Reproductive Biology and Spawning Patterns of Polynemus melanochir: Implications for Conservation and Population Management
Abstract
Understanding the reproductive biology of Polynemus melanochir is essential for conservation purposes and a sustainable management perspective for this species in the Mekong River system. The current study investigates patterns of ovarian histology in this species along with spawning season, fecundity, and maturity stages in female specimens caught in the Hau River. The study was carried out from March 2024 to February 2025, providing insights into the species’ reproductive biology and its ecological implications. Specimens were caught with various fishing gears (trawl net and gill net) and ranged in size from 10.8 to 27.5 cm (18.8 ± 0.2 SE), while their weights varied between 8.05 and 132.37 g (45.06 ± 1.76 SE). Macroscopic observation of gonads enhanced by histological analysis enabled us to identify five stages of ovarian maturation, offering valuable information for population monitoring. The gonadosomatic index (GSI) and frequency of maturity stages indicated that reproductive activity was at its highest level in March, which was symbolized by a peak for GSI (GSI = 3.74 ± 0.89), as spawning likely occurred from December to April. Absolute fecundity ranged from 2,726 to 647,306 eggs per female (252,853 ± 41,185 SE), while relative fecundity averaged 2,696 ± 376 SE eggs per gram of body weight. Fecundity exhibited a strong correlation with total length (r2 = 0.70) and body weight (r2 = 0.72), highlighting possible size-based reproductive patterns. The estimated first maturity size was 16.98 ± 0.23 SE cm, suggesting the need for size-selective conservation measures. These findings certainly add knowledge to our understanding of the reproductive biology of P. melanochir as they contribute to its conservation and management perspectives within the Mekong Delta. Future research should, however, explore habitat preferences and environmental influences on reproductive success to support biodiversity conservation efforts.
1. Introduction
Globally, the threadfin fish family (Polynemidae) comprises nine genera with 41 species [1], primarily distributed in brackish and marine waters of tropical and subtropical regions [2]. This family includes economically important species that serve as valuable food sources for humans. The blackhand paradise fish (Polynemus melanochir Valenciennes, 1831), also known as Polynemus borneensis or Polynemus paradiseus, belongs to the family Polynemidae [3]. P. melanochir is widely distributed in the Mekong River region, including Cambodia and southern Vietnam, as well as Kalimantan (Malaysia and Indonesia) [4–6]. This species inhabits bottom layers of freshwater and brackish water environments, as it is used to temperatures ranging from 24°C to 28°C [7]. The maximum length recorded for P. melanochir is 25 cm [8]. This species has a wide mouth and is classified as a carnivorous fish based on its relative gut length index, with males generally possessing longer intestines than females do [9]. P. melanochir exhibits diverse migratory strategies, reflecting its adaptability to varying environmental conditions and flexibility in migration behavior [10].
Previous studies on P. melanochir have been carried out, focusing on the species’ taxonomy, distribution patterns, feeding ecology, and genetics [4, 9, 11, 12]. This species is ecologically and economically valuable for its overall importance in Southeastern Asia’s freshwater and brackish water ecosystems’ functioning and for its usefulness to fisheries. Instead, knowledge of its reproductive biology remains incomplete.
The main objective of the current study was to deal with the reproductive biology of P. melanochir occurring within the Mekong River basin. However, a specific goal was to address this knowledge-gap situation, describing ovarian external and internal modifications throughout the reproductive process, unveiling the spawning season, telling about the length at which the fish reach maturity, and see whether or not fecundity was in line with P. melanochir’s prolific nature.
2. Materials and Methods
2.1. Sample Collection and Fish Identification
Fish samples were collected from March 2024 to February 2025 along the Hau River at two sites (Figure 1): Cai Rang, Can Tho, which has freshwater year-round, and Long Phu, Soc Trang, which experiences saltwater intrusion during the wet season, which occurs from February to April annually. Each month, fish samples were collected once at each study site using various fishing gears to ensure that the capture of individuals covered a wide range of sizes. After collection, the fish were identified using description and identification keys fitting for fishes of the Mekong Delta [5] to ensure accurate identification and to avoid confusion with morphologically similar species. Accurately identified specimens were then preserved in 4% formalin solution before they were transported to the laboratory for further analysis. Since all the samples in this study were obtained from deceased fish, no authorization for animal research was required under the relevant regulations.
2.2. Determination of Reproductive Characteristics
Morphological characteristics, including total weight (W) and total length (TL), were recorded. The total weight was measured to the nearest 0.01 g, while the TL was measured with an accuracy of 0.1 cm. Specifically, the fish was placed on a measuring board consisting of a plastic sheet (40 × 30 cm) with a 30 cm ruler affixed at the center. The fish’s snout was aligned with the starting mark of the ruler, and TL was measured from the snout to the end of the caudal fin. Meanwhile, the fish was cleaned and dried before its weight was measured using an AND analytical balance (EK-610).
The fish were then dissected to examine the ovaries and determine the maturation stage. The ovaries were carefully removed, rinsed, and weighed (Wg, 0.001 g) before being preserved in 4% formalin (Dinh and Nguyen [14]).
The histological preparation of P. melanochir gonadal tissue was conducted following the double-staining method of Carleton et al. [15] and the gonadal histology protocol for fish described by Dinh and Nguyen [14]. This process consisted of six steps: fixation, dehydration, paraffin infiltration, embedding, sectioning, and staining. Specifically, the ovaries were fixed in 1% formalin for 24 h after collection. They were then rinsed under running tap water for at least 8 h to remove residual formalin. The samples were dehydrated by sequential immersion in alcohol solutions ranging from 50% to absolute ethanol, followed by N-butanol and xylene. Subsequently, the samples were embedded in a mixture of beeswax and paraffin (ratio 1:4) at 70°C. After 24 h of embedding, the tissues were sectioned into 4–5 µm slices using a microtome cutter (Sakura, Japan). The tissue sections were then mounted on glass slides using an albumin solution. Finally, the samples were stained using the double-staining technique with hematoxylin and eosin Y. The histological slides were sealed with Canada balsam for long-term preservation.
Oocyte diameters were measured using an ocular micrometer and observed under an OLYMPUS CX41 microscope. Histological slides of ovarian developmental stages were sequentially observed under a light microscope and photographed using ImageView software Version 2.4.0. Different types of oocytes were identified based on the descriptions of Dinh and Nguyen [14], and their diameters were measured using an objective micrometer.
The spawning season of P. melanochir was determined based on the frequency of gonadal maturation stages and the temporal variation of the gonadosomatic index (GSI) [16]. The GSI was calculated using the formula proposed by Lloret and Rätz [17]: GSI (%) = (Wg/W) ∗ 100, where Wg is the gonad weight and W is the total body weight of the fish.
The length at first maturity (Lm) was defined as the TL at which 50% of individuals reach sexual maturity. For that purpose, specimens that reached Maturation stage III and over were selected. This parameter was estimated using the logistic equation [18]: P = 1/(1 + exp[−r × (TL − Lm)]), where P is the proportion of individuals with gonads at Stage III or higher in each TL group, r is the coefficient of the equation, TL is the TL of the fish, and Lm is the Lm.
Absolute fecundity (AF) was determined by counting the total number of eggs in the ovary using a Motic stereomicroscope. Each Stage IV ovary was weighed (Wg, 0.0001 g) using an OHAUS analytical balance (PR224/E) before estimating the AF. Due to the high number of oocytes in each ovary, three subsamples (anterior, middle, and posterior regions) were selected to estimate each individual’s potential fecundity (PF). Each subsample was weighed, and the number of oocytes was counted. Finally, PF was calculated using the formula of Bagenal and Braum [19]: PF = n × (total ovary weight/subsample weight), where n represents the number of oocytes in the subsample.
The relative fecundity (RF) was calculated following the formula by Bagenal and Braum [19]: RF = AF/W, where RF is the RF (number of eggs per gram of body weight); AF is the AF (total number of eggs in the ovary), and W is the total body weight of the fish.
2.3. Data Processing and Statistical Analysis
The GSI and oocyte diameters at different stages of gonadal development were tested for normality using the Kolmogorov–Smirnov test. The Kolmogorov–Smirnov test was applied to compare data by month, and the Mann–Whitney test was used to compare data by season [20]. Similarly, the Kruskal–Wallis test was used for non-normal distributions to assess differences among oocyte types. Logarithmic regression was used to analyze the correlation between fish body size (TL and W) and PF [21]. All data were analyzed using SPSS V.21, with a statistical significance of 5%.
3. Results
3.1. Size of Specimens and Size of Oocytes at Different Developmental Stages of Ovaries
Between March 2024 and February 2025, a total of 211 female P. melanochir specimens were collected, with TL ranging from 10.8 to 27.5 cm (mean ± SE: 18.8 ± 0.2 cm) and W ranging from 8.05 to 132.37 g (mean ± SE: 45.6 ± 1.77 g). The highest mean body weight was recorded in September 2024 (65.91 ± 11.56 g), while the lowest was observed in July 2024 (20.30 ± 1.10 g). The highest mean TL was found in February 2025 (21.4 ± 0.6 cm) (Table 1). The raw data used for the analysis are available in the Supporting information (Row data.xlsx) (available here).
| Time | N | W | TL | ||
|---|---|---|---|---|---|
| Mean ± SE | Min–max | Mean ± SE | Min–max | ||
| March 2024 | 31 | 54.51 ± 6.41 | 17.96–132.37 | 19.3 ± 0.6 | 14.5–26.1 |
| April 2024 | 49 | 41.59 ± 1.90 | 20.52–66.95 | 18.8 ± 0.3 | 15.0–22.3 |
| May 2024 | 26 | 41.26 ± 2.58 | 15.54–66.90 | 18.7 ± 0.4 | 14.1–22.3 |
| June 2024 | 14 | 37.38 ± 2.94 | 19.00–58.96 | 18.4 ± 0.5 | 15.2–21.8 |
| July 2024 | 12 | 20.30 ± 1.10 | 14.92–28.15 | 15.4 ± 0.3 | 14.0–17.5 |
| August 2024 | 6 | 38.08 ± 11.97 | 14.89–81.09 | 17.4 ± 1.6 | 14.0–22.9 |
| September 2024 | 9 | 65.91 ± 11.56 | 8.05–119.33 | 20.7 ± 1.5 | 10.8–25.7 |
| October 2024 | 17 | 43.58 ± 7.14 | 16.85–115.23 | 18.2 ± 0.9 | 14.0–25.8 |
| November 2024 | 17 | 41.02 ± 6.88 | 17.98–121.83 | 18.6 ± 0.8 | 15.0–27.5 |
| December 2024 | 6 | 52.60 ± 10.23 | 21.77–80.78 | 19.8 ± 1.2 | 15.5–23.0 |
| January 2025 | 15 | 60.16 ± 8.85 | 11.20–115.08 | 19.8 ± 1.2 | 12.0–25.3 |
| February 2025 | 9 | 64.10 ± 5.26 | 37.07–79.82 | 21.4 ± 0.6 | 18.5–23.7 |
| Total | 211 | 45.6 ± 1.77 | 8.05–132.37 | 18.8 ± 0.2 | 10.8–27.5 |
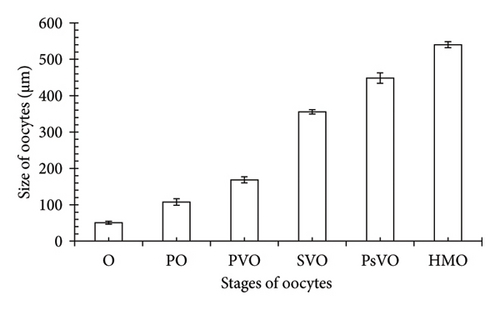
The current study showed that the sizes of the oocyte types do not follow a normal distribution (Kolmogorov–Smirnov test, KS = 0.21, p < 0.01) and increased progressively from oogonia (O) to hydrated oocytes (HMOs) (Kruskal–Wallis H, χ2 = 174.05, p < 0.001), indicating a systematic development pattern. Specifically, the average values of the oocyte types were as follows: O (50.84 ± 0.77 SE μm), primary oocyte (PO) (107.57 ± 1.62 SE μm), primary vitellogenic oocyte (PVO) (168.78 ± 1.51 SE μm), secondary vitellogenic oocyte (SVO) (355.59 ± 1.11 SE μm), postvitellogenic oocyte (PsVO) (448.73 ± 2.60 SE μm), and HMO (540.11 ± 1.48 SE μm) (Figure 2).
3.2. External Morphology of Gonads and Patterns of Ovaries as Histologically Revealed
-
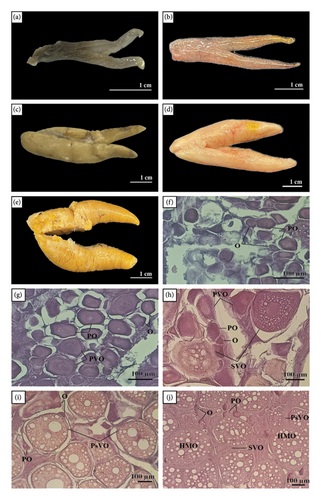
Stage I (immature stage): At this stage, the ovary was small, elongated, and dark red. The surface of the ovary was smooth, and there was no clear separation of the ovarian lobes. The ovary measured approximately 2.5–3 cm in length, with a maximum width of 0.3–0.5 cm. Histologically, O and a few POs were observed. This stage represented the initial phase of development, where the ovary showed no signs of maturity.
-
Stage II (early developing stage): The ovary grew slightly in size, with a light pink or beige color. The surface remains smooth but becomes tighter compared to the previous stage. There was no significant change in the ovary’s length, but the width increased to about 0.5–0.7 cm. Histological observation revealed an increase in the number of POs and the appearance of PVOs. This indicated the early development of the eggs, as the oocytes began to grow but without significant vitellogenin accumulation (the yolk precursor).
-
Stage III (mid-developing stage): The ovary was considerably larger, with a light yellow color. The surface became taut, and light lobulation began to appear. The overall size increased, with the length measuring approximately 3–3.5 cm and a maximum width of 1.0–1.2 cm. Histologically, there was an increase in PVOs and the appearance of SVOs. Yolk accumulation began, indicating significant development of the oocytes.
-
Stage IV (mature stage): The ovary reaches its maximum size, with a pink-orange or deep orange color. The surface of the ovary became distinct, and the ovarian lobes were fully developed. The ovary reached its maximum size for the reproductive cycle, measuring around 5-6 cm in length and 2.5–3 cm in width. Histological examination showed a predominance of SVOs and PsVOs. This indicated that the eggs were fully developed and ready for ovulation.
-
Stage V (spawning stage): The ovary appeared dark yellow and was larger but no longer as taut as in the previous stage. The surface became irregular, with some areas showing shrinkage or mild necrosis. After egg release, the ovary showed signs of contraction, with a length reduced to about 4–5 cm and a width of 2–2.5 cm. Histological observation showed a significant decrease in PsVOs, while necrotic structures (HMOs) began to appear. This was the spawning stage, where the ovary started to degenerate and prepare for the next reproductive cycle.
3.3. Spawning Season
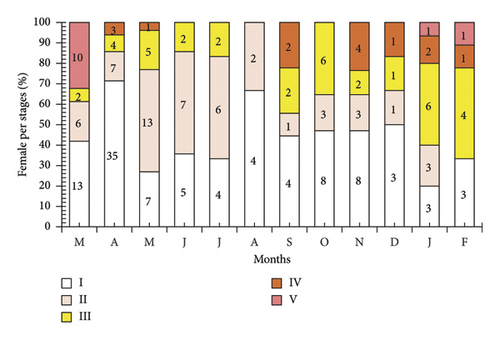
The results from the observation of the frequency of ovarian stages in P. melanochir showed significant changes over the 12-month study period. Stage I appeared most frequently in March 2024, May 2024, June 2024, and July 2024, and then gradually decreased. Stage II was presented throughout the 12 months, with the highest frequency in May 2024, June 2024, July 2024, and October 2024, followed by a decrease. Stage III occurred sporadically in most months, with the highest frequencies in April 2024, June 2024, October 2024, November 2024, and February 2025. Stage IV displayed a low frequency, being recorded only in April 2024, September 2024, October 2024, November 2024, December 2024, and February 2025. Stage V appeared the least, only present in March 2024, December 2024, January 2025, and February 2025, with a very low frequency. Overall, Stages I and II predominated for most of the year, particularly in the middle of the year, while Stages III, IV, and V gradually increased toward the end of the year and the beginning of the next, reflecting the seasonal variation in ovarian development (Figure 4).
Although the frequency of ovarian stages suggested that the reproductive season of the fish may occur during the early months of the year (dry season), it was still unclear which specific months are involved. Therefore, the GSI index was further used to accurately determine the reproductive season of P. melanochir. The results showed that the GSI did not follow a normal distribution (KS = 0.04, p < 0.01). The GSI values fluctuated significantly during the study months (χ2 = 32.66, p = 0.01, Figure 5), reflecting the species’ reproductive cycle. In March 2024, the GSI reached its highest level, with 3.74 ± 0.89%, suggesting this might be the peak reproductive period. However, by April 2024, the GSI dropped sharply below 0.5%, indicating the end of the reproductive process as the gonads degenerated after the eggs were released. From May to October 2024, the GSI remained low, fluctuating between 0.3% and 1.0%, indicating that females were recovering and gradually developing their ovaries, with some minor but insignificant fluctuations. From November 2024 onwards, the GSI began to increase, starting at around 1.0% in November and reaching approximately 2.5%–3.0% in January and February 2025, reflecting ovarian development in preparation for the next reproductive season. In addition, the GSI index showed significant seasonal differences (Mann–Whitney U, Z = 1.52, p < 0.01), with higher values during the dry season (1.41 ± 0.28 SE) compared to the wet season (0.73 ± 0.15 SE). Overall, the GSI was high during the dry season, peaking in March 2024, then decreasing sharply and remaining low for most of the year before gradually increasing from November, indicating the approach of a new reproductive cycle.
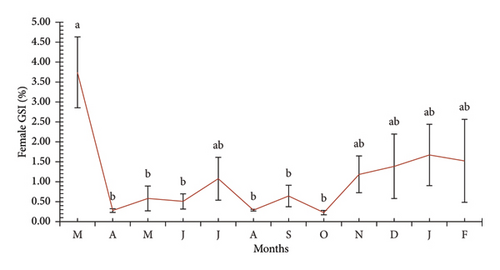
3.4. The First Maturity Length
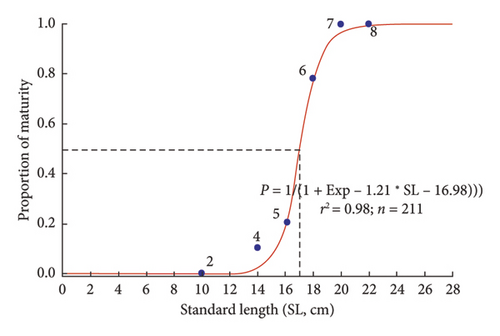
The first maturity length was defined as the length at which 50% of the individuals reach reproductive maturity, corresponding to the midpoint of the sigmoid curve. This position marks the size threshold at which half of the population begins to participate in reproduction, serving as an essential indicator in reproductive biology research and fisheries management. The results showed that the first maturity length of P. melanochir was 16.98 ± 0.23 SE cm (Figure 6).
3.5. Fecundity
AF (PF) and RF exhibited significant variation in P. melanochir. Specifically, the average value of PF was 252,853 ± 41,185 SE eggs/female. PF showed considerable variability among individuals, with the smallest individual having a PF of 2726 eggs (SL = 13.8 cm and W = 42.75 g), while the largest individual had a PF of up to 647,306 eggs (SL = 18.5 cm and W = 115.08 g), indicating that some individuals have superior reproductive potential. Meanwhile, RF had an average value of 2696 ± 376 SE eggs/gram of female body weight, ranging from 64 to 5625 eggs/gram of female body weight, reflecting differences in reproductive strategies among individuals. Overall, AF exhibited large fluctuations, reflecting the relationship between fish size and egg production, while RF also showed variability, albeit at a lower level, indicating the influence of body mass on reproductive capacity.
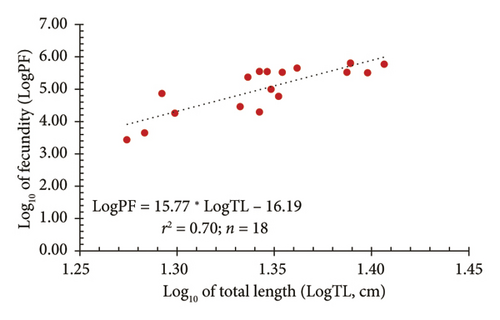
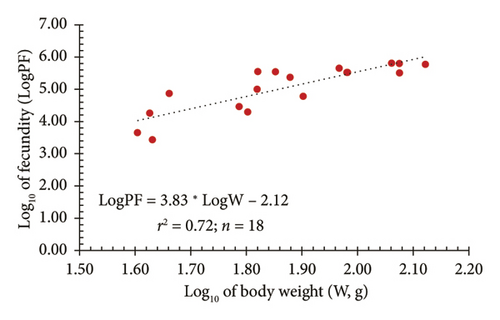
In addition, a strong relationship between AF (PF) and both TL and W was observed through logarithmic regression models. Figure 7(a) illustrates the relationship between the logarithm of fecundity (LogPF) and the logarithm of TL (LogTL), with the regression equation showing a positive correlation and a determination coefficient of r2 = 0.70. This indicated that 70% of the variation in fecundity can be explained by TL. Meanwhile, Figure 7(b) shows the relationship between the LogPF and the logarithm of body weight (LogW), with a determination coefficient of r2 = 0.72, suggesting that 72% of the variation in fecundity can be explained by body weight. These results indicated that fecundity tends to increase with both length and weight; however, body weight provided a higher level of explanation, reflecting a stronger relationship between weight and reproductive capacity in fish.
4. Discussion
Polynemus melanochir has a significant commercial value in the fisheries sector, as documented in previous studies [1, 3, 22], and it has not yet been subjected to overexploitation [23]. However, ecological pollution negatively impacts this species, with mesoplastic particles detected in its digestive tract [24]. Sustainable management and conservation efforts are essential to maintaining biodiversity and ensuring the long-term viability of regional fisheries.
Meanwhile, information on reproductive biological characteristics, including histological structure, reproductive mode and season, fecundity, and its relationship with fish length and weight, egg diameter, and Lm of this species remains unknown. Understanding reproductive strategies is crucial in fisheries management [25, 26]. Research on ovarian development serves as a foundation for understanding reproductive capacity and strategies in fish. According to Nikolsky [27], direct observations of gonadal characteristics and histological analyses can help determine the developmental stages of fish gonads. The development of reproductive organs in fish is closely related to population sustainability and contributes to the management of aquatic resources. Specifically, in the case of Periophthalmus chrysospilos, investigating ovary development elucidates reproductive mechanisms and provides critical information on optimal timing and conditions for spawning [28]. Another key aspect is determining the “Lm,” a fundamental parameter in fisheries management [29]. This measure represents the size at which fish begin to reproduce, which is essential for ensuring that populations have not been subject to overexploit before individuals reach reproductive maturity [30]. Lm is species-specific and may vary depending on environmental conditions and reproductive behavior [31]. Accurately determining this parameter provides essential data for conservation policies, thereby minimizing the negative impacts of early exploitation.
To address these gaps in reproductive biology research on P. melanochir, this study aims to describe the histological structure of the ovary across different developmental stages, determine the spawning season through the analysis of the GSI and the monthly frequency of ovarian developmental stages, assess both absolute and RF of females and their relationship with body size, and determine the Lm of the species. The findings provide essential data for fisheries resource management, support sustainable harvesting strategies, recommend minimum catch size and closed fishing seasons during spawning periods, and propose conservation measures to protect P. melanochir populations within the Mekong River region.
The histological structure of the ovaries in P. melanochir was divided into five developmental stages, from immature to spawning. The ovaries had a tubular structure, containing oocytes at various developmental stages, with the size of the oocytes gradually increasing as maturation progresses. The color of the ovaries in P. melanochir also changed significantly through the stages, from bright red in Stage I to dark yellow in Stage V. This color variation helped to distinguish the ovaries from the testes, especially in smaller stages. The color changes in the ovaries of P. melanochir differ significantly from those of other fish species. In Mystus mysticetus, the ovaries also undergo color changes, but in a different sequence: pale yellow in Stages I-II, dark yellow in Stage III, and dark red in Stages IV-V [32]. In contrast, the ovaries of Mystus gulio change from light pink in Stage I to orange in Stage IV and dark red in Stage VI [33]. Some species in the genera Acentrogobius and Glossogobius, such as Acentrogobius viridipunctatus, Glossogobius sparsipapillus [34], and Glossogobius giuris [35], show less pronounced color changes, with a pale yellow in Stage I, transitioning to bright yellow in Stage V. For Periophthalmus chrysospilos, the color change in the ovaries is similar, although not as pronounced [34]. This comparison shows that each fish species exhibits a unique pattern of ovarian color change, reflecting differences in reproductive traits and survival strategies. Compared to other species in the Polydactylus genus, such as P. paradiseus, P. melanochir has larger oocytes, indicating differences in reproductive strategy. When compared to marine species such as Atule mate [36] or Amphiprion polymnus [37], the ovarian development of P. melanochir follows a similar process but with varying maturation times, reflecting adaptations to freshwater and brackish environments. In comparison to freshwater species such as Systomus rubripinnis [38] and Acentrogobius viridipunctatus [28], the ovarian histological structure shows similarities in developmental stages. Understanding the structure and reproductive seasons of the ovaries is crucial for determining breeding protection periods and setting minimum harvesting sizes, contributing to the management and conservation of aquatic resources.
The spawning season of P. melanochir tended to concentrate during the dry season, peaking in March. This was a distinctive feature compared to other fish species in the region. Compared to species in the Mekong Delta, most fish species studied reproduce during the wet season, from June to December. For example, Glossogobius sparsipapillus [39] and Periophthalmus chrysospilos [34] all have a spawning season that lasts from June to October. Some other species, such as Mystus mysticetus [32] and Acentrogobius viridipunctatus [28], may spawn later, extending into October or December, but their reproduction still primarily occurs during the wet season. An interesting exception is Butis koilomatodon, which can reproduce year-round without a clear seasonal pattern [40]. This suggests a different reproductive strategy, possibly linked to its habitat. On the other hand, Glossogobius giuris has two distinct reproductive phases, one in April in freshwater environments and another in September in brackish water, indicating reproductive flexibility according to ecological conditions [35]. Notably, a species within the same genus, Polynemus paradiseus, spawns in June, aligning with the start of the wet season [41]. This highlights a significant difference between the two species, which could result from adaptation to different environmental conditions. Several other fish species distributed in various marine regions around the world also exhibit peak spawning seasons during the dry period, such as Atropus atropos [42], Decapterus macarellus [43], and Siganus canaliculatus [44]. In summary, P. melanochir has a reproductive season during the dry season, setting it apart from most fish species in the region that tend to spawn in the wet season. This may be an adaptive mechanism to optimize larval survival in more stable environmental conditions, minimizing the impacts of strong currents and abrupt changes in salinity during the wet season. This characteristic helps distinguish P. melanochir from other species and could play an essential role in managing and conserving aquatic resources.
The Lm was a critical biological indicator that helps assess a fish species’ reproductive strategy, reflecting its growth and reproductive potential in the natural environment [45]. P. melanochir’s first maturation length ranged from 10.2 to 12.5 cm. When compared with other species in the dataset, it is observed that P. melanochir has a relatively lower Lm compared to some marine species such as Galeoides decadactylus (18.7 cm) [46], Decapterus macarellus [43], and Atropus atropos [42]. However, it is higher than some smaller species such as Glossogobius giuris (4.82–6.14 cm) [35] or Butis koilomatodon (4.8–6.7 cm) [40]. The first maturation length of P. melanochir holds ecological significance. Since this species spawns in the dry season, reaching sexual maturity between 10 and 12 cm allows individuals to start reproducing earlier, ensuring the population persists under more stable environmental conditions compared to the wet season. This could be an adaptive mechanism to optimize the survival rate of offspring in environments with stable salinity and flow conditions. From a conservation and resource management perspective, determining the Lm of P. melanochir is crucial for establishing sustainable harvesting regulations. If individuals are caught before reaching Lm, the population could decline due to insufficient reproduction [47]. Therefore, measures such as setting a minimum harvesting size (> 12.5 cm) or prohibiting fishing during the spawning season could help maintain sustainable fish stocks. Overall, the first maturation length of P. melanochir reflects a distinctive reproductive strategy, with a size that is not too large but sufficient to ensure effective reproduction under specific environmental conditions. Compared to other fish species, this characteristic gives P. melanochir an advantage in maintaining its population without needing to reach an excessively large size, while also adapting to the ecological conditions of its habitat.
Reproductive potential is a crucial factor in fish biology, reflecting the ability to regenerate populations as well as the reproductive strategy of each species. In P. melanochir, the AF had an average value of 252,853 ± 41,185 eggs per female, ranging from 2726 to 647,306 eggs per individual. The RF varied from 64 to 5625 eggs per gram of body weight, with an average of 2696 ± 376 eggs per gram. These results indicated that P. melanochir exhibited high fecundity, particularly in larger individuals. When compared to other fish species, the difference in fecundity became apparent: Butis koilomatodon (3085–32,087 eggs per female) [40], Glossogobius sparsipapillus (17,918–28,700 eggs per female) [39], Periophthalmus chrysospilos (2614–23,465 eggs per female) [34], Glossogobius giuris (5118–100,003 eggs per female) [35], Acentrogobius viridipunctatus (5481–130,683 eggs per female) [28], Mystus mysticetus (4470–22,457 eggs per female) [32], Atropus atropos (70,709 eggs per female) [42], Galeoides decadactylus (179,447 eggs per female) [46], and Alepes djedaba (309,916 eggs per female) [48]. Notably, Polynemus paradiseus, another species in the same genus found in the Shibsa River in Bangladesh, has a much lower fecundity, with only about 32,410 eggs per female [41]. Overall, P. melanochir demonstrated higher fecundity than most of the compared fish species, particularly in terms of maximum PF values. This trend might have reflected a reproductive strategy characterized by many eggs, thereby increasing offspring survival chances. The relationship between PF and TL and body weight also indicated that body size substantially impacted fecundity in this species. Specifically, 70% of PF variation could be explained by fish length, whereas 72% could be explained by fish weight, highlighting a strong dependence on size factors. These findings suggested that P. melanochir exhibited a reproductive strategy with higher fecundity than most other species in the comparison list. This characteristic might have served as an adaptive trait ensuring population persistence in its natural habitat.
5. Conclusion
This study has provided critical reproductive data for P. melanochir that directly informs aquaculture and fisheries management practices. The detailed analysis of ovarian development, spawning seasons, and fecundity offers valuable insights for aquaculture broodstock management and seed production. Moreover, determining size at first maturity underscores the importance of implementing size-based fishing regulations to protect wild stocks and support recruitment for aquaculture purposes. The strong correlations between fecundity and fish size highlight the potential for selecting larger females for broodstock, enhancing overall seed production. To further develop sustainable aquaculture and fisheries management strategies, future research should focus on optimizing breeding and rearing techniques and investigating habitat preferences and recruitment dynamics. Integrating these findings into practical management plans will contribute to the long-term sustainability of fish populations and support the livelihoods of communities dependent on these resources and increase aquaculture outcomes in the region.
Disclosure
The manuscript is authentic and is not currently submitted, reviewed, or published in any other journal.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by the Master, PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), code VINIF.2024.ThS.52.
Acknowledgments
We thank the local fishermen for their assistance in fish collection and Can Tho University for its support.
Supporting Information
This file contains the raw data used for statistical analysis presented in the manuscript, including total length, total weight, ovarian weight, GSI, month, site, and season.
Open Research
Data Availability Statement
The raw data supporting the findings of this study are provided as supporting information with this article.




