Molecular Basis of Aggressiveness in Pituitary Adenomas and Its Association With the Immune Microenvironment
Abstract
Background: Pituitary adenomas (PAs) are common intracranial tumors, and their aggressive phenotype exhibits a poor prognosis. We aimed to explore the aggressive feature of PAs and discover novel diagnostic markers.
Method: The datasets of GSE260487 and GSE169498, which contained invasive and noninvasive samples, were downloaded from the Gene Expression Omnibus (GEO) database. Aggressive phenotype-related gene modules were classified using the “WGCNA” package. Differentially expressed genes (DEGs) in each module were identified by the “limma” package. Next, a protein–protein interaction (PPI) network was used in the construction and identification process of key genes, and the CytoHubba tool was utilized to analyze the subnetwork and select the top 10 genes. Diagnostic markers were selected using two machine learning algorithms: support vector machine (SVM) and Lasso. Finally, the ESTIMATE and “GSVA” were applied for immune infiltration assessment.
Results: WGCNA showed that the turquoise module was closely associated with the aggressive phenotype and enriched in neural differentiation and cell migration pathways. A total of 521 DEGs were intersected with the turquoise module genes to obtain 187 overlapping genes, from which 10 hub genes related to tumor proliferation were selected to develop a PPI network. Next, we determined MYH7 as an accurate diagnostic marker, and the immune infiltration analysis revealed that MYH7 expression was negatively correlated with stromal score and immune score but positively correlated with the infiltration of antitumor cells.
Conclusion: We developed a novel marker with a strong diagnostic performance for PAs, providing novel insights for the detection and individualized treatment of PAs.
1. Introduction
Human pituitary adenoma (PA) is the third most prevalent intracranial neoplasms following meningiomas and gliomas, accounting for 17% of all brain tumors [1, 2]. While the majority of PA cases are benign, PA frequently causes clinically significant symptoms due to the overproduction of pituitary hormones and/or local occupation of the tumor mass [3]. Based on the clinical manifestations, PA could be classified into the following subtypes: prolactinomas (PRL, also known as prolactin-secreting PAs), clinically nonfunctioning pituitary adenomas (NFPAs), growth hormone (GH)–secreting adenomas, adrenocorticotropin hormone (ACTH)–secreting adenomas (also known as Cushing’s disease), and thyroid-stimulating hormone (TSH)–secreting adenomas. Some other rare PAs produce clinically relevant luteinizing hormone (LH) and follicle stimulating hormone (FSH) [3]. Current therapies for PAs are limited, with somatostatin analogues and dopamine agonists as two major drugs for the treatment of PAs. Specifically, dopamine agonists are usually the first choice for hormonal and tumor size control in PRL-secreting PAs, while somatostatin analogues are often used for GH-producing PAs and post-transsphenoidal surgery treatment [3]. However, not all PA patients can benefit from these methods, which demands the identification of potential effective therapeutic biomarkers for individualized treatments.
PAs can be divided according to size as microadenomas (< 10 mm), macroadenomas (≥ 10 mm), or giant adenomas (≥ 30 mm). Based on invasiveness, PAs can be further divided into noninvasive PAs and invasive PAs. A study revealed that 25%–55% of PAs exhibit invasion to bone, dura, or surrounding anatomical structures [4]. However, most noninvasive PAs have benign behaviors even with dural invasion. A study reported that the incidence of malignant pituitary tumors with systemic and meningeal metastases is notably low (0.2%) [5]. In addition, some invasive PAs with highly aggressive characteristics display distinct clinical behaviors in comparison to benign PAs and and are characterized by the invasion of nearby anatomical structures, early postoperative recurrence, and treatment resistance. The World Health Organization (WHO) has published a classification criterion that, in addition to benign and malignant PAs, invasive PA, a type of atypical adenoma, exhibits aggressive behaviors, high mitotic index, extensive p53 nuclear staining, and Ki67 labeling index (LI) > 3% [6]. It has been found that invasive PA shows carcinoma morphological and histological features and has craniospinal or distant metastases, fatal outcomes, and poorer prognosis. Therefore, the investigation of the underlying pathogenesis of PAs with aggressive characteristics could facilitate the discovery of novel molecular targets and improvement of the prognosis of PAs [7].
This study downloaded both invasive and noninvasive PA samples from public databases and conducted weighted gene coexpression network analysis (WGCNA) to explore the aggressive features of PAs. The “limma” R package was used for filtering differentially expressed genes (DEGs), which were then intersected with aggressive genes in the module. The common genes were subjected to support vector machine (SVM) and Lasso analyses to determine potential diagnostic markers. Finally, we used the receiver operating characteristic (ROC) curve to assess the diagnostic efficiency of the identified biomarkers, and the correlation between the biomarkers and immune infiltration was also investigated. Our findings provided some insights for the diagnosis and individualized treatment of aggressive PAs.
2. Materials and Methods
2.1. Data Sources
Datasets of GSE260487 and GSE169498 were obtained from the Gene Expression Omnibus (GEO) database (https:/http://www.ncbinlm.nih.gov/geo/) [8]. Specifically, GSE260487 included 15 invasive PA samples and 17 noninvasive PA samples, and GSE169498 contained 49 invasive and 24 noninvasive PA samples.
2.2. Differential Expression Analysis
For identifying the DEGs, we applied the “limma” R package to perform differential expression analysis between invasive and noninvasive PA samples in the GSE169498 cohort [9], with adj.p val < 0.05 and |log2FC| > 1 as the threshold value [10].
2.3. WGCNA
WGCNA was used to identify gene modules linked to aggressive phenotype in the GSE169498 cohort using the “WGCNA” R package [9]. Firstly, the optimal soft threshold (β) was determined by the pickSoftThreshold function for scale independence and adjacent mean analysis to ensure a scale-free network (log(k) was positively correlated with the log(P(k)) and the correlation coefficient was greater than 0.85). Then, the expression matrix was converted into an adjacency matrix, which was further transformed to a topological matrix (TOM). Hierarchical clustering (average-linkage) was performed for identifying coexpression modules based on the optimal soft threshold (β) and TOM. After using the dynamic shear method to identify gene modules, the eigengenes of each module were calculated for further clustering analysis, and the modules with close distance were merged into a new module under height = 0.25, deepSplit = 2, and min ModuleSize = 50 [11].
2.4. Protein–Protein Interaction (PPI) Network Analysis
STRING (https://cn.string-db.org/, Version 12.0), a PPI network retrieval tool, was used to analyze the genes. Corresponding PPI networks were visualized using Cytoscape software (Version 3.9.1), and the CytoHubba algorithm was employed for identifying the subnet [12, 13].
2.5. Machine Learning for Screening Diagnostic Markers
The recursive feature elimination (RFE) function with svmLinear method in the “caret” R package and the 10-fold CrossValidation (cv) algorithm was applied to screen the key markers [14]. Lasso regression of “glmnet” R package was used for identifying the features (key parameters: nfolds = 10, family = “binomial”) [14]. Then, the diagnostic performance of the markers was assessed based on the ROC curve using the “timeROC” R package [15].
2.6. Immune Infiltration Analysis
Immune infiltration was evaluated using the ESTIMATE method [7]. Single-sample gene set enrichment analysis (ssGSEA) scores of 28 types of tumor-infiltrating lymphocytes (TILs) were calculated based on the marker genes from a previous study utilizing the “GSVA” R package [16].
2.7. Statistical Analysis
All statistical data were analyzed in R language (Version3.6.0). Wilcoxon’s test was used to calculate the difference between two sets of continuous variables. The correlation matrix was developed by the Pearson method. The data with p < 0.05 were considered statistically significant ( ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001).
3. Results
3.1. Identifying the Aggressive Feature-Related Gene Modules for the PA Samples
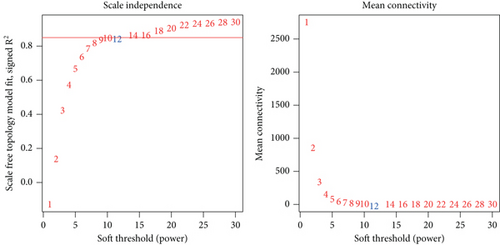
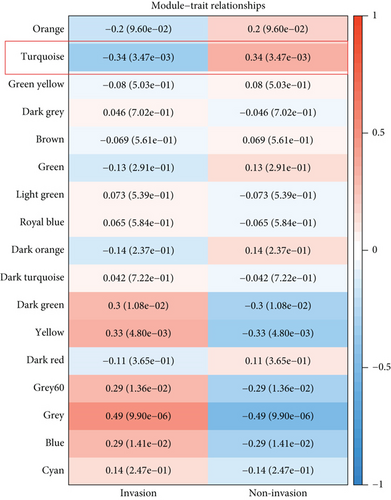
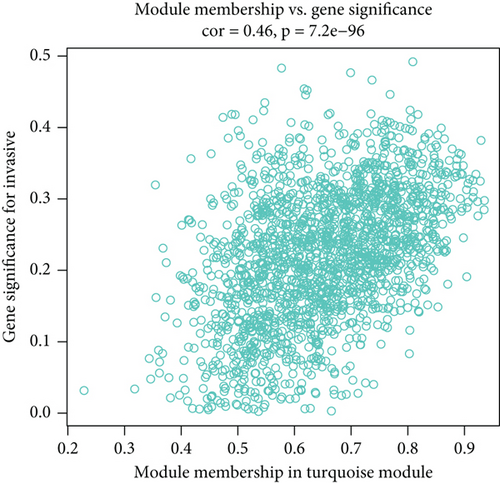
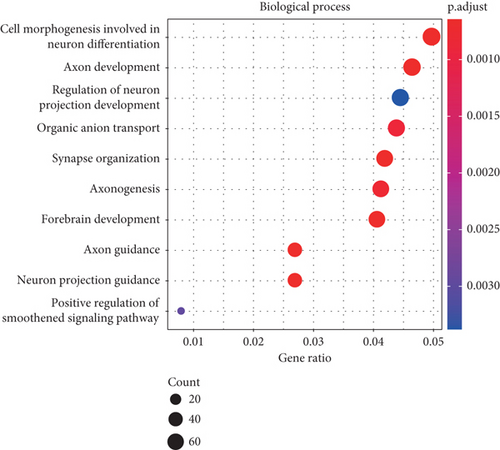
WGCNA was performed to identify gene modules linked to the invasiveness phenotype. The scale independence and adjacent mean plot showed that the scale-free topological fitting index was stable when the soft threshold was 12 (Figure 1a). Based on the soft threshold, hierarchical clustering of the genes was performed for classifying the coexpression gene modules (Figure 1b). Correlation analysis demonstrated that the turquoise module was significantly closely associated with the invasiveness phenotype (p < 0.05, Figure 1c,d). Enrichment analysis in biological process (BP) term of the turquoise module genes demonstrated that the module was significantly enriched in several BPs related to neural differentiation and cell migration, including cell morphogenesis involved in neuron differentiation, axon development, and organic anion transport process (Figure 1e).

3.2. Screening Key Genes
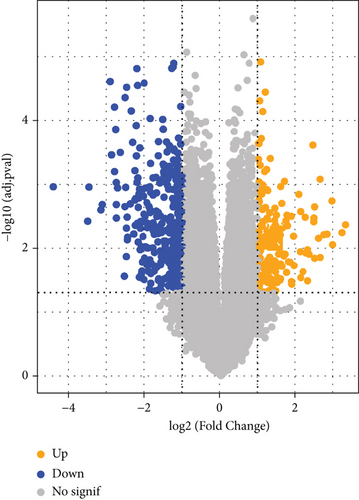
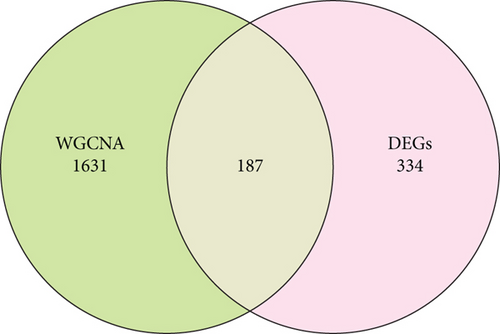
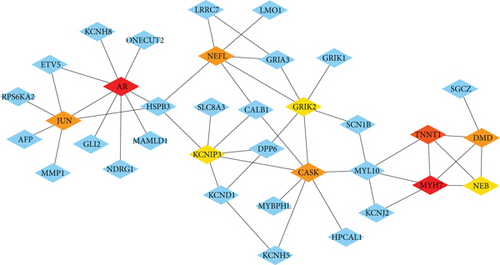
A sum of 521 DEGs in the GSE169498 cohort was identified (Figure 2a). There were 187 overlapping genes in the intersection between the turquoise module genes and the DEGs (Figure 2b). Subsequently, based on the PPI network, we identified the Top 10 key genes from the 187 overlapping genes (Figure 2c), including AR, JUN, NEFL, MYH7, KCNIP3, GRIK2, CASK, TNNT1, NEB, and DMD. These genes may play an important role in the aggressive progression of PAs.
3.3. Machine Learning Algorithms Selected Potential Diagnostic Markers
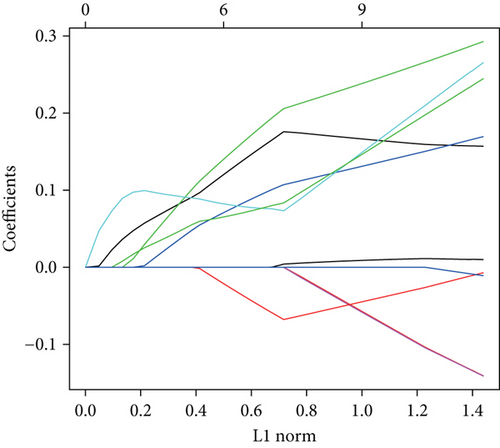
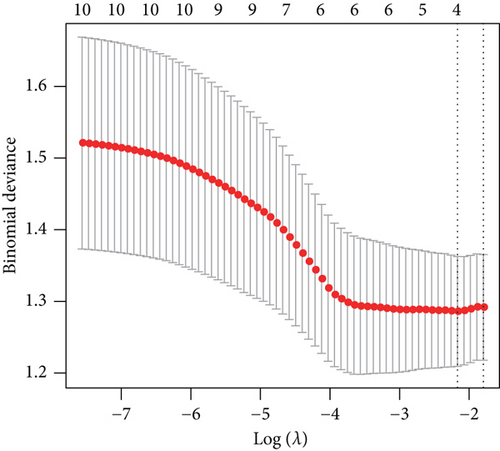
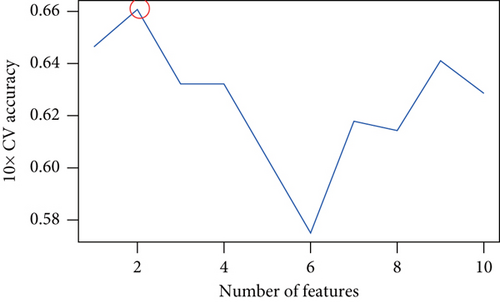
The aforementioned 10 key genes were used for further analysis. Lasso Cox regression analysis was used for selecting the key candidate genes. The λ for the optimal number of feature genes in the model was determined according to the Lasso coefficient and regularization trajectories (Figure 3a). Further, the RFE method was used to select the number of features, and the SVM model determined that the model had the highest cross-verification accuracy when the number of features was 4 (Figure 3b). Finally, we identified MYH7 as the diagnostic marker based on the results of the two machine learning algorithms (Figure 3c).
3.4. MYH7 Was the Biomarker With a High Diagnostic Efficiency
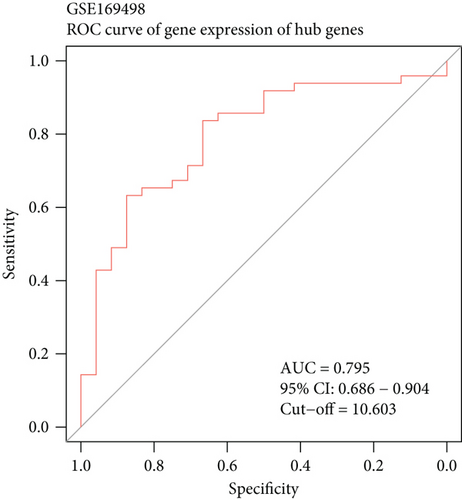
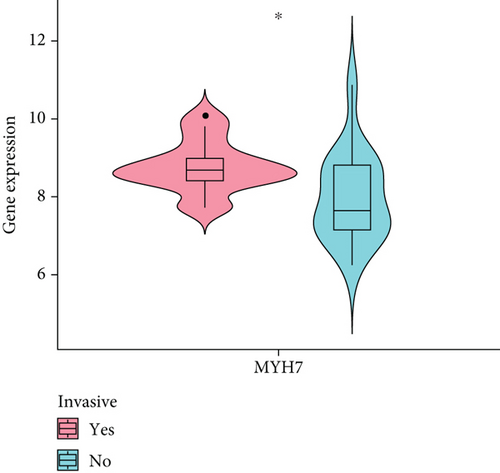
The diagnostic efficiency of MYH7 in the GSE169498 cohort was analyzed through the ROC analysis. The results showed that the area under curve (AUC) value of the diagnostic marker was 0.8 (Figure 4a), indicating a high classification effectiveness of MYH7 in PA diagnosis. Meanwhile, we observed that the expression level of MYH7 was significantly upregulated in the invasive phenotype group (p < 0.05, Figure 4b). Consistent results were observed in the validation set of GSE260487, with an AUC = 0.74 and significantly overexpressed MYH7 in the invasive group (p < 0.05, Figure 4c,d).


3.5. MYH7 May Promote the Invasive Properties of PAs by Influencing the Function of Antitumor Cells
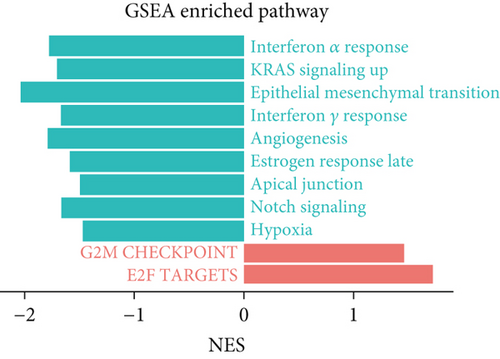
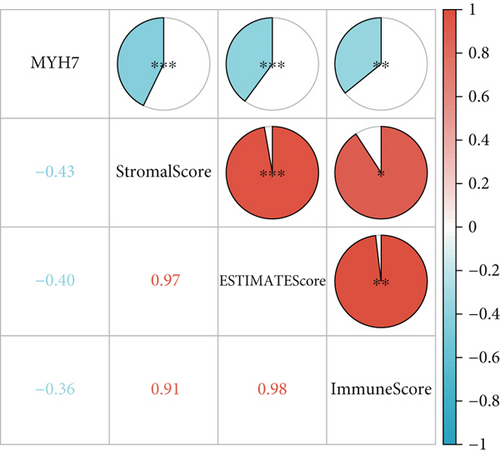
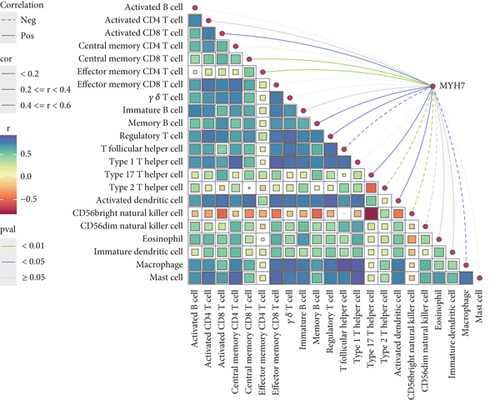
Gene set enrichment analysis (GSEA) of invasive and noninvasive pituitary tumors revealed that proliferation-related pathways like G2M CHECKPOINT and E2F TARGETS were significantly enriched in the invasive samples (Figure 5a). After calculating the immune infiltration score, correlation analysis revealed that the expression of MYH7 was significantly negatively correlated with the StromalScore, ImmuneScore, and ESTIMATEScore, with a correlation coefficient of −0.43, −0.36, and −0.40, respectively (p < 0.01, Figure 5b), indicating that the expression of MYH7 was associated with the suppressive tumor microenvironment of the invasive PAs. Additionally, the correlation analysis between the expression of MYH7 and the infiltration of a variety of immune cells demonstrated that MYH7 was positively correlated with CD8 T cells, memory B cells, and regulatory T cells but was negatively correlated with T helper cell, Type 2 T helper cell, and macrophage in the invasive PA samples (p < 0.05, Figure 5c). This suggested that MYH7 may promote the invasiveness of PAs by influencing the function of antitumor cells.
4. Discussion
PAs are common intracranial neoplasms originating from adenohypophysis cells. Somatostatin analogues and dopamine agonists are the first- or second-line drugs for PAs [17]. Furthermore, the clinical treatment of many PAs remains difficult due to the invasive nature of the tumors. In some cases, the tumor is unresectable or is too small to be detected. Moreover, stereotactic radiosurgery with adjuvant chemotherapy is not completely effective in achieving biochemical remission and tumor control. A study reported that the 10-year recurrence rate of the tumor is 7%–12% [18]. Therefore, this study explored the aggressive features of PAs to discover potential biomarkers for its diagnosis and treatment [19].
After screening the DEGs and performing PPI analysis, AR, JUN, NEFL, MYH7, KCNIP3, GRIK2, CASK, TNNT1, NEB, and DMD were selected as the hub genes in the aggressive PA samples. The high expression of AR mediated the PRL-PA dopamine agonist resistance through reducing the level of reactive oxygen species (ROS) [20]. JUN is a proto-oncogene that regulates cell proliferation and apoptosis [21], and NEFL could promote the invasion and migration of esophageal squamous carcinoma through activating the EGFR/AKT/S6 pathway [22]. Silencing of KCNIP3 enhances the epithelial–mesenchymal transition and proliferation via activating the Wnt/β-catenin pathway in papillary thyroid carcinoma [23]. GRIK2 maintains the function of urothelial carcinoma stem-like cells [24]. CASK acts as a tumor promoter in pancreatic cancer [25]. TNNT1 is regulated by miR-873 to promote colorectal cancer progression [26]. These findings suggested that the screened hub genes were closely associated with the migration and invasion of PAs and could serve as potential target genes for the treatment of PAs.
MYH7 encodes the heavy chain of myosin and plays an important role in cardiac and skeletal muscle contraction [27]. Variation of MYH7 can cause hypertrophic cardiomyopathy (HCM) [28], and MYH7 has also been considered the hub gene in oral cancer [29]. We utilized two machine learning algorithms to identify MYH7 as a diagnostic marker with a high diagnostic accuracy in PAs. Similar findings in a previous study revealed that downregulated MYH7 is a biomarker for the poor prognosis of head and neck squamous cell carcinoma (HNSCC), and that MYH7 promotes CD4+ T cell activation in cancer [30]. In addition, we also found that the expression of MYH7 was negatively correlated with the stromal score and immune score and positively correlated with the infiltration of antitumor cells, including CD8 T cells and Memory B cells. This indicated that the expression of MYH7 aggravated the exhaustion of antitumor immune cells for a tumor-suppressive microenvironment, which may contribute to the aggressive phenotype of PAs. Overall, we identified MYH7 as a novel diagnostic marker with a high diagnostic accuracy for PAs, and MYH7 may be associated with a tumor-suppressive microenvironment through promoting the exhaustion of immune cells.
5. Conclusion
The present study explored the aggressive features of PA patients and constructed a PPI network containing the key hub genes with the potential to serve as the therapeutic targets for PAs. Importantly, MYH7 was identified as an accurate diagnostic marker for PAs, providing some novel insight for further study.
5.1. Limitation
There were some limitations in this study. First, the study only used two public datasets (GSE169498 and GSE260487) with a small sample size and limited data sources, which may influence the robustness of MYH7 as a diagnostic factor. In the future, we will validate the expression of MYH7 and its diagnostic efficacy by including independent multicenter clinical samples and large-scale patient clinical follow-up data and by measuring its protein level. In addition, this study identified MYH7 as a key gene related to the aggressive phenotype of PAs employing two machine learning algorithms, but we lacked in vitro or in vivo experiments to verify its specific function in PA cells. Follow-up studies will use cell line and animal models to overexpress and interfere with MYH7 as well as cell function experiments to further clarify its mechanism of action in PAs. Finally, this study only used the ssGSEA algorithm to analyze the immune cell infiltration characteristics, but this method does not provide data about the spatial distribution in real tumor tissues and cannot distinguish the functional status of immune cells. Therefore, further studies will employ spatial transcriptomics or single-cell RNA sequencing techniques to analyze the spatial relationship between MYH7 expression region and the location of immune cell infiltration and their association with immune escape or depletion status.
Nomenclature
-
- PAs
-
- pituitary adenomas
-
- DEGs
-
- differentially expressed genes
-
- PPI
-
- protein–protein interaction
-
- PRL
-
- prolactin
-
- WHO
-
- World Health Organization
-
- WGCNA
-
- weighted gene coexpression network analysis
-
- SVM
-
- support vector machine
-
- Lasso
-
- least absolute shrinkage and selection operator
-
- ROC
-
- receiver operating characteristic
-
- GEO
-
- Gene Expression Omnibus database
-
- ssGSEA
-
- single sample gene set enrichment analysis
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed to this present work. X.C. and J.W. designed the study. Q.G. acquired the data. X.C. and Q.G. improved the figure quality. X.C., J.W., and Q.G. drafted the manuscript. X.C., J.W., and Q.G. revised the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 82002432) and the Natural Science Foundation of Shandong Province (Grant No. ZR2020QH179).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the GSE260487 repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE260487) and the GSE169498 repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169498).




