Determining the 90% Effective Dose of Remimazolam in Terms of Inhibiting Responses to Upper Gastrointestinal Endoscopy Insertion in Elderly Patients: A Double-Blind Study Utilizing a Biased Coin Up-and-Down Sequential Method
Abstract
Background: Remimazolam is a good option for anesthesia in elderly patients undergoing gastrointestinal (GI) endoscopy procedures because of its rapid onset, short metabolic duration, and extensively documented safety profile. However, the accurate clinical dosage of these agents has yet to be determined. The objective of this research was to examine the efficacy of the 90% effective dose (ED90) of remimazolam in suppressing the responses of elderly patients during the insertion phase of upper GI endoscopy.
Methods: We enrolled 53 individuals aged 65– 85 years who underwent upper GI endoscopy and were anesthetized with an intravenous bolus of remimazolam. After initiating an initial dose of 0.35 mg/kg remimazolam, subsequent adjustments were made on the basis of the patient’s response, employing an up-and-down sequential allocation using a biased coin design. The primary outcome was the ED90 of the remimazolam infusion for inhibiting the response to upper GI endoscope insertion. Adverse reactions during the perioperative period were observed and recorded.
Results: The ED90 of remimazolam for upper GI endoscope insertion in elderly patients was 0.400 mg/kg (95% CI = 0.348–0.524). Stable circulation was maintained in all patients, and no serious adverse events were observed during sedation. Satisfaction levels were high among the participants: Patients reported a satisfaction score of 4.98 ± 0.14 points, anesthesiologists rated their satisfaction at 4.91 ± 0.35 points, and endoscopists expressed a satisfaction level of 4.89 ± 0.38 points (based on a total score of 5 points, with a minimum of 1 point).
Conclusion: Administration of remimazolam for upper GI endoscopy in elderly patients was found to be both safe and effective. A single intravenous bolus at an ED90 dose of 0.556 mg/kg effectively suppressed the response to the procedure.
Trial Registration: Chinese Registry of Clinical Trials: ChiCTR2200062535
1. Introduction
The prevalence of gastrointestinal (GI) endoscopy diagnosis and treatment in elderly patients is increasing. However, due to age-related declines in organ function and comorbidities, sedation poses a significantly heightened risk of respiratory and circulatory complications in this population [1–3]. The short duration of action of propofol, a good hypnotic with rapid onset, makes it one of the most commonly used intravenous anesthetics during endoscopic procedures [4–8]. However, it was reported that transient hypoxia occurs in 3%–32% of patients treated with propofol sedation via GI endoscopy and that transient hypotension occurs in 7%–28% of patients [9–12]. Older patients constitute a vulnerable group that is more susceptible to hypoxia during GI endoscopy performed under drug-induced sedation [12, 13]. Indeed, older people are more vulnerable to the hemodynamic instability caused by propofol because of a decreased initial distribution volume and increased sensitivity to the drug [14–16]. It has been reported that postinduction hypotension is associated with increased mortality, and that intraoperative hypotension causes reinfarction in 20% of patients with myocardial infarction [17, 18]. During the sedation process for upper GI endoscopy, the close proximity of the procedure to the respiratory tract increases the probability of respiratory system–related adverse events [19, 20]. Overall, the occurrence of hypotension and hypoxemia following the administration of anesthesia impairs the feasibility of GI endoscopy procedures in elderly patients. Given the well-documented risks associated with propofol, our study explores remimazolam as a potentially safer alternative owing to its unique pharmacological properties and favorable safety profile.
Remimazolam, a novel short-acting gamma-aminobutyric acid (GABA) receptor agonist, exerts its sedative effects by inducing inward chloride ion flow through the activation of GABA receptors, thereby inhibiting neuronal electrical activity [21–23]. The advantages of remimazolam include rapid onset of action, short recovery time, stable hemodynamics, and minimal hepatic and renal metabolism [21, 24–26]. Compared with propofol, the administration of remimazolam during outpatient GI endoscopy has been shown to maintain stable respiratory and circulatory function without compromising recovery quality [27, 28]. Given the aforementioned advantages of remimazolam, its potential as a superior option for sedation during surgical procedures in elderly patients may be considered. According to the manufacturer’s instructions, the dose of remimazolam is limited owing to the long induction time and difficulty in achieving the desired target sedation depth [2]. Therefore, a more reasonable dose on the basis of body weight is recommended [29]. Limited research has been conducted on the utilization of remimazolam in elderly patients undergoing gastroscopy procedures, and the optimal dose for inducing sedation in individuals aged older than 65 years remains uncertain. The objective of this study was to ascertain the 90% effective dose (ED90) of remimazolam for elderly patients aged 65–85 years by employing a biased coin design, which offers superior accuracy in estimating the ED90 compared with an up-and-down method [30, 31].
2. Materials and Methods
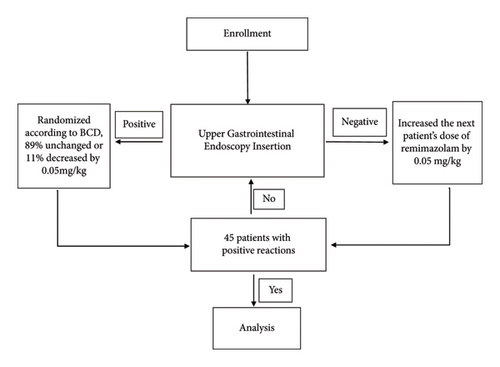
The flow diagram of this study is provided in Figure 1.
2.1. Study Population
This was a single-center, randomized controlled trial that was registered with the Chinese Clinical Trial Registry on August 11, 2022. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. All participants provided written informed consent prior to participation. This project was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LZ24H090002 and the Zhejiang Province Traditional Chinese Medicine Science and Technology Project under Grant No. 2023ZL595.
Informed consent was obtained from each patient. During the trial, the anesthetists performed sedation in a blinded manner. The sedative medications were prepared by specialized anesthesia nurses and then drawn into a 20-mL syringe, which was subsequently transferred to the anesthesiologist for administration.
A total of 53 patients underwent upper GI endoscopy under sedation at Shulan Hospital from January 2022 to August 2023. The eligibility criteria were as follows: (1) aged between 65 and 85 years; (2) American Society of Anesthesiologists (ASA) I–II classification; (3) body mass index (BMI) ranging from 18 to 25 kg/m2; (4) no history of language disorders; and (5) able to participate effectively in the examinations. Patients were ineligible for the study if they met any of the following criteria: (1) chronic pain managed with the prolonged use of analgesics or psychiatric medications such as opioids, NSAIDs, sedatives, or antidepressants; (2) alcohol addiction; (3) history of abnormal recovery from surgical anesthesia; (4) history of hypertension with systolic blood pressure (SBP) exceeding 180 mmHg or diastolic blood pressure (DBP) exceeding 110 mmHg; (5) chronic lung disease with oxygen saturation (SpO2) below 95%; (6) history of central nervous system disease; (7) recent use of sedative drugs within 24 h prior to surgery; (8) anticipated difficult airway, allergy, or contraindication to relevant anesthetic medications; (9) emergency examination; (10) pregnancy or parturition; (11) clinical or imaging evidence of gastric obstruction, intestinal anatomical changes, high risk of bleeding, or unstable circulation; and (12) participation in other clinical studies within the preceding 3 months.
The criteria for withdrawal were as follows: (1) complications such as reflux aspiration, anaphylactic shock, laryngeal spasm, or similar adverse reactions following sedative administration, rendering the patient unable to complete the experimental procedure; and (2) life-threatening conditions such as GI bleeding, perforation, or similar critical events during the endoscopy process, leading to discontinuation of the examination.
2.2. Procedures
All patients commenced fasting and liquid fasting for 8 h and 3 h prior to surgery, respectively, and preoperative medication was not administered. Upon admission, the patients were subjected to continuous ECG monitoring, blood pressure measurement of the right upper limb, and pulse oxygen saturation (SpO2) monitoring of the left index finger. Venous access to the left upper limb was established via continuous infusion of 0.9% sodium chloride solution at a rate of 5–6 mL/kg/h. Following the oral administration of lidocaine gel, the patient was placed in the left lateral decubitus position. Oxygen inhalation was administered via a nasal catheter at a rate of 3 L/min, and relevant emergency drugs and equipment were prepared.
The induction process began following the initial blood pressure assessment. A predetermined volume of 30 mL of remimazolam was administered intravenously at a controlled rate over a period of 30–40 s by a proficient anesthesiologist. The anesthesiologists assessed the anesthetic impact and monitored any unfavorable reactions. Subsequently, upper GI endoscopic procedures were initiated by a senior gastroenterologist when the patient’s Modified Observer’s Assessment of Alerts/Aedations (MOAA/S) score was equal to or less than 3. The patient’s physical response during placement of the upper GI endoscope was observed and documented.
Physiological parameters, including heart rate (HR), SBP, DBP, mean blood pressure (MBP), SpO2, and respiratory rate (RR) (measured via impedance pneumography), were monitored. Furthermore, circulatory and respiratory variables were assessed and documented at various time points: before induction (T0), after induction (T1), during upper GI endoscopy insertion (T2), and at endoscope withdrawal (T3). Any instances of movement or anesthesia-related complications were noted.
Following the surgical procedure, the patient was transferred to the postanesthesia care unit (PACU). A dedicated anesthesia nurse monitored and documented the patient’s vital signs and level of consciousness via the MOAA/S score, the pain level, and the presence of nausea, vomiting, dizziness, gait, and other pertinent conditions. In addition, the nurse assessed the satisfaction of the patients, anesthesiologists, and operating physicians. The discharge criterion for the PACU was an Aldrete score of 10, and the timing of discharge was noted. The same nurse subsequently conducted a 24 h postdischarge follow-up with each patient via telephone.
Remedial measures: In cases in which physical activity reactions during upper GI endoscopy hindered the examination, anesthesia failure was considered to have occurred. To address this, remedial sedation via remimazolam was administered at a rescue dose of 0.05 mg/kg. After a one-minute observation period, the endoscopic procedure was carried out, and the data were assessed. If sufficient sedation was not achieved after three additional doses of remimazolam, intravenous injection of propofol medium/long-chain fat emulsion (1–2 mg/kg) was administered as a rescue measure to facilitate completion of the examination.
2.3. Clinical Outcomes and Safety Assessments
The primary endpoint was successful placement of the upper endoscope in the cardia. The secondary endpoints were body movement, respiratory depression (i.e., RR < 10 beats/min), hypoxemia (i.e., SpO2 < 95%), hiccups, hypotension (i.e., SBP < 90 mmHg), hypertension, and arrhythmia. The scoring criteria for anesthesia satisfaction from both doctors and patients constitute a comprehensive system for evaluating the effectiveness of anesthesia and patient experience. The doctor’s scoring criteria focus on the technical aspects of the anesthesia process and are divided into five levels. The highest level, five points, represents “very satisfied,” indicating a smooth induction of anesthesia without agitation, moderate depth during maintenance, and a stable recovery period with normal monitoring. As the score decreases, the instability and discomfort during anesthesia gradually increase until reaching one point for “very dissatisfied,” which signifies anesthesia failure or severe complications that threaten the patient’s safety. In contrast, the patient’s scoring criteria emphasize personal feelings and experiences, also using a five-point scale. A score of five points for “very satisfied” means that the patient felt minimal pain and discomfort during anesthesia and recovered quickly with no side effects. As the score decreases, patients may express dissatisfaction with details such as waiting time before anesthesia or minor discomfort after anesthesia, culminating in one point for “very dissatisfied,” which represents extreme dissatisfaction with the anesthesia process, possibly due to severe complications or extremely poor anesthesia effects that caused significant pain during surgery.
During the administration of anesthesia, symptomatic treatment must be provided in response to adverse reactions such as hypotension, hypoxia, and arrhythmia. In the event of hypotension, prompt administration of a 200-mL rapid infusion of 0.9% sodium chloride is recommended. If this intervention is ineffective, appropriate vasoactive medications, such as ephedrine at a dose of 6 mg or phenylephrine at a dose of 40 μg via intravenous bolus, should be administered on the basis of the specific clinical circumstances. In cases of bradycardia (HR < 50 beats/min), it is advisable to administer an intravenous injection of atropine at a dose of 0.5 mg. Furthermore, in instances of hypoxemia or respiratory depression, increasing the flow of oxygen and providing jaw support are important. If necessary, the endoscope should be temporarily suspended while mask-pressurized oxygen is administered.
This research employed a double-blind design. The dose of remimazolam administered to each patient undergoing deep sedation/anesthesia was prepared by a specialized individual and provided to the anesthesiologist responsible for administering the anesthesia. The anesthesiologist, patient, surgeon, and follow-up nurse were unaware of the specific dose of remimazolam. A positive reaction was characterized by the absence of choking, coughing, nausea, vomiting, and/or motor response during insertion of the upper GI endoscope into the pharyngeal cavity, indicating successful anesthesia. Conversely, a negative reaction, indicating failed anesthesia, was recorded if these criteria were not met. The outcomes of the anesthesia were assessed and documented by the anesthesiologist conducting the procedure.
The biased coin up-and-down (BCUD) sequential method allows for efficient dose-finding by adaptively adjusting doses on the basis of responses, minimizing the number of subjects required while maintaining statistical rigor. In order to determine the ED90, a minimum of 45 successful sedation cases was required [32]. As a result, patients were prospectively recruited until this target was achieved. At that point, 44 sealed envelopes containing random assignments of remimazolam concentrations were disclosed. The BCUD method was employed, with a target probability (Γ) of 0.9 established for maintaining the dosage necessary for deep sedation/anesthesia, calculated using R software. Following each successful sedation, the next patient had an 11% probability (b = (1 − Γ)/Γ) of experiencing a dose reduction of 0.05 mg/kg, while there was an 89% probability of the current dose being maintained. The study concluded upon reaching 45 successful responses, with the envelopes being sequentially opened to indicate dosage adjustments (−1 for a dose reduction, 0 for no change) until the predetermined threshold was met.
2.4. Statistical Analysis
Continuous variables are presented as either the mean ± standard deviation (SD) or median and interquartile range (IQR) for normally distributed and nonnormally distributed variables, respectively. Categorical variables are reported as frequencies and percentages. Repeated measures analysis of variance was used to compare measurement data at various time points.
Numerical disparities among groups were evaluated via a t-test. The estimation was computed via R software, and the 95% confidence interval (CI) was determined from bootstrap samples through 2000 iterations. The statistical analyses were performed via SPSS Version 26.0 and R software Version 4.0.3. Graphical representations were generated via GraphPad Prism 8.
3. Results
3.1. Patient Characteristics
This study included 53 participants, with an average age of 70.1 years, and 34 (64.2%) were female. Among the cases, 17.0% were classified as ASA I, and 83.0% were classified as ASA II. In addition, 3.8% of the patients underwent simple gastroscopy and 96.2% underwent GI endoscopy. Further details can be found in Table 1.
| Item | Value |
|---|---|
| Gender, female | 34 (64.2%) |
| Age, years | 70.1 ± 3.9 |
| Height, cm | 162.4 ± 7.4 |
| Weight, kg | 60.2 ± 8.4 |
| BMI, kg/m2 | 22.7 ± 2.1 |
| ASA grade | |
| I | 9 (17.0%) |
| II | 44 (83.0%) |
| Surgery type | |
| Gastroscopy | 2 (3.8%) |
| GI endoscopy | 51 (96.2%) |
- Abbreviations: ASA = American Society of Anesthesiologists; BMI = body mass index.
3.2. Procedure Characteristics
The BCUD sequence of the remimazolam dose is shown in Figure 2, and the ED90 was 0.400 mg/kg, with a 95% CI of 0.324–0.524 mg/kg. Table 2 displays the positive response of all patients to each actual dose of remimazolam, as well as the adjusted positive response by PAVA.
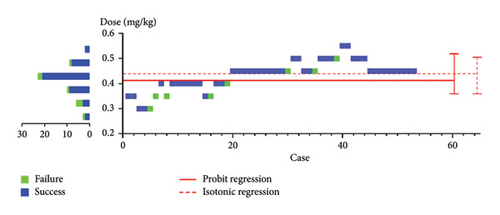
 ) indicates a negative reaction (anesthesia failure), and the blue square (
) indicates a negative reaction (anesthesia failure), and the blue square ( ) indicates a positive reaction (anesthesia success). The red line (
) indicates a positive reaction (anesthesia success). The red line ( ) indicates the estimated ED90 and 95% CI. The right ordinate shows that the sample size reached the target concentration when BCUD was used. Thus, even a study with a small sample size could effectively obtain an accurate ED90.
) indicates the estimated ED90 and 95% CI. The right ordinate shows that the sample size reached the target concentration when BCUD was used. Thus, even a study with a small sample size could effectively obtain an accurate ED90.| Remimazolam dose (mg/kg) | Number of successfully placed endoscopes (n) | Total cases (n) | True-positive rate | Adjusted positive rate |
|---|---|---|---|---|
| 0.30 | 2 | 3 | 0.667 | 0.583 |
| 0.35 | 3 | 6 | 0.500 | 0.583 |
| 0.40 | 9 | 10 | 0.900 | 0.900 |
| 0.45 | 21 | 23 | 0.913 | 0.901 |
| 0.50 | 8 | 9 | 0.888 | 0.901 |
| 0.55 | 2 | 2 | 1.000 | 1.000 |
Table 3 summarizes the anesthesia-related adverse reactions that occurred. All 53 patients completed the endoscopic procedure and follow-up. Six patients experienced hypotension, 1 patient experienced bradycardia, 7 patients experienced hiccups, and 2 patients experienced hypoxia.
| Adverse reactions | Elderly patients aged 65–85 years (n = 53) |
|---|---|
| Total | 22 (41.5%) |
| Hypotension | 6 (11.3%) |
| Anoxia | 2 (3.7%) |
| Respiratory depression | 0 |
| Injection pain | 2 (3.7%) |
| Hiccups | 7 (13.2%) |
| Nausea and vomiting | 0 |
| Bradycardia | 5 (9.4%) |
3.3. Anesthesia Satisfaction
As presented in Table 4, a total of 53 satisfaction surveys were completed by the patients, anesthesiologists, and endoscopists, and the scores ranged from 1 to 5. The overall patient satisfaction score was 4.98 ± 0.14, the anesthesiologist satisfaction score was 4.91 ± 0.35, and the endoscopist satisfaction score was 4.89 ± 0.38. In addition, there was one patient whose anesthesia satisfaction score was less than 4 points. The patient was unable to successfully undergo the gastroscopy procedure, despite receiving three additional doses of remimazolam for sedation and ultimately required propofol to complete the gastroscopy examination.
| Anesthesia satisfaction score (points) | Patients (n) | Anesthesiologists (n) | Endoscopists (n) |
|---|---|---|---|
| 5 | 52 | 49 | 48 |
| 4 | 1 | 3 | 4 |
| 3 | 0 | 1 | 1 |
| 2 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 |
3.4. Anesthesia Recovery
There were 2 (3.8%) patients who underwent simple gastroscopy, and 51 (96.2%) patients who underwent GI endoscopy. The recovery time of seven individuals exceeded 30 min (Table 5).
| Events | Time (min) |
|---|---|
| Gastroscopy operation time | 7.8 ± 4.3 |
| GI endoscopy operation time | 20.0 ± 1.3 |
| Gastroscopy anesthesia recovery time | 29.5 ± 2.2 |
| GI endoscopy anesthesia recovery time | 16.8 ± 1.4 |
- Note: The anesthesia recovery time was the time from endoscope withdrawal to an Aldrete score of 10. The operation time was the time from endoscope insertion to endoscope withdrawal.
3.5. Circulating and Respiratory System Activity During Anesthesia
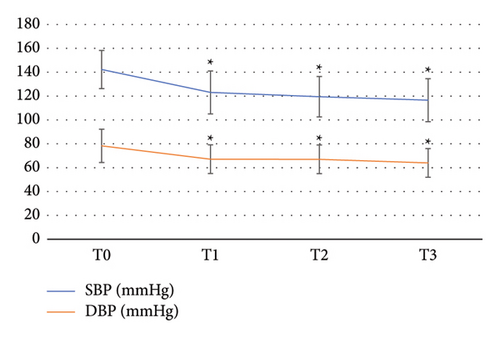
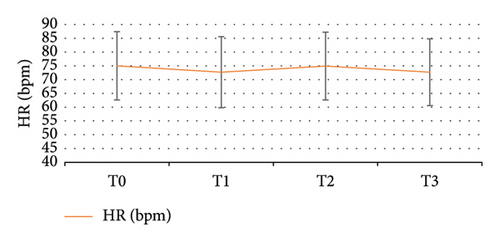
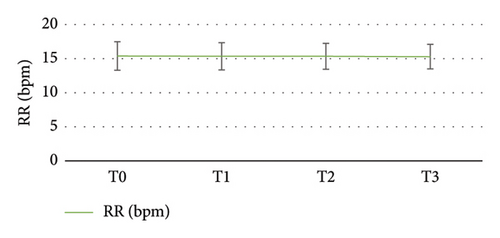
There were four significant time points for observation: prior to induction (T0), following induction (T1), during upper GI endoscopy insertion (T2), and at the withdrawal of the endoscope (T3). Figure 3 illustrates the alterations in SBP and DBP at each time point, indicating a slight decrease in blood pressure following induction. Repeated measures analysis of variance revealed a significant difference in SBP between T1, T2, and T3 compared with T0 (p < 0.05), but no significant variance was found among T1, T2, and T3, indicating a stable postbaseline SBP level. The fluctuations in HR at each observation time point are depicted in Figure 4, demonstrating an increase after induction, yet the mean HR at each time point remained within the normal range. Repeated measures analysis of variance indicated no statistically significant differences in HR changes at any time point. The changes in the RR at each observation time point are presented in Figure 5, with no significant variation throughout the diagnosis and treatment process or at any time point.
4. Discussion
To date, the application of the Dixon–Mood up-down sequential method (DM-UDM) in clinical studies aimed at determining the ED50 of remimazolam for the inhibition of various surgical stimuli has been limited. This method typically necessitates a larger sample size, often two to three times greater than that required for the BCUD sequential method. The present study aims to utilize the BCUD method to identify the ED90 of remimazolam in mitigating responses in patients undergoing upper GI endoscopic procedures. A significant advantage of this approach, when compared to traditional methodologies, is its substantially reduced sample size requirement, which enhances feasibility and minimizes patient risk, thus rendering it more ethically defensible. Furthermore, the extrapolation of the ED50 value obtained from the DM-UDM method is likely to introduce considerable inaccuracies. As a result, statisticians consistently recommend the BCUD method for achieving a more precise estimation of the higher quantiles of the ED [30].
We observed that the ED90 induction dose for remimazolam, which effectively inhibits movements in elderly patients undergoing gastroscopy, was 0.4 mg/kg (95% CI 0.348–0.524). Independent administration of remimazolam is both safe and feasible for gastrectomy in elderly patients. In our previous studies, we discovered that the ED90 of remimazolam for inhibiting upper GI endoscopic implantation reactions in adults aged 18–65 years was 0.556 mg/kg, with a 95% CI ranging from 0.399 to 0.578. This dosage exceeds the recommended amount for elderly individuals. Remimazolam is a benzodiazepine with a unique mechanism of action that involves rapid, nonhepatic, and nonrenal metabolism, primarily through tissue esterases. This distinct metabolic pathway indicates that remimazolam is not significantly impacted by age-related reductions in hepatic or renal function, which often affect the clearance and elimination of many other drugs in elderly individuals. As a result, the pharmacokinetics of remimazolam is relatively unaffected by age, allowing similar doses to be used across different age groups. In addition, the study revealed that age had a minor influence on the drug’s efficacy and safety profile. These findings suggest that the use of remimazolam in elderly patients is safe and effective, with no significant escalation in associated complications compared with that in younger adults [33]. While the ED90 (the dose required to inhibit upper GI endoscopic implantation reactions in 90% of patients) for remimazolam in adults aged 18–65 years was 0.556 mg/kg, the dose used in elderly patients (0.4 mg/kg) was slightly lower [33]. This could be because elderly patients may be more sensitive to the sedative effects of benzodiazepines due to changes in pharmacodynamics related to aging, such as decreased body mass, altered receptor sensitivity, and changes in drug distribution. Therefore, a slightly lower dose may be sufficient to achieve the desired level of sedation in elderly patients.
An increasing number of elderly patients are undergoing GI endoscopy examinations; these patients often present with comorbidities such as cardiovascular or respiratory diseases or experience electrolyte imbalances due to colon preparation, which renders them more vulnerable to circulatory system depression during surgical anesthesia. In our previous study involving adults aged 18–65 years, a total of seven patients (13%) exhibited mild hypotension [33]. This study demonstrated that among elderly patients, 6 (11.3%) exhibited transient hypotension following the administration of remimazolam during gastroscopy procedures. However, these hypotensive episodes spontaneously resolved after insertion of the endoscope, indicating that remimazolam ensures relatively stable hemodynamics and a low frequency of adverse reactions when used as an anesthetic agent for gastroscopy in elderly patients. The reduced occurrence of hypotension has significant advantages for postoperative recovery and long-term prognosis [34]. Hence, remimazolam is suitable for anesthesia during gastroscopy in geriatric patients and has a favorable impact on the prompt postoperative recovery of elderly individuals. In contrast to our previous trials, a higher incidence of bradycardia was observed in the elderly population (5 [9.4%] vs. 1 [1.9%] for individuals aged 65–85 years vs. adults aged 18–65 years). However, no statistically significant difference was found. This finding is likely attributed to the heightened vulnerability of elderly patients to hemodynamic side effects, necessitating meticulous attention during the sedation process [33, 35].
In the present study, hypoxemia occurred in two elderly patients aged 65–85 years during sedation with remimazolam and was effectively alleviated by implementing the jaw thrust maneuver. In a previous study involving 54 adult patients aged 18–65 years, no instances of hypoxemia were observed. The RRs at various time points in the present study were comparable to those of elderly patients (approximately 15 breaths per minute), with no statistically significant differences [33]. In contrast to propofol, remimazolam is safe and efficient for sedation in elderly patients undergoing GI endoscopy, with a decreased incidence of sedation-related adverse effects, particularly hemodynamic events and respiratory depression [36].
Compared with that in adults, the incidence of hiccups in elderly population was significantly lower (7 [13.2%] vs. 13 [24.1%] for individuals aged 65–85 years vs. adults aged 18–65 years). Benzodiazepines have been implicated in the occurrence of drug-induced hiccups, although the exact underlying mechanism has not been fully elucidated [37]. Further investigations are warranted to address this issue. Given the potential for hiccups to increase the likelihood of gastroesophageal reflux, caution should be exercised when administering remimazolam to patients who are susceptible to reflux and aspiration, such as those with gastroesophageal reflux disease (GERD), which coincidentally also contributes to the onset of hiccups [37].
The average awakening time for gastroscopy in this study was approximately 30 min; for colonoscopy, it was approximately 17 min. Compared with previous reports involving propofol and midazolam for GI endoscopy procedures, the efficacy of remimazolam was comparable for geriatric gastroscopy anesthesia [22, 38]. In this study, two patients underwent gastroscopy for a duration of approximately 8 min, whereas 51 patients underwent both gastroscopy and colonoscopy for an average procedure time of approximately 20 min. The recovery time following gastroscopy anesthesia was prolonged in this study because of the shorter duration of the gastroscopy procedure after a single administration of medication. Owing to the absence of various long-term adverse reactions, such as postoperative nausea and vomiting (PONV) and postoperative delirium, the findings of the present study suggest that remimazolam is a suitable sedation option for elderly individuals. However, owing to the limited sample size in this study, validation of the incidence of adverse reactions in elderly patients using remimazolam necessitates a larger sample size.
The present study demonstrated that intravenous administration of remimazolam, on the basis of body weight and loading dose, effectively induced rapid and controllable sedation. This finding is particularly noteworthy as it responds to a pressing requirement for safe and effective anesthesia management in geriatric patients, who frequently face an elevated risk of complications attributable to their age and possible preexisting health issues.
This study has certain limitations, such as the use of a sample of relatively healthy elderly patients undergoing elective surgery. Thus, future investigations should prioritize high-risk elderly individuals, super-elderly individuals, and frail-elderly individuals. In addition, the study’s short-term follow-up period may have precluded the detection of delayed or long-term adverse effects associated with the use of remimazolam. Furthermore, the potential for observer bias within the procedural setting cannot be overlooked, as it may have influenced the assessment of outcomes and introduced another layer of complexity in interpreting the results. To mitigate this, more rigorous blinding procedures and standardized assessment protocols should be implemented in future studies. Finally, our study did not yield comprehensive pharmacokinetic data, which is typically crucial for accurately characterizing and comprehending the dose-exposure-response relationship. This deficiency has impeded our capacity to thoroughly interpret the findings, especially concerning the necessity and optimal timing for supplemental doses of remimazolam. Consequently, our conclusions may be constrained in their ability to offer definitive recommendations regarding dosage adjustments or the optimization of therapeutic outcomes. Before proposing any alterations to clinical practice, it is essential to undertake further large-scale multicenter studies to corroborate our findings.
5. Conclusion
The use of remimazolam in elderly patients undergoing upper GI endoscopy is safe and effective, and the ED90 of a single intravenous bolus of remimazolam that suppresses the response to this procedure in older adults is 0.4 mg/kg, with a 95% CI between 0.348 and 0.524 mg/kg.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Chaoliang Zhang and Pengfei Yin are co-first authors.
Funding
This project was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LZ24H090002 and Zhejiang Province Traditional Chinese medicine science and technology project under Grant No. 2023ZL595.
Acknowledgments
This project was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LZ24H090002 and Zhejiang Province Traditional Chinese Medicine Science and Technology Project under Grant No. 2023ZL595.
Open Research
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.




