SGLT2 Inhibitors Increase Hemoglobin and Hematocrit Levels in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis
Abstract
What Is Known and Objective: Sodium-glucose cotransporter 2 (SGLT2) inhibitors improve renal and cardiovascular outcomes and have been reported to have a positive impact on anemia, a common and challenging condition in patients with chronic kidney disease (CKD). The aim of this study is to evaluate the effects of SGLT2 inhibitors on anemia in patients with CKD.
Methods: We performed a systematic review and meta-analysis of randomized controlled trials. Changes in hemoglobin (Hb) and hematocrit (Hct) levels were assessed in participants treated with SGLT2 inhibitors—empagliflozin, dapagliflozin, or canagliflozin—and compared with those receiving control treatments. Statistical analyses were performed using a fixed-effects model or a random-effects model, depending on the heterogeneity. Sensitivity analyses were also conducted to evaluate the impact of each study on the meta-analysis. Publication bias was examined using Begg’s and Egger’s tests.
Results and Discussion: Five studies assessing Hb levels were included. SGLT2 inhibitors significantly increased Hb levels compared with controls (standard difference in means [SE] = −0.350; 95% confidence interval [CI] −0.401–−0.299). Similarly, in three studies evaluating Hct levels, SGLT2 inhibitors significantly increased Hct levels compared with controls (SE = −0.453; 95% CI −0.829–0.077).
What Is New and the Conclusions: This systematic review confirms that SGLT2 inhibitors effectively improve anemia in patients with CKD. We recommend considering SGLT2 inhibitors as a treatment option for CKD patients, not only for their renal and cardiovascular benefits but also for their reliability in addressing anemia.
1. What Is Known and the Objective
Chronic kidney disease (CKD) impacts multiple organ systems, leading to a wide range of complications and significant health challenges [1]. Anemia is a common complication of CKD, primarily caused by a relative deficiency in erythropoietin production [2]. The prevalence of anemia increases with disease progression, affecting more than 50% of patients with CKD G5, despite treatment with erythropoietin-stimulating agents and iron supplements [3]. Left untreated, anemia exacerbates left ventricular hypertrophy, worsens heart failure, and may increase the risk of cardiovascular disease, stroke, and mortality [4].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors including empagliflozin, dapagliflozin, or canagliflozin have gained attention not only for their role in managing diabetes mellitus but also for their protective effects on renal and cardiovascular outcomes [5–9]. In addition, people with diabetes have a twice as high chance of acquiring cardiovascular disease compared to those without it [10]. Interestingly, recent evidence has highlighted their potential to correct anemia, as demonstrated by a study reporting the anemia-correcting effect of dapagliflozin in patients with type 2 diabetes [11]. A meta-analysis has also investigated the impact of SGLT2 inhibitors on hemoglobin (Hb) and hematocrit (Hct) levels in patients with type 2 diabetes [12]. However, these studies did not focus specifically on patients with CKD.
Some studies have explored the effect of SGLT2 inhibitors on anemia-related parameters in CKD patients [13–15]. While these studies reported similar findings, the anemia-related outcomes were not primary endpoints. One trial, despite its small sample size, demonstrated increases in reticulocyte count, Hb, Hct, and erythropoietin as primary endpoints in CKD patients treated with canagliflozin [16]. Although the results of these clinical studies have been reported individually, they have not been quantitatively integrated into a single conclusion. Therefore, the evidence is somewhat weak for definitively asserting the anemia-related effects of SGLT2 inhibitors in CKD patients.
This study aimed to evaluate the impact of SGLT2 inhibitors on anemia in CKD patients through a systematic review and meta-analysis. Specifically, we assessed changes in Hb and Hct levels in participants treated with SGLT2 inhibitors compared to control groups. Given the importance of anemia treatment in CKD patients, the new evidence we intend to present through this study will have substantial clinical implications.
2. Materials and Methods
2.1. Search Strategy
We conducted a comprehensive search of online databases, including PubMed, EMBASE, Cochrane Library, and Web of Science. The following search terms were used: “empagliflozin,” “dapagliflozin,” “canagliflozin,” “SGLT2 inhibitors,” “kidney failure,” “renal failure,” “Chronic Kidney Disease,” “kidney disease.” We searched the bibliographies of the retrieved articles. The relevant reviews were manually searched to identify additional eligible studies. No restrictions were applied to the type of publication, and the search was completed on February 13, 2024.
2.2. Selection of Studies
The review and selection process for inclusion in the systematic review was performed independently by two authors. The inclusion criteria were as follows: (1) prospective controlled trials; (2) Use of empagliflozin, dapagliflozin, or canagliflozin; (3) patients with CKD; and (4) anemia-related data (e.g. Hb, Hct, and ferritin). Other SGLT2 inhibitors were excluded as they are not indicated for patients with CKD.
Disagreements regarding article inclusion were resolved through discussion. For trials with multiple publications, data were extracted from the most comprehensive report, with additional publications used solely for clarification.
The study protocol for this meta-analysis was registered in the International Prospective Register for Systematic Reviews (PROSPERO).
2.3. Data Extraction
Two authors independently reviewed the full text of each included study. The following data were extracted: number of participants, patient characteristics, treatment details (dose regimen and periods), and anemia-related outcomes.
2.4. Risk of Bias (RoB) Assessment
The RoB in the included studies was assessed independently by two authors using the Cochrane RoB 2 criteria: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result [17]. Discrepancies between reviewers were resolved through discussion.
2.5. Meta-Analysis and Statistical Analysis
We compared changes in Hb and Hct levels between participants treated with SGLT2 inhibitors (empagliflozin, dapagliflozin, or canagliflozin) and those treated with placebo or other hypoglycemic agents. Statistical analyses were performed using a fixed-effects model (Mantel–Haenszel method) or a random-effects model (DerSimonian–Laird method), depending on the heterogeneity of the data [18, 19]. Sensitivity analyses were conducted by sequentially excluding each study to evaluate its impact on the meta-analysis result.
Study heterogeneity was assessed using the χ2 test (employing Q statistics) and quantified by calculating I2 values [20]. Publication bias was examined using Begg’s rank-correlation test and Egger’s regression test [21, 22].
All statistical analyses were performed using Comprehensive Meta-analysis Software (Version 2; CMA 26526; Biostat, Englewood, NJ, USA). Statistical tests were two-sided, and a value of p < 0.05 was considered statistically significant. Additionally, the PRISMA 2020 checklist was used as presented in Supporting Table S1.
3. Results
3.1. Study Characteristics and RoB
A total of 1631 articles were identified in the literature. After removing duplicates, 1167 articles remained for title and abstract screening. Of these, 1056 articles were excluded, and the full texts of the remaining 111 articles were assessed for eligibility. An additional 104 articles that did not meet the inclusion criteria were excluded, resulting in seven articles being included in this systematic review (Figure 1). One article was excluded from the meta-analysis due to an inconsistent data format [23].
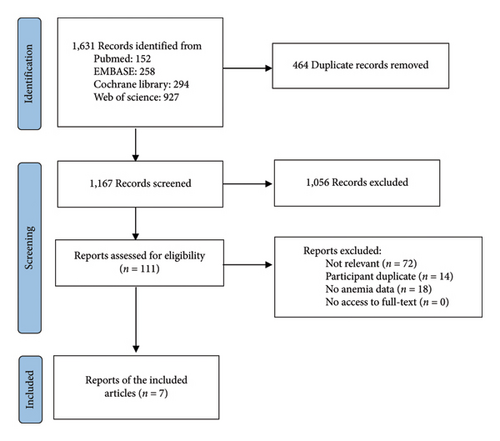
Details of each study are presented in Table 1. The severity of CKD varied slightly among the included studies. In three studies, patients with CKD along with type 2 diabetes or cardiovascular disease were included (Table 1).
| Study | Participants | Duration | Intervention | No (M/F) | Change in hemoglobin (SD or 95% CI) | Change in the hematocrit (SD or 95% CI) | Risk of bias |
|---|---|---|---|---|---|---|---|
| Yale et al. [23] | T2DM and eGFR 30–50 | 52 weeks |
|
|
|
|
Low risk |
| Wanner et al.; EMPA-REG outcome [13] | T2DM, CVD, and eGFR < 60 | 30 months | EMPA 10 or 25 PLacebo |
|
|
|
Low risk |
| Takashima et al. [14] | eGFR 45–89 | 52 weeks |
|
|
|
|
Low risk |
| Pollock et al.; DELIGHT [15] | eGFR 25–75 and UACR 30–3500 | 24 weeks |
|
|
|
|
Low risk |
| Cherney et al.; DIAMOND [24] | eGFR > 25 and UACR 500–3500 | 6 weeks ∗∗ |
|
|
|
|
Low risk |
| Hiramatsu et al. [25] | T2DM and eGFR 30–60 | 36 months |
|
|
|
|
Some concerns |
| Koshino et al.; DAPA-CKD [26] | eGFR 25–75 and UACR 200–5000 | 32 months |
|
|
|
|
Low risk |
- Abbreviations: CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); T2DM, type 2 diabetes mellitus; UACR, urinary albumin-to-creatinine ratio (mg/g).
- ∗Presented as mean percent change.
- ∗∗Crossover trial.
The RoB assessments for each study, including domain judgments and their support rationale, are provided in Table 1 and Supporting Table S2.
3.2. Meta-Analysis of Hb and Hct Levels
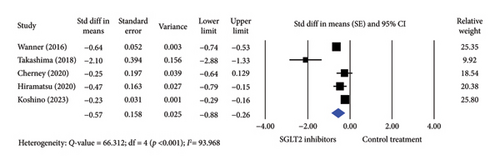
Five studies were included in the analysis of Hb levels, comprising 3326 participants treated with SGLT2 inhibitors and 2798 participants in control groups. Treatment with SGLT2 inhibitor significantly increased Hb levels compared to controls using a random-effects model (standard difference in means [SE] = −0.350; 95% confidence interval [CI] −0.401–0.299) (Figure 2). A significant difference was also observed when the fixed-effects model was applied (SE = −0.575; 95% CI −0.884–0.265).
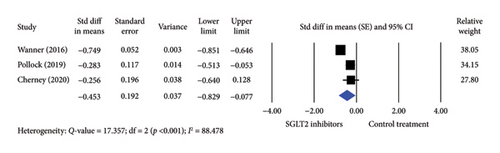
For Hct levels, three studies involving 1339 participants treated with SGLT2 inhibitors and 785 controls were included. SGLT2 inhibitor treatment significantly increased Hct levels compared to controls using a random-effects model (SE = −0.453; 95% CI −0.829–0.077) (Figure 3). Similarly, a significant difference was observed with a fixed-effects model (SE = −0.648; 95% CI −0.739–0.557).
3.3. Sensitivity Analyses and Publication Bias
Sensitivity analysis was performed by recalculating all findings after sequentially omitting each study included in the meta-analysis. The results remained consistent throughout this process (data available upon request). Publication bias was assessed using Begg’s and Egger’s tests. Both tests revealed no evidence of publication bias (Supporting Table S3).
4. Discussion
This systematic review and meta-analysis examined the effects of SGLT2 inhibitors, including empagliflozin, dapagliflozin, or canagliflozin, in patients with CKD. Our findings demonstrated that Hb and Hct levels significantly increased with SGLT2 inhibitor therapy compared to controls.
The observed increase in Hb and Hct levels with SGLT2 inhibitors is associated with renal and cardiovascular protection, likely explained by improved tissue oxygen delivery [27, 28]. The diuretic effect of SGLT2 inhibitors, which reduces plasma volume, may also contribute to this increase [29, 30]. In addition, these drugs may stimulate erythropoietin secretion by decreasing renal fibrosis and enhancing the viability of erythropoietin-secreting cells [27, 31].
Previous studies have shown that the anemia-correcting effects of SGLT2 inhibitors vary with the duration of treatment. For instance, one clinical trial reported a gradual increase in Hct levels with dapagliflozin, reaching a maximum approximately 4 months after treatment initiation [26]. Another study administering 100 mg of canagliflozin daily reported Hb increases of 5.3% and 6.5% and Hct increases of 6.0% and 6.6% from baseline at 26 and 52 weeks, respectively [23, 32]. In our meta-analysis, most included studies had treatment durations ranging from 6 to 36 months, except for one study with a 6 week follow-up period [24]. This allowed us to evaluate Hb and Hct levels after the effects of SGLT2 inhibitors had stabilized.
As shown in Table 1, a study comparing two doses of canagliflozin found greater increases in Hb and Hct levels with the 100 mg dose compared to the 300 mg dose [23]. These results aligned with the findings from the same participants at week 26 [32]. A meta-analysis of patients with type 2 diabetes also revealed that only dapagliflozin showed a dose-dependent increase in Hct levels, whereas no significant differences were observed with varying doses of canagliflozin, empagliflozin, and ipragliflozin [12]. Although higher doses of SGLT2 inhibitors may be necessary for other clinical outcomes, their incremental benefit in anemia correction appears minimal.
This study has several limitations. First, changes in Hb and Hct levels were not the primary outcomes in the included studies. Second, the analysis did not account for the range of comorbidities among participants. Third, significant heterogeneity was observed, likely due to variations in study protocols. It is necessary to conduct follow-up studies that address these limitations in order to evaluate the effectiveness of SGLT2 inhibitors for anemia treatment in CKD patients.
5. What Is New and the Conclusion
In conclusion, the results of our meta-analysis revealed a significant effect of SGLT2 inhibitors in increasing Hb and Hct levels in patients with CKD compared to controls. In particular, the anemia-correcting benefits can be achieved with relatively low doses, minimizing potential toxicity. Therefore, SGLT2 inhibitor treatment is significantly helpful for patients with CKD to correct anemia and its effect on renal or cardiovascular outcomes.
Nomenclature
-
- CKD
-
- Chronic kidney disease
-
- CI
-
- Confidence interval
-
- Hb
-
- Hemoglobin
-
- Hct
-
- Hematocrit
-
- SGLT2
-
- Sodium-glucose cotransporter 2
-
- SE
-
- Standard difference in means
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
H.D.C. and S.W.L. searched and collected the data. H.D.C. conceived the study and performed the analysis. H.D.C. and S.W.L. wrote and reviewed the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government, Ministry of Science, and ICT (Grant No. 2022R1F1A1074583).
Supporting Information
Table S1. PRISMA checklist.
Table S2. Risk of bias.
Table S3. Publication bias.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.




