Exploring the Antibiofilm and Antibacterial Potential of Datura stramonium and Prosopis farcta Against Gram-Positive and Gram-Negative Bacteria
Abstract
Background: The rise of drug-resistant bacteria poses a significant challenge to global healthcare, underscoring the urgent need for new therapeutic interventions. In this study, ethanolic extracts of Datura stramonium and Prosopis farcta, sourced from Northern Iran, were examined for their antibacterial properties against Staphylococcus aureus, Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Methods: The antibacterial activities of the medicinal extracts were examined in this study using disc diffusion, microbroth dilution, and agar well diffusion methods against both Gram-positive and Gram-negative microorganisms. In addition, the extracts were assessed for toxicity using the MTT assay on HT29 cells. The biofilm-inhibitory effects of the extracts were also investigated.
Results: The results showed that the ethanolic extracts from D. stramonium and P. farcta have strong antibacterial activity against S. aureus. However, their activity against P. aeruginosa and A. baumannii is less pronounced. The extracts showed significant antibiofilm capabilities against the tested bacteria at both MIC and 2× MIC concentrations (p < 0.05). The MTT test showed HT29 cells were more sensitive to P. farcta extract than D. stramonium, especially at 200 μg/mL concentration.
Conclusion: The findings demonstrate that ethanolic extracts of P. farcta and D. stramonium exhibit significant antibacterial activity, particularly against S. aureus, while also effectively disrupting bacterial biofilms. These results suggest that these extracts possess considerable potential as alternative therapeutic agents against drug-resistant bacterial infections. Further investigation is warranted to elucidate the specific mechanisms of action and to explore their efficacy in clinical applications.
1. Background
The misuse of antibiotics has precipitated the emergence of antimicrobial resistance, significantly challenging human civilization. The escalating issue of antibiotic resistance among microbial pathogens is a global concern that affects all nations and poses an imminent threat to public health [1]. Projections indicate that by 2050, infections attributed to these resistant microbes could emerge as a leading cause of mortality worldwide [2]. Increasingly, bacteria are demonstrating resistance to existing antibiotics, largely attributed to their capacity to exchange antibiotic resistance genes among themselves [3].
In addition, biofilm formation is a critical bacterial strategy that contributes to drug resistance, as antimicrobials struggle to penetrate biofilm-protected bacterial cells effectively [4]. This resistance encompasses a variety of agents, including antibiotics and chemical disinfectants such as chlorine bleach and glutaraldehyde [5]. Biofilms not only diminish the effectiveness of antibiotics but also compromise host immune responses, conferring a greater resistance than that observed with singular antibiotic resistance mechanisms [6].
The urgent challenge of antibiotic resistance has captured the attention of healthcare professionals and researchers, particularly in the context of treating bacterial infections that are resistant to multiple drugs. This pressing issue has ignited a growing interest in innovative therapeutic modalities, prominently featuring the exploration of herbal extracts as potential adjuncts in combating bacterial infections and the looming threat of antibiotic resistance [3].
Plant extracts and their secondary metabolites are increasingly utilized in various drug combinations. Traditional medicine, which incorporates the use of diverse medicinal plants, animals, and fungi, has historically served as a foundational approach to preventing and treating numerous ailments. The rich bioactive compounds present in plants make them particularly attractive as therapeutic agents [7, 8]. A multitude of studies have substantiated the antimicrobial and biological properties of numerous medicinal plants [9–11], demonstrating the efficacy of these compounds in inhibiting growth and promoting the death of pathogenic microorganisms [12].
Among these plants, Prosopis has garnered significant attention in recent years for its antibacterial properties within traditional medicine [12, 13]. Various studies have assessed the antibacterial efficacy of extracts from Prosopis spp. against different bacterial strains [12, 14, 15], including investigations into the effectiveness of methanolic extracts from Prosopis juliflora leaves and ethanolic extracts of its alkaloids against a range of human pathogenic bacteria [16, 17]. These antibacterial activities have been associated with the presence of phenolic acids, flavonoids, and alkaloids, secondary metabolites found in these plants [18, 19]. P. farcta, specifically, boasts a rich history in traditional medicine, where it has been employed to treat a variety of diseases and disorders. Recent research has underscored its significant proliferative and angiogenic properties, along with its antioxidant capacity and reported antitumor effects [20–22].
Furthermore, the pharmacological and phytochemical attributes of the Datura genus, belonging to the family Solanaceae, have been extensively researched [23, 24]. Investigations have revealed that this genus possesses antimicrobial, anti-inflammatory, and cytotoxic activities, in addition to analgesic properties (such as narcotic effects). The diverse metabolic effects observed in this genus result from the presence of a wide array of secondary metabolites, including steroids, phenolic compounds, fatty acids, anolides, and lactones [25].
Given the medicinal significance of D. stramonium and P. fracta, the present study investigated the antibacterial effects of their ethanolic extracts on the growth and biofilm formation of five species of pathogenic bacteria: Staphylococcus aureus, Escherichia coli, Acinetobacter baumannii, Klebsiella pneumonia, and Pseudomonas aeruginosa. In addition, we investigated the toxicity of these extracts on human colon cancer.
2. Methods
2.1. Ethics Statement
Iran University of Medical Sciences, Tehran, Iran’s ethics committee confirmed this study (IR.IUMS.REC.1398.134).
2.2. Plant Collection and Extraction
Two plant species exhibiting healthy leaves, roots, and stems were selected and collected from the northern provinces of Iran [26]. P. fracta and D. stramonium were identified and placed in the Histogenotech Company in Tehran, Iran (Dr. S. Bolori) and Mazandaran University of Medical Sciences Herbarium (Dr. A. Mahmoudi Otaghvari). The voucher specimens for P. fracta and D. stramonium were H9922 and 9124, respectively. The plants were rinsed with distilled water to eliminate pesticides, toxins, and other trash before making the ethanolic extract. They were oven-dried at 80°C. Finally, the dried plant material was crushed into smaller particles to increase solvent interaction and extraction efficiency [26]. The Soxhlet extraction method was employed to prepare the extracts, as Sales and Shariat described [27]. In a stock solution of D. stramonium and P. fracta extracts, 20 mg of each was dissolved in 500 μL of solvent (DMSO 5% for D. stramonium and sterile distilled water for P. fracta). Microbial assays were performed at 50, 25, 12.5, 6.25, and 3.1 mg/mL dilutions.
2.3. Determination of Antimicrobial Activity
2.3.1. Bacterial Strains
Five pathogenic bacterial strains, S. aureus ATCC 6538, E. coli ATCC 35218, A. baumannii ATCC 19606, K. pneumonia ATCC 13883, and P. aeruginosa ATCC 27853, were included in this study. These strains were sourced from the microbiology laboratory at the College of Medicine, Iran University of Medical Sciences, Tehran, Iran. Following overnight incubation at 37°C on Mueller–Hinton broth (MHB) (Merck, Germany), the turbidity of the bacterial suspension was adjusted to 0.5 McFarland (1.5 × 108 CFU/mL), measured at OD 625 nm using a spectrophotometer (Sigma 3-30K, USA) [26, 28].
2.3.2. Disc Diffusion and Agar Well Diffusion Methods
The disk diffusion method was done as outlined by Aiyegoro et al. [29]. In brief, after incubation for 24 h at 37°C in Nutrient broth medium, under sterile conditions, 100 μL of the prepared bacterial suspension was spread across MHA plates in three different directions. Sterilized paper discs of Whatman No. 1 filter paper (6 mm in diameter) were placed on the inoculated agar plates after being impregnated with varying concentrations of the ethanolic extracts. The plates were incubated for 18–24 hours at 37°C. The diameter of the inhibition zones was measured in millimeters using calipers. Tetracycline disks were utilized as positive controls, while the solvent and DMSO served as negative controls [30–32].
The agar well diffusion technique disseminated 500 μL of microbial suspension at 1.5 × 106 CFU/mL per isolate across two MHA plates for each bacterium. On the agar surface, 5 mm wells were spaced 2 cm apart. For each well, 50 μL of each ethanolic extract concentration was applied. The extract solvent (sterile distilled water or 5% DMSO) was a negative control, while tetracycline was a positive control. After 24 h of 37°C incubation, well inhibition zones were observed [27].
2.4. Determination of MIC and minimum bactericidal concentration (MBC)
The MIC of each plant extract was determined using microbroth dilution. Ten serial concentrations (0.09, 0.19, 0.39, 0.78, 1.56, 3.1, 6.25, 12.5, 25, and 50 mg/mL) were produced in MHB medium in a 96-well plate for each extract. A 1:100 dilution of the bacterial suspension was obtained by pipetting 150 μL of saline solution with the inoculum into 15 mL of sterile broth, yielding a final inoculum density of 1.5 × 106 CFU/mL. Inoculate 10 μL of suspension into wells and incubate for 24 h at 37°C. The first well without turbidity was found by macroscopic examination of the MIC. The lowest extract concentration that totally suppressed observable growth was the minimal inhibitory concentration. The negative control was MHB medium alone, while the positive control was MHB medium inoculated with the tested bacterium. All extract and bacteria tests were done in triplicate [28, 33].
The MBC for each extract was determined following the method described by Mishra [34]. Inoculum from the wells exhibiting no growth in the MIC experiment was subcultured onto freshly prepared Nutrient agar plates. The highest extract concentration that yielded no bacterial colonies (i.e., without visible growth on the plate) was designated as the MBC [3].
2.5. Assessment of Established Biofilms
2.6. Evaluation of the Cytotoxicity Effect of the Extract
2.7. Statistical Analysis
Statistical data analysis was performed using SPSS software (Version 22). Each test was conducted in triplicate to minimize measurement errors in assessing the antibacterial properties of the extracts. The antibacterial assay values were expressed as the mean ± standard error (SE), with statistical significance determined at P < 0.05.
3. Results and Discussion
The global burden of infectious diseases, particularly in developing nations, is a widely recognized concern. The rise of antimicrobial resistance presents a pressing issue, attributed not only to substandard drug manufacturing, patient nonadherence to treatment regimens, and inappropriate use of antimicrobials but also to the emergence of spontaneous mutations within microbial populations. In response to this challenge, researchers are investigating medicinal plants as potential sources of antimicrobial compounds, aiming to meet the urgent need for novel antimicrobial agents [39].
The results of this study on the antibacterial effects of ethanolic extracts of D. stramonium and P. fracta against pathogenic bacteria are presented in Tables 1 and 2 and Figure 1. The study utilized the disc diffusion method, the agar-well diffusion assay, and MIC determination. A direct correlation was observed between the concentration of the extract and the diameter of the inhibition zone. One-way analysis of variance (ANOVA) revealed a significant difference in the mean diameter of the nongrowth zones among the bacteria studied at concentrations of 50, 25, 12.5, 6.25, and 3.1 mg/mL, with a significance level of 95% (P value < 0.001). Pairwise comparisons were conducted using Tukey’s multiple comparison test. In addition, the study found significant differences in the ratios of S. aureus to P. aeruginosa for the D. stramonium extract and S. aureus to A. baumannii for the P. fracta extract (P value < 0.0001).
| Method | Bacterial strains | Control + | Control − | Concentration of extract (mg/mL) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| 50 (mg/mL) | 25 (mg/mL) | 12.5 (mg/mL) | 6.25 (mg/mL) | 3.1 (mg/mL) | |||||
| Disk diffusion | K. pneumoniae | 0 | 2 | 2.17 ± 0.29 | 1.83 ± 0.29 | 1.52 ± 0.23 | 1.23 ± 0.25 | 1.00 | < 0.001 |
| E. coli | 0 | 2 | 2.27 ± 0.25 | 2.10 ± 0.17 | 1.83 ± 0.29 | 1.62 ± 0.13 | 1.27 ± 0.25 | ||
| S. aureus | 0 | 3 | 4.07 ± 0.12 | 3.87 ± 0.23 | 3.67 ± 0.29 | 3.43 ± 0.06 | 3.03 ± 0.06 | ||
| A. baumannii | 0 | 1.25 | 1.83 ± 0.14 | 1.58 ± 1 = 0.14 | 1.40 ± 0.10 | 1.17 ± 0.06 | 1.03 ± 0.06 | ||
| P. aeruginosa | 0 | 0 | 0.57 ± 0.06 | 0.47 ± 0.06 | 0.23 ± 0.06 | 0.07 ± 0.12 | 0 | ||
| Agar well diffusion | K. pneumoniae | 0 | 2 | 7.27 ± 0.25 | 5.87 ± 0.15 | 5.87 ± 0.15 | 5.57 ± 0.12 | 5.47 ± 0.06 | |
| E. coli | 0 | 2 | 10.17 ± 0.76 | 9.83 ± 0.29 | 9.77 ± 0.40 | 9.43 ± 0.51 | 9.00 | ||
| S. aureus | 0 | 3 | 13.37 ± 0.32 | 12.67 ± 0.58 | 10.50 ± 0.50 | 9.33 ± 0.58 | 9.23 ± 0.40 | ||
| A. baumannii | 0 | 1.25 | 6.25 ± 0.25 | 6.00 | 5.67 ± 0.29 | 4.50 ± 0.50 | 3.33 ± 0.29 | ||
| P. aeruginosa | 0 | 0 | 2.83 ± 0.29 | 2.17 ± 0.29 | 1.00 | 0.93 ± 0.12 | 0.10 ± 0.17 | ||
| Method | Bacterial strains | Control + | Control − | Concentration of extract (mg/mL) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| 50 (mg/mL) | 25 (mg/mL) | 12.5 (mg/mL) | 6.25 (mg/mL) | 3.1 (mg/mL) | |||||
| Disk diffusion | K. pneumoniae | 0 | 2 | 2.17 ± 0.29 | 1.83 ± 0.29 | 1.52 ± 0.23 | 1.23 ± 0.25 | 1.00 | < 0.001 |
| E. coli | 0 | 2 | 2.27 ± 0.25 | 2.10 ± 0.17 | 1.83 ± 0.29 | 1.62 ± 0.13 | 1.27 ± 0.25 | ||
| S. aureus | 0 | 3 | 4.07 ± 0.12 | 3.87 ± 0.23 | 3.67 ± 0.29 | 3.43 ± 0.06 | 3.03 ± 0.06 | ||
| P. aeruginosa | 0 | 1.25 | 1.83 ± 0.14 | 1.58 ± 1 = 0.14 | 1.40 ± 0.10 | 1.17 ± 0.06 | 1.03 ± 0.06 | ||
| A. baumannii | 0 | 0 | 0.57 ± 0.06 | 0.47 ± 0.06 | 0.23 ± 0.06 | 0.07 ± 0.12 | 0 | ||
| Agar well diffusion | K. pneumoniae | 0 | 2 | 4.83 ± 0.29 | 4.57 ± 0.51 | 4.33 ± 0.29 | 4.08 ± 0.38 | 4.00 | |
| E. coli | 0 | 2 | 5.00 | 4.75 ± 0.25 | 4.58 ± 0.14 | 4.22 ± 0.20 | 4.13 ± 0.23 | ||
| S. aureus | 0 | 3 | 6.23 ± 0.25 | 6.20 ± 0.20 | 5.63 ± 0.15 | 5.33 ± 0.29 | 5.07 ± 0.12 | ||
| P. aeruginosa | 0 | 1.25 | 7.00 | 6.75 ± 0.25 | 6.67 ± 0.14 | 6.50 | 6.07 ± 0.12 | ||
| A. baumannii | 0 | 0 | 0.67 ± 0.14 | 0.58 ± 0.14 | 0.47 ± 0.06 | 0.37 ± 0.12 | 0 | ||
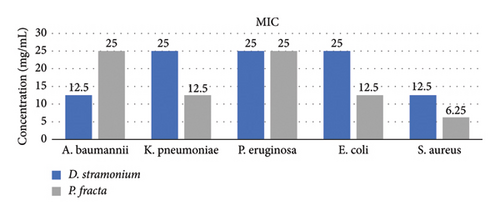
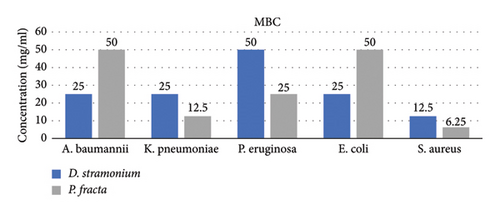
The findings from the MBC/MIC tests indicated that the D. stramonium extract exhibited the most pronounced antibacterial effect on S. aureus, with MIC and MBC values of 12.5 mg/mL while displaying the least effect on P. aeruginosa (MIC: 25 mg/mL and MBC: 50 mg/mL). Conversely, the P. fracta extracts demonstrated the highest efficacy against S. aureus (MIC and MBC: 6.25 mg/mL) and the lowest efficacy against P. aeruginosa/A. baumannii (MIC: 25 mg/mL and MBC: 50 mg/mL). The statistical analysis of the mean MIC and MBC of both extracts among the tested bacteria was significant at the 95% confidence level, with a P value of less than 0.001 (Figure 1). To date, the antimicrobial activity of P. fracta and D. stramonium against various bacterial strains has been documented in several studies [8, 33, 40, 41]. According to the study conducted by Saeedi, the antimicrobial activity of D. stramonium and P. farcta extracts was evaluated against S. aureus. [42]. They also reported that the MIC of P. farcta extract was 25 ppm. However, our study found the MIC of P. farcta extract to be 6.25 ppm. This significant difference in results may be attributed to variations in the origins and sources of both the Prosopis species and the bacteria. Comparative analysis indicated that the D. stramonium extract exhibited larger inhibition zones than the P. farcta extract. Notably, across various concentrations, D. stramonium demonstrated the most significant effect against S. aureus (mean of 3.74 in the disc diffusion method and 11.02 in the agar well diffusion method) and the least effect on P. aeruginosa (mean of 0.26 in the disc diffusion method and 7.03 in the agar well diffusion method) (Table 1). Conversely, the most pronounced inhibitory effect of the P. farcta extract was observed against S. aureus (mean of 3.61 in the disc diffusion method and 5.69 in the agar well diffusion method) (Table 2).
The varying composition and concentration of bioactive secondary metabolites in the extracts may be the underlying cause of the observed differences. A study conducted in Ethiopia by Arage et al. [43] on methanol extracts of Artemisia absinthium, D. stramonium, and Solanum anguivi demonstrated varying degrees of antibacterial activity among these species, which aligns with our findings. In the research conducted by Mazandaran et al. [30], significant antimicrobial activity was observed in P. juliflora (another species of Prosopis) against various microorganisms. It was speculated that flavonoids and other phenolic compounds contributed to the observed bioactivity.
Both Gram-positive and Gram-negative bacteria were susceptible to these ethanolic extracts; however, Gram-positive cells exhibited greater susceptibility. The most potent antibacterial activity of P. fracta and D. stramonium was observed against S. aureus. Conversely, the extracts demonstrated the lowest antibacterial activity against A. baumannii and P. aeruginosa. These results align with those reported by Yeganegi et al. [44], Yazdi and Behbhani [45], and Sureshjani [46], indicating that Gram-negative bacteria are generally more resistant to antimicrobials. Differences in cell structure likely explain the varying susceptibilities of microorganisms to different antimicrobial agents. The absence of an outer membrane in Gram-positive bacteria allows for a higher penetration rate of active compounds. In contrast, the lipopolysaccharide layer present in the outer membrane of Gram-negative bacteria significantly increases their impermeability [47]. This finding is supported by a study conducted by Jafari-Sales and Pashazadeh [47], which investigated the antibacterial effects of saffron petal methanolic extract on several standard strains of pathogenic bacteria. Their results indicated that the bacteria most susceptible to saffron petal extract were S. aureus and Bacillus cereus, while P. aeruginosa and E. coli exhibited the highest resistance. In contrast, our study found that E. coli was highly sensitive to all solvent extracts of D. stramonium, a finding previously reported by Idris and Adam [32].
3.1. Investigating the Inhibitory Effect of Extracts on Biofilm Formation
Figure 2 illustrates the impact of D. stramonium and P. farcta extracts on inhibiting biofilm formation in five tested bacteria. Both extracts, at MIC and 2 × MIC concentrations, exhibited significant biofilm inhibitory effects against these strains (P < 0.05). The inhibition percentages ranged from 27% for D. stramonium (with the highest efficacy observed against S. aureus and E. coli) to 40% for P. farcta (most effective against S. aureus).
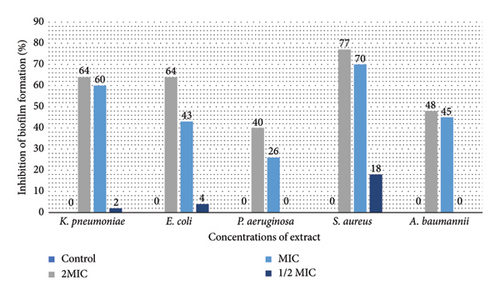
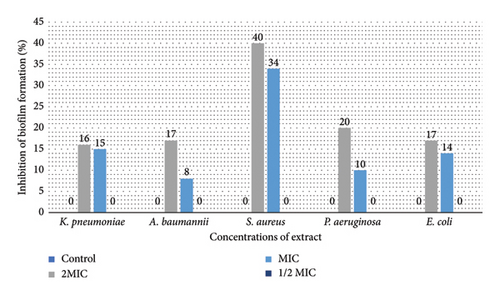
In biofilms, the extracellular polymeric matrix of bacteria facilitates their attachment to both living and inert surfaces [48]. In addition to developing resistance to antibiotics, microorganisms residing within biofilms often exhibit resistance to the antiseptics and disinfectants commonly employed [49]. Recent research has highlighted that natural compounds rich in secondary plant metabolites demonstrate inhibitory effects on biofilm growth, prompting scientists to explore herbal treatments as a means to combat biofilms. Plant extracts are favored due to their efficacy, natural origins, and nontoxic nature when targeting biofilms. Within these extracts, bioactive compounds play a crucial role in exhibiting antibiofilm activity [49]. The inhibitory effects of D. stramonium on biofilm formation are likely attributed to its bioactive metabolites. This hypothesis warrants further investigation through advanced quantitative and qualitative analyses. It is important to note that there is limited literature on the antibiofilm properties of D. stramonium and P. farcta extracts. Mandal documented the antibiofilm effects of D. stramonium against Candida albicans, identifying a novel hydroxyproline-rich glycopeptide that effectively eradicated biofilms of C. albicans, including those resistant to other treatments [50]. Few studies on Prosopis suggest that certain species within this genus (e.g., P. juliflora) possess antibiofilm activity [51, 52]. Based on the current information, this appears to be the inaugural report on the antibiofilm properties of ethanolic extracts derived from P. farcta and D. stramonium. This underscores the novelty and significance of the findings regarding the antibiofilm activity of these plant extracts. Further research could provide valuable insights into their potential applications in addressing biofilm-related challenges.
4. MTT Assay
The graphical representation in Figure 3 illustrates the results of screening the cytotoxic effects of various concentrations (25, 50, 100, and 200 μg/mL) of the respective extracts on the HT29 cell line. The results indicate that higher extract concentrations correlate with increased toxicity towards cancer cells. Notably, the cells showed signs of cell death, particularly at the highest concentration of 200 μg/mL. This observation aligns with the findings of Pourali et al. [53], which demonstrated that the viability of cancer cell lines decreased as the concentrations of the extract were elevated. In addition, they reported that the Tribulus terrestris L. fruit extract did not exhibit lethal effects at lower concentrations. The results revealed that the extracts exhibited low toxicity at lower concentrations, with cell survival remaining above 90%. Research by Merza et al. [54] indicated that the effect of D. stramonium extract was dependent on the duration of exposure rather than the dosage. At both low and medium concentrations, this extract demonstrated high cell viability after 24 h. In this study, the IC50 concentration of P. fracta extract was determined to be 200 μg/mL after 24 h, indicating a higher sensitivity of colon cancer cells to this extract compared with D. stramonium. Khodaei et al. found that P. farcta displayed greater cytotoxicity against HT-29 cells than Teucrium polium after 48 and 72 hours of treatment [55].
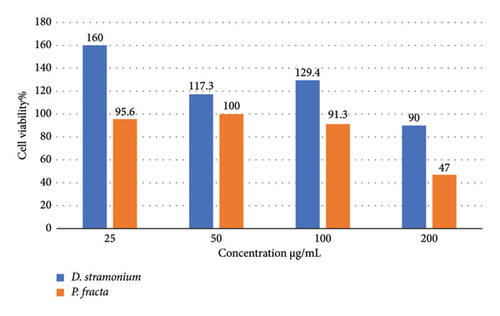
Although the anticancer properties of P. farcta and D. stramonium [56–58] have been investigated on different cells, the findings of this study did not bring statistically significant results. This suggests that relying solely on this method may overlook important factors and is insufficient on its own. Therefore, it is advisable to conduct supplementary tests in future research. These could include examining the inhibitory effects of extracts on DNA synthesis, cell proliferation, and apoptosis, which should be employed in conjunction with the current method. Furthermore, it is recommended that the cytotoxic effects in cancerous mouse models be explored.
5. Conclusion
This research underscores the remarkable inhibitory effects of P. farcta and D. stramonium extracts on the growth and biofilm formation of certain Gram-positive and Gram-negative bacteria. However, it is noteworthy that different parts of the plants may exhibit varying concentrations and proportions of phytochemicals. Consequently, it is advisable to conduct future phytochemical screenings of these extracts.
Disclosure
A preprint has previously been published [59], and this is an updated version of the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.S. and N.A. initiated the idea of this study. S.S. and M.M. developed the first and final draft of the manuscript. S.S. designed and checked all figures and tables. S.S., M.K., and S.M. wrote the manuscript and carried out the data analysis. All authors reviewed and contributed to the revisions and finalized the drafts.
Funding
This work was supported by the Microbial Biotechnology Research Center, Iran University of Medical Sciences, Tehran, Iran (grant numbers 97-4-73-12910).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




