Nutritional Retention in Frozen Tubifex (Tubifex tubifex) Stored at −20°C: A Comparative Analysis of Storage Durations up to 12 Weeks
Abstract
The utilization of live Tubifex (Tubifex tubifex) as a feed source in ornamental fish aquaculture is widely recognized for its high nutritional value. However, feeding live tubifex worms come with a higher risk of transmitting parasites, and precautions must be taken. So, necessitate feeding strategies have to be adopted which includes dietary diversification such as usage of tubifex in frozen form. This study aimed to evaluate the nutritional stability of frozen Tubifex over a 12-week period (85 days), stored at −20°C. Weekly evaluations were conducted to measure changes in key nutritional parameters, including crude protein, moisture, lipid, and ash content. The results indicated that crude protein content remained stable (p > 0.05) for the first 5 weeks, while lipid content showed no significant changes (p > 0.05) until the seventh week. Additionally, ash content was stable (p > 0.05) until the ninth week of storage. These findings suggest that frozen Tubifex can be safely stored for up to 36 days without significant nutritional degradation, offering a viable alternative to live feed in ornamental fish aquaculture this research supports the use of frozen Tubifex by mitigating the risks associated with live feed while ensuring optimal nutritional delivery to aquatic organisms.
1. Introduction
The rising global population and increasing demand for protein sources have driven advancements in commercial fish and shellfish production through aquaculture. As a result, the aquaculture sector has been growing at a faster pace than other animal food industries and is expected to become a key provider of high-quality protein in the near future [1]. Global aquaculture production reached an unprecedented 130.9 million tonnes, with 94.4 million tonnes being aquatic animals, accounting for 51% of the total aquatic animal production [2]. This rapid growth calls for sustainable feeding practices that minimize economic losses and environmental impact. In this context, nutritional research is essential to ensure animals receive high-quality feed to meet the needs of the expanding aquaculture industry [3]. Among the various feeding strategies employed in aquaculture, the use of live food, particularly Tubifex worms (Tubifex tubifex), has gained popularity due to their high nutritional value and ability to stimulate natural feeding behaviours in fish [4]. Tubifex worms are Oligochaeta annelids that thrive in environments rich in organic matter, such as sediments in freshwater and brackish ecosystems [5]. Their soft bodies are composed primarily of protein and essential fatty acids, making them an ideal food source for both juvenile and adult fish, especially in Aquariculture [6, 7]. Tubifex worms, also known as sludge worms, are a popular live food source in aquaculture, especially for young fish fry and small ornamental species [8]. These worms, which thrive in organically rich environments such as sewage and ponds, are known for their high protein content, making them an excellent source of nutrition [9]. The benefits of using Tubifex worms as natural feed include enhanced growth and health of fish larvae and cost efficiency in production. According to researcher [10], tubifex is rich in omega-3 (18:3 and 20:5) and omega-6 (18:2 and 20:4) fatty acids at rates of 18% and 22%, respectively, though it lacks DHA (22:6). Additionally, they are rich in fatty acids, promoting the maturation of fish. Aquaculture enthusiasts and fish breeders often prefer live Tubifex because they stimulate natural hunting behaviours in fish and provide an easily digestible and nutrient-rich food source, which promotes faster growth and enhances overall health.
One of the most significant challenges associated with the use of live Tubifex is the variability in its availability. Seasonal fluctuations, environmental changes, and overharvesting can lead to shortages of live feed, impacting fish farms and aquarists [11]. The potential for contamination is another major issue. Since Tubifex worms are often harvested from polluted environments, they can harbour pathogens, parasites, and toxins that pose risks to fish health and water quality [3]. The introduction of harmful microorganisms into aquaculture systems can lead to disease outbreaks, compromising fish stocks and resulting in economic losses. Additionally, maintaining live Tubifex in tanks requires careful attention to avoid introducing waste or decaying organic matter, which can lead to water fouling and additional management issues [12].
To mitigate these challenges, there is a growing interest in exploring alternative feeding strategies, including the use of frozen Tubifex [6]. Freezing is an effective preservation method that can significantly extend the shelf life of Tubifex while maintaining its nutritional quality [13]. The freezing process helps eliminate pathogens and parasites that may be present in live worms, reducing the risk of disease transmission to fish [14]. Furthermore, frozen Tubifex offers practical advantages, as it can be stored for extended periods and easily portioned, making it convenient for aquarists and fish farmers alike [15]. There are several advantages of using frozen tubifex as a food source for fish. Frozen tubifex worms are easy to store in the freezer and can be used whenever needed [16]. Unlike live tubifex, which require specific care and maintenance, frozen tubifex eliminates the need for feeding, housing, and handling live worms. It also eliminates the risk of entry of harmful parasites or diseases associated with live worms into the aquarium. It provides a safe and hygienic food option for fish, reducing the likelihood of contamination or infection.
Existing literature on the nutritional composition of Tubifex highlights its rich protein content, which typically ranges between 50% and 70% on its dry weight, making it an excellent protein source for fish [17]. However, there is limited research regarding the impact of freezing on the nutritional quality of Tubifex over time. Studies have shown that freezing can affect the texture and palatability of certain types of live food, but comprehensive investigations into the specific changes in nutritional composition during storage are lacking. Although freezing has been proposed as a solution, limited research exists on the long-term nutritional stability of frozen Tubifex. This study aims to fill this gap by evaluating the nutritional retention of frozen Tubifex over a 12-week storage period. The implications of this research extend beyond the immediate benefits of using frozen Tubifex as a feed source. By establishing the viability of frozen Tubifex as a reliable alternative, this study supports the sustainability of aquaculture practices by reducing reliance on live food and mitigating the risks associated with harvesting Tubifex from natural environments. Moreover, the findings will contribute to the broader understanding of feed quality management in aquaculture, emphasizing the importance of nutrition in promoting the health and growth of aquatic organisms.
2. Materials and Methods
2.1. Sample Collection and Preparation
A total of 600 g of live Tubifex worms were purchased from Silversea Aquarium, a local aquarium shop in Mumbai. The worms were carefully collected and transported under live conditions to the Aquaculture Laboratory at the ICAR-Central Institute of Fisheries Education (CIFE), Mumbai. The worms were transported in well oxygenated containers to overcome the transportation mediated stress. Upon arrival at the laboratory, the worms were thoroughly rinsed to eliminate any debris or contaminants. This collection process was carried out with strict protocols to maintain the vitality of the worms for the planned research activities. A sample of 5 g of Tubifex worms was taken and placed into each cell (5 cm × 5 cm × 5 cm) of an ice tray, to which 5 μL of water was added. Each ice tray with 9 cells. This process was repeated for all 13 ice trays (each ice tray contains 9 cells), which were prepared for subsequent analysis during the experiment. Each tray, containing the appropriate amount of sample, was frozen at a consistent temperature of −20°C to preserve the integrity of the worms throughout the study. The trays should be labelled with the freezing date and treatments numbers. To evaluate the nutritional value and proximate composition of the frozen Tubifex, analyses were conducted at every week. For each sampling, one ice tray with frozen tubifex (each replicate 15 g) was used to ensure accuracy and reliability in the results. This experimental design allowed for systematic monitoring of any changes in the nutritional profile of Tubifex during the storage period is Completely Randomised Design (CRD) includes 13 treatments with 3 replicates, ensuring a comprehensive understanding of its suitability as a preserved feed source for aquaculture (Table 1). The use of replicates helped ensure that the findings were statistically robust and representative of the overall trends observed throughout the freezing period.
| Treatments | Amount of sample (g) | |||
|---|---|---|---|---|
| T1 | 45∗ | T1R1 | T1R2 | T1R3 |
| T2 | 45∗ | T2R1 | T2R2 | T2R3 |
| T3 | 45∗ | T3R1 | T3R2 | T3R3 |
| T4 | 45∗ | T4R1 | T4R2 | T4R3 |
| T5 | 45∗ | T5R1 | T5R2 | T5R3 |
| T6 | 45∗ | T6R1 | T6R2 | T6R3 |
| T7 | 45∗ | T7R1 | T7R2 | T7R3 |
| T8 | 45∗ | T8R1 | T8R2 | T8R3 |
| T9 | 45∗ | T9R1 | T9R2 | T9R3 |
| T10 | 45∗ | T10R1 | T10R2 | T10R3 |
| T11 | 45∗ | T11R1 | T11R2 | T11R3 |
| T12 | 45∗ | T12R1 | T12R2 | T12R3 |
| T13 | 45∗ | T13R1 | T13R2 | T13R3 |
- ∗Each ice tray had 9 cells, with 5 g of sample each.
- #Three cells in each ice tray constitute one replicate of that particular treatment (from each replicate 15 g of sample has been used for analysis).
2.2. Proximate Composition Analysis
2.3. Statistical Analysis
The experimental data collected were subjected to statistical analysis using the Statistical Package for the Social Sciences (SPSS), version 27.0. To assess the significance of variations among the different sample groups, a one-way Analysis of Variance (ANOVA) was performed. This method was used to determine whether there were statistically significant differences (p < 0.05) between the means of the various samples over the course of the experiment. To further evaluate and pinpoint which specific groups differed, Duncan’s Multiple Range Test (DMRT) was employed as a post hoc analysis. This test helped to clarify where the significant differences occurred among the means of the samples, providing a more in-depth understanding of the variations. Statistical significance was established at a 5% probability level (p < 0.05), ensuring that any differences detected were not due to random chance. All experimental results were expressed as mean values accompanied by their corresponding standard errors (mean ± SE), which provided a measure of variability and reliability for each data set. The use of these statistical techniques allowed for a robust and thorough interpretation of the data, ensuring accurate conclusions about the effects of the experimental treatments.
3. Results and Discussion
3.1. Proximate Composition
The nutrient composition of live tubifex and frozen tubifex (evaluated over 12 weeks at 7-day intervals) was represented in Table 2 and Figures 1, 2, 3, 4. The proximate composition of frozen Tubifex was assessed at 7-day intervals, continuing up to the 85th day (12 weeks) of storage. Over the course of this study, aimed at evaluating the shelf-life and nutritional stability of frozen Tubifex, several key observations were made. Freezing is widely used in industries that process meat, fish, and other animal-based proteins, as it helps maintain quality over longer periods [19]. This method provides numerous benefits, such as minimal changes to the product’s size and only slight alterations to its colour, flavour, and texture.
| Nutrients | Composition (%) |
|---|---|
| Moisture | 85.8 ± 0.02 |
| Crude protein | 6.98 ± 0.01 |
| Fat | 2.68 ± 0.1 |
| Ash | 4.01 ± 0.04 |
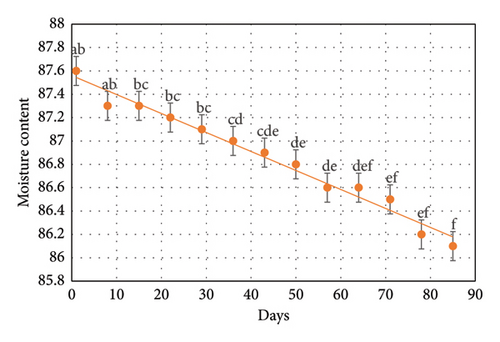
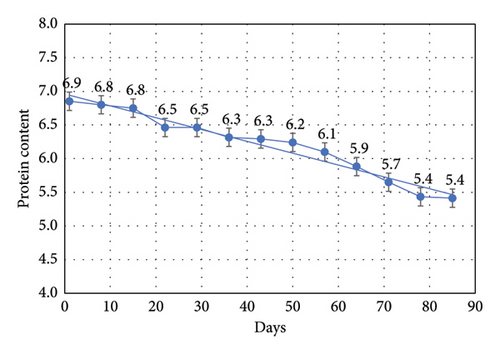
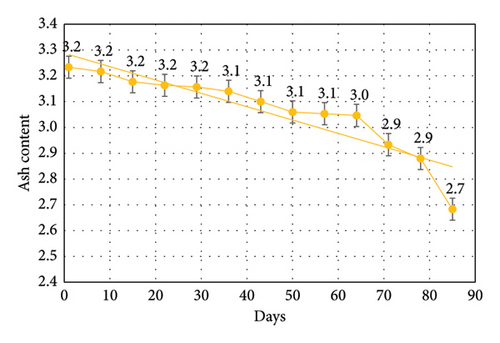
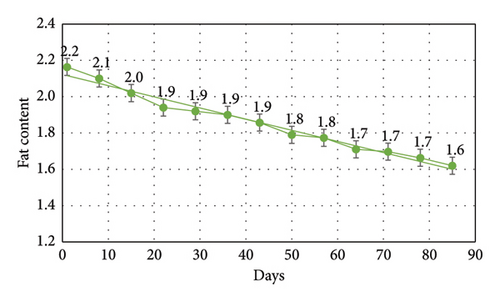
3.2. Moisture Content
Regular measurements indicated that moisture levels (Figure 1) remained relatively stable (p > 0.05) during the early weeks (up to 22nd days) of storage, though minor fluctuations were noted as the storage period extended. The observed changes may result from protein denaturation during the freezing process [20]. Protein denaturation can affect several muscle properties, including texture, water-holding capacity, colour, and flavour. Additionally, moisture transfer from the Tubifex during freezing process is often unavoidable [21]. Drip loss during the thawing process can also contribute to the reduction in moisture levels [22]. Numerous researchers have also noted that the moisture content in meat/fish samples tends to decrease during frozen storage [23–25].
3.3. Crude Protein Content
The crude protein content of frozen tubifex was analysed from 1st to 12th week of experimental period which was represented in Table 3 and Figure 2. A notable and statistically significant (p ≤ 0.05) reduction in crude protein content was observed from the first week of freezing to the 12th week of storage. The crude protein content, a critical indicator of the nutritional quality of Tubifex as fish feed, remained stable up to the 2nd week (15 days), but declined significantly thereafter. This decline may be attributed to protein denaturation and leaching of amino acids during freezing [20, 26]. Similar results have also been reported by several authors, including tilapia fish [27] and Puntius species [28]. The decline in protein may be explained by the denaturation of fish proteins, resulting from changes in the chemical composition and protein breakdown. Protein denaturation involves the disruption of its secondary, tertiary, and quaternary structures, simplifying it into a polypeptide chain [29]. In line with the current results, research by Kanwal [30] also observed a significant reduction in protein content during frozen storage in sea bass (Dicentrarchus labrax). This aligns with our findings, suggesting that frozen storage over time can lead to a gradual decline in protein levels, primarily due to the loss of soluble nutrients.
| Freezing time | Nutrient composition | |||
|---|---|---|---|---|
| Moisture | Crude protein | Fat | Ash | |
| 1st day | 87.63 ± 0.1f | 6.85 ± 0.0a | 2.16 ± 0.04a | 3.23 ± 0.03a |
| 8th day (1st week) | 87.30 ± 0.1ef | 6.80 ± 0.1a | 2.10 ± 0.05ab | 3.22 ± 0.01ab |
| 15th day (2nd week) | 87.30 ± 0.1ef | 6.75 ± 0.1ab | 2.02 ± 0.01ab | 3.18 ± 0.03bc |
| 22nd day (3rd week) | 87.23 ± 0.1def | 6.46 ± 0.1bc | 1.94 ± 0.03bc | 3.16 ± 0.02bc |
| 29th day (4th week) | 87.10 ± 0.1de | 6.46 ± 0.1bc | 1.92 ± 0.02cd | 3.16 ± 0.02bc |
| 36th day (5th week) | 87.00 ± 0.1de | 6.32 ± 0.1c | 1.90 ± 0.02cd | 3.14 ± 0.04bc |
| 43rd day (6th week) | 86.87 ± 0.2cde | 6.29 ± 0.1d | 1.86 ± 0.02d | 3.10 ± 0.01cd |
| 50th day (7th week) | 86.83 ± 0.1cd | 6.24 ± 0.1de | 1.79 ± 0.01e | 3.06 ± 0.03cd |
| 57th day (8th week) | 86.55 ± 0.2bc | 6.10 ± 0.1e | 1.77 ± 0.01ef | 3.05 ± 0.03cd |
| 64th day (9th week) | 86.55 ± 0.2bc | 5.88 ± 0.1fe | 1.71 ± 0.01fg | 3.05 ± 0.08d |
| 71st day (10th week) | 86.53 ± 0.1bc | 5.65 ± 0.1gfe | 1.70 ± 0.02g | 2.93 ± 0.06e |
| 78th day (11th week) | 86.17 ± 0.1ab | 5.43 ± 0.0gf | 1.66 ± 0.01gh | 2.88 ± 0.04e |
| 85th day (12th week) | 86.07 ± 0.1ab | 5.41 ± 0.1gf | 1.62 ± 0.01h | 2.68 ± 0.04f |
- Note: Values are expressed as mean ± SE. a, b, c, d, e, f and g values in a column with different superscripts differ significantly (p < 0.05).
3.4. Lipid Content
Results of crude lipid content of frozen tubifex were mentioned in Table 3 and Figure 3. The lipid content of frozen Tubifex, which is crucial for energy supply in fish diets, remained stable up to the 7th week of storage. Beyond this point, a gradual decrease (p < 0.05) in crude fat was noted, likely due to slow oxidative processes occurring even at lower temperature, primarily due to losses in the triglyceride fraction [31]. Similar results have also been depicted by various authors, including crab [32], tilapia [27], and in buffalo meat [33]. This reduction in crude lipid levels is consistent with the current study’s findings on frozen Tubifex, where lipid oxidation was identified as a key factor contributing to quality degradation of many foods that contain fats and oils [13]. Fish lipids, which are rich in polyunsaturated fatty acids, are particularly vulnerable to oxidation through an autocatalytic process [34]. Lipid oxidation is a well-known process that affects the stability of fats, leading to a decrease in nutritional value and quality over time.
3.5. Ash Content
Results shown in Table 3 and Figure 4 which revealed that the ash content decreased significantly from 3.23 ± 0.03 at 1st week to 2.68 ± 0.04 on 12th week of storage at −20°C. The significant difference was observed from 10th week of storage up to which no significant difference was observed. Total ash, representing the mineral composition, was consistent up until the 9th week of storage, after which slight variations were detected. This suggests some degree of mineral leaching or changes in the organic matrix of the worms as freezing time progressed. Supporting the present study’s results, research highlights on sea bass fillets [35], as well as on Nile perch [36] and on Tilapia (Oreochromis niloticus) [37], reported a decline in total ash content during frozen storage. This reduction in ash content, which represents the mineral composition, could be due to the breakdown or migration of certain minerals during the freezing and thawing process.
These studies, in conjunction with the current findings on frozen Tubifex, highlight the impact of long-term freezing on the nutritional composition, reinforcing the importance of understanding how extended storage affects the quality of feed and food items. These findings across various studies demonstrate a broader pattern of nutrient loss during frozen storage, particularly in relation to protein, lipid, and ash content. They emphasize the need for careful handling and storage practices to preserve the nutritional integrity of frozen biological materials such as fish and feed like Tubifex. So, we can conclude that the nutrient value of frozen tubifex (protein, fat, and ash) remains unchanged up to approximately three weeks when tested up to 12 weeks (85 days). So, it is significant to state that frozen tubifex, stored for up to 36 days at a temperature below −20°C, can be safely fed to fish without loss of nutritional potency and without significant degradation in key components like protein and fat. After this period, although some changes were observed, the frozen worms remained a viable feed option up to the 85th day, particularly for less demanding applications in aquaculture. However, the gradual decline in protein and lipid content after 5 weeks highlights the need for proper storage practices to maximize shelf life. Given its stability over 36 days, there is an opportunity for commercial-scale production and distribution of frozen Tubifex as a standardized feed product. Industry stakeholders can invest in optimizing freezing and storage techniques to further extend shelf life and maximize benefits.
4. Conclusion
The study highlights the importance of regular monitoring and proper storage conditions to maximize the shelf life of frozen Tubifex while maintaining its nutritional benefits, making it a reliable alternative feed source when live worms are not available. Finally, we can conclude saying this that the frozen tubifex which is stored at −20°C for 5th week (36 days) is suitable to feed fishes particularly aquarium fishes. The exploration of frozen Tubifex as a viable option presents an opportunity to enhance the sustainability and safety of fish farming practices especially in ornamental fish farming. By investigating the nutritional stability of frozen Tubifex, this study aims to provide aquarists and fish farmers with essential information that will aid in the effective management of fish nutrition, ultimately contributing to the overall health and productivity of aquaculture systems. For optimal results, we recommend using frozen Tubifex within this period and ensuring proper storage conditions to minimize nutrient loss. This study supports the adoption of frozen Tubifex in aquaculture to enhance sustainability and reduce risks associated with live feed.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Guntapalli Sravani: data curation, investigation, biochemical analysis, software and writing – original draft. Paramita Banerjee Sawant: conceptualization, supervision and writing – review and editing. Sukham Munilkumar: supervision, methodology, writing – review and editing. Gouranga Biswas: supervision, software, data curation. Shamna N.: supervision, writing – review and editing. Subam Debroy: supervision and visualization. Vikas Kumar Ujjania: formal analysis, Debajit Sarma: formal analysis Kurapati Nagendrasai: data curation, Kamil Akamad D.: visualization, manuscript editing and Neerudu Harika: formal analysis.
Funding
This study was funded by the Indian Council of Agricultural Research, Central Institute of Fisheries Education (ICAR-CIFE), Mumbai, Maharashtra.
Acknowledgments
The authors would like to thank Director and Vice-Chancellor, ICAR-Central Institute of Fisheries Education, Mumbai, India, for the necessary support and encouragement.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




