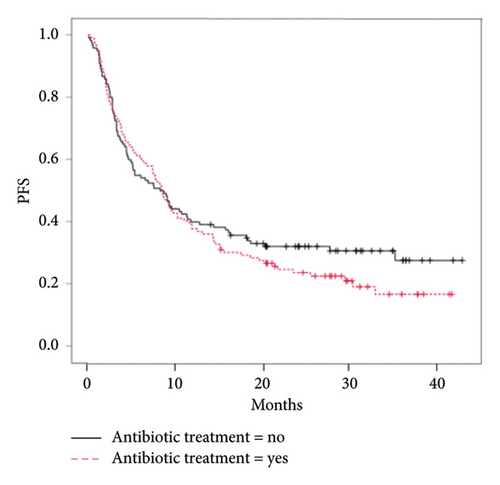
The 49.8% (119) of the 239 patients who received immunotherapy also received antibiotic therapy (ATC J01) within the time interval considered. ICIs efficacy analyses revealed no significant difference in OS between subjects who received antibiotics (median OS: 16.6 months [IC 95%:11.8–24.8]) and those who did not (median OS: 13.9 months [IC 95%: 11.3–19.6], p value = 1.0) (Figure 2(a)). No statistically significant difference was observed in PFS of patients who received antibiotics (median PFS: 8.5 months [IC 95%: 7.0–10.1], p = 0.33) compared with those who did not (median PFS: 8.3 months [IC 95%: 4.8–11.8], p value = 0.33) (Figure 2(b)).
Further survival analyses were performed by categorizing patients into subgroups based on their pathology. In melanoma patients, although the results are not statistically significant, the trend of the Kaplan–Meier curves points out unfavorable clinical outcomes in terms of OS and PFS, in subjects who received antibiotics compared with those who did not (Supporting Figure 1). In contrast, subjects affected by NSCLC show an opposite trend, reporting better OS and PFS in those patients receiving both ICIs and antibiotic therapy (Supporting Figure 2). Multivariate analysis in the melanoma subgroup showed that the presence of metastasis and increasing ECOG-PS (1-2) values were correlated with unfavorable estimates of OS and PFS (Supporting Tables 1 and 2). Even in NSCLC patients, ECOG-PS values of 1-2 correlate with unfavorable OS, while the presence of ADRs is associated with longer survival estimates (Supporting Tables 3 and 4). The same analyses were also performed in renal and skin cancer cohorts; however, the results did not reach statistical significance due to the small number of subjects.
3.2.2. Effects of Antibiotics Exposure on ICIs Efficacy
The potential correlation between immunotherapy efficacy and the level of antibiotic exposure was investigated. Median value of antibiotic exposure was calculated for each subgroup as a percentage value of the ratio between days of antibiotic therapy/days of ICI treatment and used as a cutoff to divide patients into two cohorts: subjects with antibiotic exposure above or below the cutoff. Median value of antibiotic exposure, calculated for the 119 study subjects who received antibiotics, was 7%. The median OS was 8.8 (95% CI: 5.02–11.8) and 31.05 months (95% CI: 24.81–NA) in subjects with exposure > 7% and < 7%, respectively (p value = 0.00000002) (Figure 3).
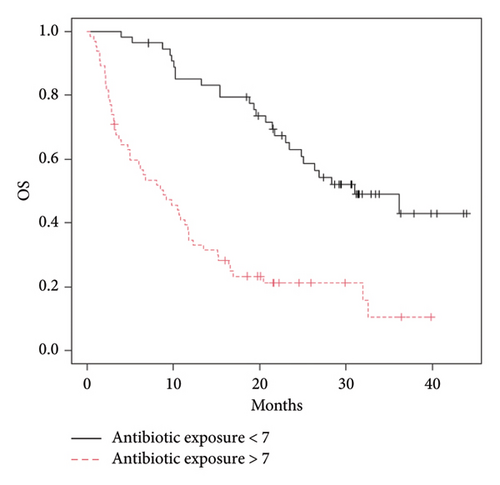
Subgroup analysis confirmed that each subgroup is representative of the whole population since the antibiotic exposure rate decreases in patients treated with ICIs, OS increases. Median antibiotic exposure in subjects affected by melanoma (N = 34) concomitantly immune treated was 4%, slightly lower than the value calculated for the total study population. Lower antibiotic exposure in melanoma patients exerts a favorable effect on both OS and PFS (Figure 4).
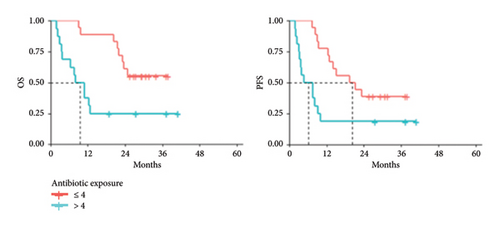
Median PFS was 6.10 (95% CI: 3.22–NA) and 20.31 months (95% CI: 12.89–NA) in patients with antibiotic exposure > 4% and ≤ 4%, respectively (p value = 0.0073). Median OS was also significantly increased in patients affected by melanoma with lower antibiotic exposure rate (p value = 0.0036). Melanoma cohort was further investigated, in terms of OS, comparing patients with high antibiotic exposure (> 4%) to those who did not receive any antibiotics. As shown in Supporting Figure 3, the subgroup of patients with antibiotic exposure > 4% showed an unfavorable OS profile, 10.1 months (95% CI: 4.0–NA), compared with those who did not undergo antibiotic therapy (median not reached).
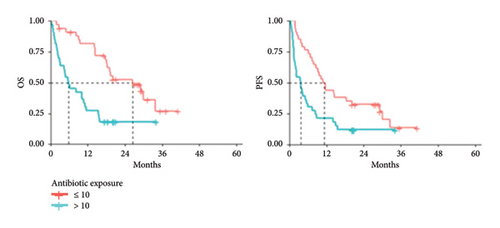
Subjects diagnosed with NSCLC (N = 67), undergoing immunotherapy and receiving antibiotics reported a median antibiotic exposure value of 10%, suggesting that lung cancer patients are more prone to antibiotic use compared with melanoma patients. Median OS was 5.86 (95% CI: 3.19–11.51) and 26.58 months (95% CI: 18.39–NA) in subjects with antibiotic exposure > 10%, compared with those with exposure ≤ 10% (p value = 0.0001). Similarly, PFS was 3.65 (95% CI: 1.94–7.34) and 11.32 months (95% CI: 8.59–29.11) in subjects with antibiotic exposure > 10% compared with those with exposure ≤ 10% (p value = 0.0030) (Figure 5), further supporting that high antibiotic exposure is associated with unfavorable clinical outcomes.
Despite their limited number, univariate analyses performed on patients with renal carcinoma yielded interesting significant results. Median antibiotic exposure value was 7%, intermediate between melanoma and NSCLC. Median OS and PFS were then calculated comparing antibiotic exposure values: median OS was 9.84 (95% CI: 1.38–NA) and 25.20 months (95% CI: 9.57–NA) in subjects with antibiotic exposure > 7% compared to those with antibiotic exposure ≤ 7%, respectively, although not statistically significant (p value = 0.7297). Median PFS was significantly higher in subjects with antibiotic exposure ≤ 7% compared with those with exposure > 7%: antibiotic exposure 12.88 (95% CI: 2.53–NA) versus 2.04 months (95% CI: 1.38–NA), respectively (p value = 0.0476) (data not shown).
Multivariate analyses in NSCLC and melanoma subgroups confirmed that antibiotic exposure is a significant factor in estimating OS and PFS: a high antibiotic exposure ratio is found to be an unfavorable predictor for OS and PFS outcomes. Multivariate analysis in the renal and squamous cell skin carcinoma subgroups was not performed due to their small sample sizes.
3.2.3. Efficacy Analysis of Patients Treated With ICIs in 2020–2021
To assess secondary objectives, immunotherapy efficacy analysis in terms of OS and PFS was also performed. The median OS and PFS in the overall population were 14.41 (95% CI: 11.64–18.42) and 8.42 months (95% CI: 6.68–10.10), respectively.
Further analyses on disease site subgroups showed that OS of melanoma patients was 27.40 months (95% CI: 20.39–NA), including 41 deaths during observation period, while median PFS was 15.03 months (95% CI: 9.44–NA). Median OS of NSCLC patients treated with ICIs was considerably lower at 10.66 months (95% CI: 8.22–15.43) compared with melanoma, including 85 deaths among 115 patients observed; median PFS was 5.30 months (95% CI: 4.14–8.19), with 97 patients experiencing disease progression (DP). Patients affected by renal carcinoma had a median OS of 17.40 months (95% CI: 9.84–NA) and a median PFS of 5.07 months (95% CI: 2.93–25.20). Finally, squamous cell skin carcinoma patients exhibited a median OS of 11.64 months (95% CI: 6.81–NA), including 15 deaths among 21 total patients, and a median PFS of 9.21 months (95% CI: 4.38–NA), with 16 patients experiencing PD during analysis (Supporting Table 5).
3.3. Analysis of the Most Widely Used Antibiotic Classes in Cancer Patients Treated With ICIs, Identified According to ATC Level IV
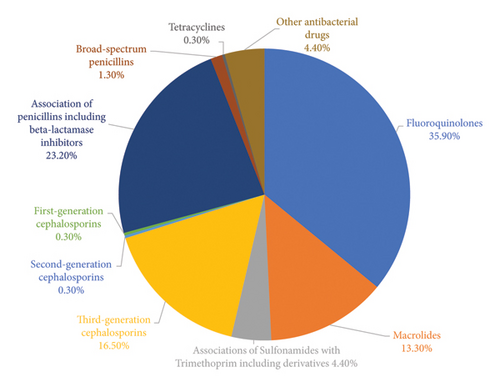
The analysis of the most common classes of antibiotic drugs prescribed within the 119 eligible patients was performed by assessing the percentage distribution of antibiotic dispensations based on the different pharmacological class identified according to the ATC level 4. As shown in Figure 6, the four most prescribed antibiotic classes are fluoroquinolones (J01MA, 35.9%), penicillin in combination with beta-lactamase inhibitors (J01CR, 23.2%), third-generation cephalosporins (J01DD, 16.5%), and macrolides (J01FA, 13.3%).
Although we found no significant differences in OS and PFS considering the overall population of patients on immunotherapy treatment who concomitantly received antibiotics versus the population who did not, the analysis by antibiotic classes showed that patients who concomitantly assumed penicillins and macrolides had better outcomes in terms of OS than patients who concomitantly assumed antibiotics of other classes. Median OS was significantly higher in the subgroup of subjects who concomitantly assumed penicillins and macrolides compared with those who assumed other classes of antibiotics, 32.00 (95% CI:21.71–33.50) versus 13.26 months (95% CI: 15.91–22.68) (p = 0.023), respectively (Supporting Figure 4).
3.4. Analysis of Treatment Discontinuation Causes
Out of 239 subjects, 18 patients were still ongoing while 221 (92%) discontinued immunotherapy due to PD in 125 patients (57%), toxicity in 7 (3%), death in 36 subjects (16%), and other causes in 51 patients (23%); 2 patients were then lost to follow-up. Among others, the most common reason reported by physicians is “treatment completion” as the patient is free from recurrence. Other reasons for interruption include patient’s decision or therapeutic pause.
3.5. ADR Analysis in the Study Population
The analysis of ADR occurrence in the cohort of patients undergoing ICI treatment and antibiotic therapy compared with those who did not receive antibiotics showed similar results. Considering the subgroup of patients who received antibiotics, out of 119 subjects, 82 (69%) experienced ADRs; similarly, 82 patients (68%) out of 120 who did not receive antibiotic therapy experienced ADRs.
4. Discussion
ICIs significantly improved prognosis in various tumor diseases, leading to their exponential use in oncology [3]. Numerous randomized clinical trials and retrospective analyses evaluated the efficacy and toxicity profile of these drugs [8, 9].
With the limit of a monocentric retrospective analysis including a heterogeneous population in terms of treatment line and disease site, this work aimed at investigating clinical outcomes resulting from immunotherapy in melanoma, squamous cell carcinoma, NSCLC, and renal carcinoma. Results obtained are aligned with current literature evidence in real practice for the disease sites investigated [10–13] and confirm that survival outcomes in melanoma patients were among the most favorable, while NSCLC patients had the least favorable prognosis. Also, in renal cancer, data of survival are comparable to pivotal trials [14]. Real-world evidence for cemiplimab in squamous cell carcinoma is limited, but published studies suggest encouraging results [15].
Several drugs used concomitantly with ICIs have been investigated for their potential impact on cancer patients’ clinical outcomes but understanding factors influencing variable ICI response still remains challenging [16]. Among the limitations of this study, the lack of consideration for the impact of severe comorbidities on antibiotic use and clinical outcomes in cancer patients is recognized. Future investigations should aim to address this aspect to enhance the robustness of the findings.
Recent focus on gut microbiome’s role in immunity and ICI activity suggests that a favorable diversity and enrichment of microbiota correlates with higher efficacy. Otherwise, antibiotic intake during immunotherapy may affect efficacy due to microbiota disruption. As antibiotics are widely used drugs and known, particularly the broad-spectrum ones, to cause long-term perturbation of gut microbiota [16], they are largely studied, especially when used concurrently with cancer therapies. Our study does not report significantly different outcomes in terms of OS and PFS in subjects undergoing ICIs treatment who received antibiotics, compared with those who did not, although subgroup analysis revealed varied impacts based on cancer site and antibiotics exposure. Furthermore, subgroup analyses showed that, unlike patients with melanoma and renal carcinoma, in NSCLC patients, antibiotic therapy concomitant with ICIs did not have a negative impact on survival. Similar results have already been found in NSCLC patients: it has been hypothesized to be due to the coexistence of the tumor with several factors (e.g., smoking and COPD) that predispose more to lung infections, which may benefit from major antibiotic treatment. These infections, particularly when severe, may significantly benefit from antibiotic treatment. This has probably contributed to the results heterogeneity described above and may suggest an explanation for the outcomes observed in our analysis in this setting of patients [15]. However, Cortellini et al., in their retrospective analysis on NSCLC patients treated with pembrolizumab in monotherapy, demonstrated a significant negative correlation of antibiotics to reduced probability of radiological response, a higher risk of disease progression and to a higher risk of death, producing further evidence about antibiotics immune-modulatory effect [6], as such detrimental effects on overall response rate (ORR), PFS, and OS were not observed within patients enrolled in the chemotherapy arm. Similar results have been obtained in a retrospective analysis of the pooled data from the phase II POPLAR and phase III OAK trials. The analysis suggests that antibiotics use in metastatic NSCLC patients is associated with poor OS and may influence ICIs efficacy [17]. Similar results were observed by Geum M.J. et al., in advanced NSCLC patients treated with nivolumab in monotherapy, who have significantly poorer OS when treated concurrently with antibiotic therapy, with no effect on PFS [18]. All authors agree that dysbiosis caused by antibiotics may adversely affect ICIs effectiveness, further supporting the hypothesis that antibiotics might act as immune modulators rather than by masking an unrecognized association with underlying adverse prognostic features [6]. However, although these papers consider immunotherapic agents most commonly used in this setting of patients, only few papers stratify patients basing on antibiotic exposure and classes, which in our study proved to be significant in terms of OS when comparing macrolides and penicillins to other classes. It is well known indeed that different classes of antibiotics, such as broad-spectrum antibiotics used in patients with infections at high risk of resistance, have a greater ability to disrupt the gut microbiota and this is more likely to influence clinical response to immunotherapy treatments. According to Qiu et al., we found that penicillins, widely used antibiotics, worsen the outcomes of NSCLC patients treated with immunotherapy compared with other antibiotic classes [19]. This could be attributed to the β-lactamase enzymatic activities for carbohydrate degradation, which leads to imbalance of glucose metabolism and immunosuppressive tumor microenvironment remodeling, which in turn, results in immune evasion [20]. Moreover, amoxicillin has been found to significantly reduce gene expression of major histocompatibility complex classes I and II, which participate in the adaptive immune system promoting cancer immune evasion [21, 22].
Such heterogeneous results, depending on the tumor site and antibiotic class, highlight that these factors are not detrimental in predicting immunotherapeutic treatment outcomes but other factors must be taken into account, as the exposure to antibiotics and the timing of antibiotic administration with respect to immunotherapy. Recent studies investigating antibiotic therapy impact during ICIs treatment assessed antibiotic exposure’s role, indicating worsening OS and PFS in patients with high antibiotic exposure [5, 7].
Our analysis, in line with current literature [16, 18, 23], confirmed that prolonged antibiotic administration significantly impacts patients’ survival and further subgroup analysis confirmed that each subgroup is representative of the whole population, except for renal and squamous cell skin carcinomas, due to their limited sample size. The importance of antibiotic exposure factor in relation to the ability of antibiotics to alter gut microbiota has been considered in several studies, demonstrating that gut and throat microbiota show a dramatic disruption 1 week after the start of antibiotic therapy and remain perturbed, in some cases, for up to 4 years posttherapy [24]. Moreover, a decline in the clonal diversity of isolated Bacteroides, the main components of the bacterial flora of mucous membranes, starts after 7 days of antibiotic treatment and persists for up to 2 years after treatment [25]. As our study lacks the analyses of the dynamic microbiota changes during antibiotics therapy, we can only speculate that antibiotics may interfere with microbiota, exerting an impact on systemic immunity, thus resulting in a reduced immunotherapy effectiveness.
An additional important aspect to consider is the timing of antibiotic therapy initiation with respect to immunotherapy treatment. It is well known how antibiotics initiated before immunotherapy negatively affects its outcomes. In particular, many studies show that antibiotic therapy has a negative impact if started in the timeframe between 60 or even worse if started 30 days before immunotherapy treatment or during the first 30 days of treatment [16, 23, 26–28]. One hypothesis that may explain the radiologically assessed refractoriness to ICIs is that antibiotics may exert a “priming” effect on cancer-specific immunity by altering gut microbiota [16]. These results should lead to some caution in evaluating the timing of immunotherapy initiation in those patients who have started antibiotic therapy within the previous 60–30 days but also during the first 30 days of treatment.
For all these reasons, antibiotic stewardship is crucial, given oncology patient susceptibility to infections and the emergence of multidrug-resistant organisms [29]. The study also analyzed antibiotic prescriptions in oncology patients receiving ICIs, with fluoroquinolones being most commonly prescribed, aligning with guidelines for high-risk oncology patients [30, 31]. The reported causes for immunotherapy discontinuation are consistent with published literature [32–34]. Interestingly, despite 50% of the patients reported ADRs, only 3% of ICI discontinuation was toxicity related. We can conclude that prompt management of ICI-related toxicities contributes to treatment success.
5. Conclusions
Prolonged antibiotic exposure during ICIs therapy may negatively impact the efficacy. Emerging evidence highlights the critical impact of factors such as timing, class, and exposure level of antibiotics, as well as tumor site and staging. Higher antibiotic exposure consistently correlates with poorer survival and reduced immunotherapy effectiveness. Thus, managing antibiotic exposure is fundamental for ICI treatment optimization. Further research is needed to promote targeted antibiotic stewardship to enhance patient outcomes and fight resistance.
Ethics Statement
The study received approval from the local Ethical Committee on April 24th, 2023 (Internal Review Board prot.nr 2022-57). Study procedures were conducted according to Good Clinical Practice and the Helsinki Declaration. Data collection was also conducted in accordance with current legislation on the processing of personal data in the context of studies promoted by Scientific Institute for Research, Hospitalization, and Healthcare (IRCCS).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Marina Coppola and Alberto Russi contributed to the study conception and design. Camilla Saran and Giulia Zanchetta collected, analyzed, and discussed data interpretation. Giorgia Zorzetto and Giovanna Crivellaro contributed in data interpretation and wrote the manuscript. Chiara De Toni and Carola Cenzi performed statistical analysis. Marco Maruzzo, Giulia Pasello, Alessio Fabozzi, Elena Berti, Silvia Cognolato, Francesca Pipitone, Alberto Bortolami, Ugo Moretti, Giovanna Scroccaro, and Paola De Ambrosis critically revised the manuscript. Alice Capogrosso Sansone, Chiara Salvato, and Francesca Bano provided data from ULSS6 and ULSS2 Territorial Pharmaceutical Services. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the research.
Alberto Russi and Camilla Saran contributed equally to this paper.
Funding
This work was supported by Agenzia Italiana del Farmaco (AIFA), as part of the regional pharmacovigilance project, and promoted by the National Plan to Contrast Antimicrobial Resistance (PNCAR) 2017–2020, implemented at regional level by G.D.R. No. 1875 dated 11/22/2017. Open access funding provided by BIBLIOSAN.
Acknowledgments
The authors would like to thank Agenzia Italiana del Farmaco (AIFA) for supporting the project with pharmacovigilance funds within the National Plan to Contrast Antimicrobial Resistance (PNCAR) years 2017–2020.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data underlying the findings described in this manuscript are deposited in the Zenodo repository within the Veneto Institute of Oncology IOV IRCCS community, but they are not publicly available. The anonymized datasets are available from the corresponding author upon reasonable request.




