LysR-Type Transcriptional Regulator VirR Responds to Temperature and pH and Directly Activates the Transcription of virS-Containing Operon in Rhodococcus equi
Abstract
Rhodococcus equi—a facultative intracellular pathogen of macrophages—causes bronchopneumonia in foals and patients who are immunocompromised. Virulent strains of R. equi possess a virulence-associated plasmid, which encodes a 15- to 17-kDa surface protein called virulence-associated protein A (VapA). VapA expression is regulated by temperature and pH. Two transcriptional regulators, VirR and VirS, are involved in the transcriptional regulation of vapA. VirR regulates VapA expression through VirS. However, whether VirR directly regulates virS transcription is unclear. In this study, we examined VirR binding to the promoter region of the icgA operon, which contains virS, using the electrophoretic mobility shift assay and DNase I footprinting. VirR bound DNA fragments containing the virR-icgA intergenic region. Transcription from the promoter in this region was VirR-dependent and regulated by temperature and pH. The VirR-binding site contained the LysR-type transcriptional regulator-binding consensus motif, T–N11–A. A point mutation (L98E) in the putative ligand-binding pocket of VirR constitutively activated the icgA promoter. However, no apparent difference was observed in the electrophoretic mobility shift assay and DNase I footprinting using the icgA promoter when L98E VirR was compared with wild-type VirR. A bacterial two-hybrid system identified an interaction between VirR and RpoA. Our data reveal that VirR binds the promoter of the icgA operon and directly activates its transcription. Furthermore, the regulation of VapA expression in response to temperature and pH is mediated by VirR.
1. Introduction
Rhodococcus is a gram-positive bacterium that belongs to the family Nocardia and is widely distributed in soil. Although > 60 Rhodococcus species are reported, only Rhodococcus equi infects animals and causes disease [1]. R. equi infects horse, cattle, goat, llama, camel, alpaca, pig, boar, dog, cat, etc. [2–11]. R. equi causes severe, fatal respiratory disease in young horses < 6 months of age and is pathogenic to humans, causing pneumonia in immunocompromised individuals [12]. R. equi possesses a virulence plasmid, and plasmid-cured R. equi strains were avirulent in a foal or mouse infection model [13, 14]. R. equi is thought to have expanded the niche into various animal species by acquiring this virulence plasmid.
The virulence plasmid has a pathogenicity island (PAI) that is thought to be acquired by horizontal transmission; PAI contains multifamily genes that encode virulence-associated proteins (Vaps) [15]. Of these, vapA encodes a 15- to 17-kDa cell surface protein. The deletion of vapA reduces pathogenicity to the level of plasmid curing [13, 16–18]. R. equi can survive and multiply in host macrophages [19]. Intracellular growth depends on VapA, which affects the membrane permeability of R. equi–containing phagosomes, excluding the proton-pumping ATPase, and consequently, neutralizing the phagosome lumen [20, 21]. VapA expression is regulated by the temperature and pH of the bacterial environment. VapA expression is abolished at normal temperature (≤ 30°C) and increases at higher temperature and lower pH (e.g., 37°C and pH 6.5), which R. equi encounters in the early endosomes of macrophages [22, 23].
The transcriptional regulation of vapA involves two transcriptional regulators, VirR and VirS, which are encoded in the same PAI as vapA [24–26]. VirS is a response regulator of a two-component regulatory system and belongs to the OmpR/PhoB subfamily, although its sensor kinase is unknown [15]. The deletion of virS markedly reduces vapA promoter activity [24]. The transcription of virS is regulated by temperature and pH, and the vapA promoter is constitutively activated when VirS is constantly expressed from an exogenous promoter [24]. virS forms an operon with three upstream genes, including icgA, vapH, and orf7. The transcriptional regulation of this operon involves VirR, which is a lysR-type transcriptional regulator (LTTR) [26]. Mutations in virR markedly reduce the transcription of this operon [24].
LTTRs are the largest regulatory family in prokaryotes and are involved in regulating biological activities, such as metabolism, quorum sensing, oxidative stress response, motility, and virulence [27]. LTTRs require low molecular weight ligands, referred to as coinducers, to activate gene expression [27, 28]. Although most LTTR family members bind their target promoters in both the absence and presence of the coinducer, interactions with coinducers elicit structural changes in the LTTR–promoter complex, resulting in transcriptional activation [27].
In this study, we identified the VirR-binding site upstream of the icgA operon promoter and characterized it by electrophoretic mobility shift assay (EMSA) and DNase I footprinting. We used site-directed mutagenesis to substitute VirR residues in a region that corresponds to the ligand-binding sites of other LTTRs. L98E VirR allowed activation of the icgA operon promoter under noninducing conditions. These results suggest that VirR directly regulates the transcription of the icgA operon by binding upstream of the promoter as a positive regulator, responding to temperature and pH, probably by binding an unidentified coinducer.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
R. equi ATCC33701 was cultured on brain heart infusion (BHI) agar at 30°C. Apramycin (80 μg/mL) was added to BHI agar to select for R. equi growth. All R. equi strains were stored at −80°C in 85% BHI broth and 15% glycerol (vol/vol). Escherichia coli DH5α and ER2566 were cultured on Luria–Bertani (LB) agar or in LB broth. Ampicillin (50 μg/mL) was used when necessary. E. coli strains were stored at −80°C in 85% LB broth and 15% glycerol (vol/vol). Table S1 lists the strains used in this study.
2.2. Construction of Transcriptional Reporter Using t-Tag
The virR–icgA intergenic region (−116 to +4), fused with t-tag [25] (PicgA2-t-tag), located within two Rv3813 terminators of Mycobacterium tuberculosis [29], and flanked by the NotI site, was synthesized and cloned into pUC57-mini (pTKR918). The fragment of PicgA2-t-tag flanked by the Rv3813 terminator was excised from pTKR918 with NotI and ligated into the NotI site of pINT to create pReporter. The ATCC33701 strain or virRΔHTH strain (TKR474) was transformed with pReporter by electroporation and cultured on BHI agar with apramycin for transformant selection. The nucleotide deletion or substitution derivatives of pReporter were generated using PCR amplification with the pReporter template, and the following primer pairs are as follows: delta-35-1-delta-35-2 and delta ABS1-delta ABS2, RL1 and RL2 (Table S2), and KOD One PCR Master Mix. The PCR mix was DpnI digested for enrichment of PCR-amplified plasmids. DH5α was transformed by PCR-amplified plasmid and selected on LB agar containing ampicillin. Deletion or substitution was confirmed by sequencing. TKR474 was transformed with the resultant plasmids by electroporation.
2.3. Construction of Complement Plasmid
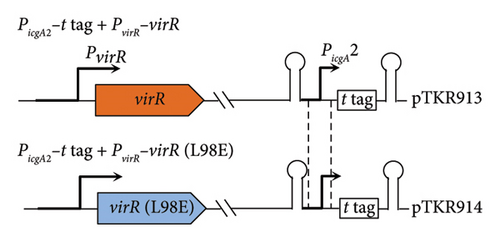
The fragment containing the virR ORF and its own promoter region was amplified by PCR using the PvirR-termF–virR-R primer pair (Table S2) and ligated into the pGEM-T Easy Vector to create pTKR509. To introduce point mutations in the virR coding sequence, PCR-mediated mutagenesis was performed using the complementary mutagenic primer pair VirR L98E-1–VirR L98E-2 (Table S2), pTKR509 template, and KOD One PCR Master Mix. The PCR mix was DpnI digested to enrich PCR-amplified plasmids. DH5α was transformed by PCR-amplified plasmid and selected on LB agar containing ampicillin. The point mutation was confirmed by sequencing. To create the integration plasmid containing PicgA2-t-tag with PvirR-virR (wild) or PvirR-virR (L98E), pTKR509 and pTKR917 were used as templates to amplify fragments containing PvirR-virR (wild or L98E) by using the virR-builderF–virR-builderR primer pair (Table S2), respectively. The resultant PCR products and EcoRV-digested pReporter were assembled by NEBuilder, according to the manufacturer’s instructions, to create pTKR913 and pTKR914, respectively. The virulence plasmid-cured derivative of ATCC33701 was transformed with pTKR913 or pTKR914.
2.4. RNA Extraction
Total bacterial RNA was isolated from 5-mL cultures grown to midlogarithmic phase (optical density at 600 nm [OD600] = 0.8). Briefly, cultures were mixed immediately with 10 mL of RNAprotect Bacteria Reagent (Qiagen) and incubated for 5 min at 20°C–28°C. Cells were harvested by centrifugation for 10 min at 5000 × g at 4°C, resuspended in 0.7 mL of RLT buffer (RNeasy Mini Kit; Qiagen), mixed with 1 g of 0.1-mm-diameter zirconia–silica beads (μT-01, Taitec), and lysed by mixing three times for 1 min in a bead beater (Taitec) at 4600 rpm. Total RNA was isolated using RNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions. To remove contaminant DNA, RNA was treated with 10 U of RNase-free DNase for 30 min at 37°C. DNase was inactivated by incubating the mixture for 5 min at 75°C.
2.5. qRT-PCR
In total, 200-ng RNA was reverse transcribed to cDNA using 6-mer oligonucleotide primers and a PrimeScript RT-PCR kit (TaKaRa), according to the manufacturer’s instructions. When the expression of t-tag was examined, cDNA was reverse transcribed using t-tag 167 R primer. Real-time RT-PCR analysis was performed in 20 μL, containing 1 × PowerSYBR Green PCR Master Mix (Applied Biosystems), 200 nM forward and reverse primers, and cDNA. The primers used are listed in Table S2. Reactions were performed with StepOne Real-Time PCR Systems (Applied Biosystems) as follows: 95°C for 10 min, 40 cycles at 95°C for 15 s, and 60°C for 1 min. Melting curve analysis was performed after each reaction to ensure the specificity of PCR products. Primer efficiency was calculated using a standard curve derived from dilutions of plasmid containing each target gene. Primer pairs with amplification efficiency ranging from 90% to 110% were used. Relative quantification was performed using standard curves generated for each gene-specific primer pair for all experiments, except one where results were normalized to gyrB levels, and analyzed using the ΔΔCT method. The values shown are relative to gyrB values.
2.6. 5′-RACE
To determine transcriptional initiation site of the icgA operon, 5′-RACE was performed with 5′-Full RACE Core Kit (TaKaRa), according to the manufacturer’s instructions. First-strand cDNA was synthesized from 3 μg of total RNA using 5′-terminal phosphorylated RT primer (Table S2). The cDNA was treated with RNase H to degrade RNA from hybrid DNA–RNA. The single-stranded cDNA was cyclized (or concatenated) with T4 RNA ligase. PCR was performed with the cyclized (or concatenated) cDNA with the A1–S1 primer pair, followed by nested PCR using the A2–S2 primer pair (Table S2). The amplified DNA fragments were ligated into pGEM-T Easy Vector.
2.7. Plasmid Integration in virRΔHTH Strain
PCR-mediated mutagenesis was used to introduce point mutations into the coding sequence of virR in pTKR528 (pINT::PvirR-virR). pTKR638 (pINT::PvirR-virR L98E), pTKR639 (pINT::PvirR-virR S100E), and pTKR640 (pINT::PvirR-virR L101E) were produced using the primer pairs VirR L98E-1-VirR L98E-2, virR A100E-1-virR A100E-2, and virR L101E-1-virR L101E-2, respectively (Table S2). Each plasmid was electroporated into TKR474 (virRΔHTH, PvapA-lacZ). Transformants were recovered on BHI agar containing 80 μg/mL apramycin.
2.8. β-Galactosidase Assay
Cells were cultured overnight at 30°C in BHI broth with shaking. Cultures were diluted 1:10 with 60 mM Tris-buffered BHI (pH adjusted to 6.5 or 8.0). Cultures were grown to OD600 = 0.5–0.7. Cells were washed twice with 0.9% NaCl, resuspended in 500 μL of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol [pH 7.6]), permeabilized with 20 μL chloroform and 35 μL of 0.1% SDS, and incubated at 28°C for 5 min with 100 μL of 13 mM 2-nitrophenyl β-D-galactopyranoside (Sigma-Aldrich, St Louis, MO, USA). The reaction was stopped by adding 250 μL of 1 M Na2CO3, and absorbance was read at 420 nm using a spectrophotometer (GENESYS 20, Thermo Fisher Scientific). The activity of each sample was calculated in Miller units as follows: 1000 × OD420/OD600 × reaction time × volume. Data represent average results of three biological replicates.
2.9. Protein Expression and Purification
DNA extracted from R. equi strain ATCC33701 was used as template. A DNA fragment containing the virR ORF was amplified by PCR using the VirR-NdeF–VirR-SapR primer pair. The PCR product was electrophoresed on 0.8% agarose gel. The gene fragment was gel extracted using MinElute kit (Qiagen), digested with NdeI and SapI, and ligated into NdeI and SapI sites of pTXB1 (New England Biolabs) to construct pTKR752. E. coli strain ER2566 was transformed with pTKR752. Bacteria were cultured in 200-mL LB broth with ampicillin to midexponential phase, incubated with 0.4 mM isopropyl-D-thiogalactopyranoside (IPTG) overnight at 18°C, harvested, disrupted by sonication, and centrifuged at 20,000 × g for 30 min at 4°C. The supernatant was loaded onto a chitin column (New England Biolabs) and equilibrated with column buffer (20 mM Tris-HCl [pH 8.0] and 500 mM NaCl). The column was first washed with column buffer, then quickly washed with cleavage buffer (column buffer + 50 mM DTT), and incubated overnight at 20°C. VirR was eluted with column buffer. Recombinant protein was dialyzed against PBS for 12 h and stored at −30°C until use. PCR-mediated mutagenesis was used to introduce point mutations into the coding sequence of virR in pTKR752 by using the VirR L98E-1–VirR L98E-2 primer pair to produce pTKR901. pTKR901 was electroporated into ER2566. L98E VirR was produced as described previously.
2.10. EMSA
The virR–icgA intergenic region was amplified by PCR using the PicgA-F–PicgA-R primer pair. The amplified DNA fragment was cloned into pGEM-T Easy Vector to construct pTKR837, which was used as template to construct the mutated versions of PicgA by PCR-based mutagenesis using the following primer pairs: delta-ABS1–delta-ABS2, delta-L1–delta-L2, delta-R1–delta-R2, and RL1–RL2 (Table S2). The binding of VirR to the icgA operon promoter was investigated using the EMSA. Probes containing the virR–icgA intergenic region were obtained by PCR using pTKR837, pTKR778, pTKR780, or pTKR781 as template. The reverse primer (PicgA-R) was FITC-labeled at the 5’ terminus (Table S2). The amplified fragments were separated by agarose gel electrophoresis, and the probes were extracted from the gel by MinElute kit. The reactions (15 μL) for measuring mobility shift contained FITC-labeled DNA probes and different concentrations of VirR in a buffer containing 10 mM Tris-HCl (pH 7.5), 100 mM KCl, and 1 mM EDTA, 5% vol/vol glycerol, 0.1 mM DTT, and 10 μg/mL BSA. Reactions were performed at room temperature for 30 min, loaded onto 6% polyacrylamide/bis (37.5:1) gels in 0.5 × Tris–borate–EDTA buffer, and run at a constant voltage of 150 V for 90 min. Gel images were acquired using a Typhoon scanner (GE Healthcare) and analyzed using ImageQuant software (GE Healthcare).
2.11. DNase I Footprinting Assays
Probes were obtained by PCR using a combination of FITC-labeled and FITC-unlabeled primers (Table S2). Binding reactions were performed in DNase I footprinting buffer (20 mM Tris-HCl, 3 mM MgCl2, 5 mM CaCl2, 100 mM NaCl, 100 mM EDTA, 50 μg/mL BSA, and 0.1 mM DTT) containing 100-ng probe and 0–0.975 μM VirR in a final volume of 100 μL. The reaction was incubated at 37°C for 30 min and maintained at 25°C for 5 min. Partial digestion of DNA was initiated by adding 1 μL of DNase I stock solution (50 μg/mL). Incubation was continued for 1 min, and the reaction was stopped by adding 100 μL of phenol–chloroform and 25 μL of stop solution (1.5 M sodium acetate, 20 mM EDTA, and 10 mg/mL tRNA). The sample was centrifuged, and the supernatant was ethanol precipitated. DNA was resuspended in 5 μL of loading buffer (0.125% bromophenol blue, 0.125% xylene cyanol, 20 mM EDTA, and 95% formamide) and separated by gel electrophoresis on a 6% polyacrylamide–urea sequencing gel. A sequencing reaction performed with the Sequenase 2.0 kit (USB), using an oligonucleotide specific for the labeled strand, was run in parallel as a size marker. Images of the gels were acquired using a Typhoon scanner (GE Healthcare) and analyzed using ImageQuant software.
2.12. Circular Permutation Analysis
Circular permutation analysis was performed to assess DNA bending by VirR using the pBend2 vector, as described [30]. The oligonucleotides PicgA-core1 and PicgA-core2 were annealed, and the resultant double-stranded DNA was ligated into SalI and BamHI sites of pBend2 to construct pTKR810. Plasmid pTKR810 was single digested with EcoRV and BamHI. Removal of restriction enzyme and buffer change were performed using a PCR purification kit (Qiagen). The binding reaction was performed using 0.75 μM VirR and 100 ng of each digest as probe. Assay conditions were identical to those for EMSA. Samples were separated on a 6% polyacrylamide native gel in 0.5 × Tris–borate–EDTA buffer at 4°C. Gels were stained with ethidium bromide, and DNA bands were visualized by UV fluorescence. The bending angle (α) was calculated using the Thompson and Landy [31] equation μM/μE = cos (α/2), where μM = mobility of the complex with protein bound at the center of DNA and μE = mobility of the complex with protein bound at the end.
2.13. Bacterial Two-Hybrid System
A bacterial two-hybrid system was used to identify interactions between VirR and RpoA. This system is based on functional complementation between two fragments (T18 and T25) of the catalytic domain of adenylate cyclase from Bordetella pertussis [32]. The plasmids pT18 and pT25 express proteins fused to the N-terminus of T18 and C-terminus of T25, respectively. virR (wild), virR (L98E), and rpoA were PCR amplified using the following primer pairs: T18-VirRF–T18-VirRR, T25-VirRF–T25-VirRR, T18-RpoAF–T18-RpoAR, and T25-RpoAF–T25-RpoAR (Table S2) and assembled into the MCS of pT25 and pT18 using NEBuilder, according to the manufacturer’s instructions. The insertions were confirmed by sequencing. Escherichia coli strain Sp850 (cya) was transformed with all combinations of pT18 and pT25 plasmids containing virR (wild), virR (L98E), or rpoA. The negative control contained Sp850 with pT18 and pT25, and the positive control was Sp850 with pT18-Zip and pT25-Zip [32]. Transformants were cultured in LB broth at 30°C with 100 μg/mL ampicillin, 30 μg/mL chloramphenicol, and 0.5 mM IPTG. β-galactosidase assays were done in triplicate; data represent average results of three biological replicate experiments.
3. Results
3.1. The icgA Operon Is Transcribed From a Promoter in the virR–icgA Intergenic Region
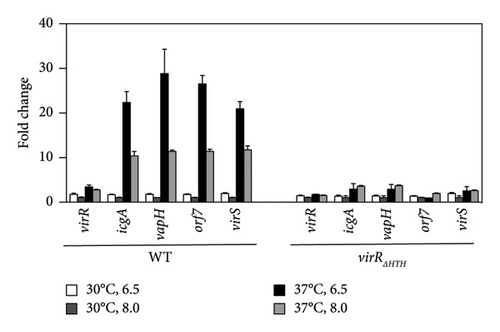
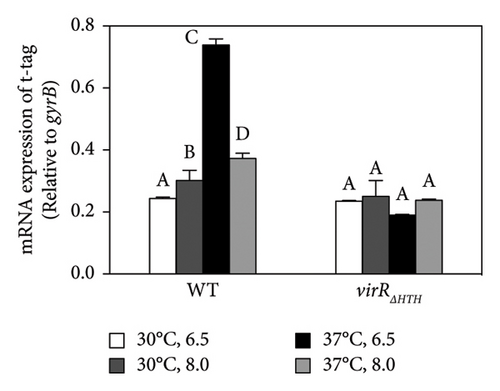
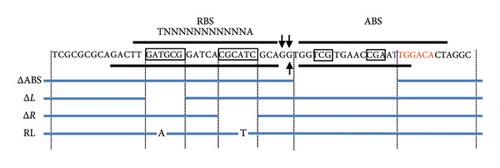
virS, which regulates vapA transcription, constitutes an operon with icgA, vapH, and orf7. The transcription of this operon is regulated by temperature and pH [24]. We confirmed the mRNA level of the four genes constituting the icgA operon (icgA, vapH, orf7, and virS) by real-time RT-PCR. The expression of icgA, vapH, orf7, and virS remarkably increased (> 21- to 29-fold) when R. equi was cultured at pH 6.5°C and 37°C compared with that at pH 8.0°C and 30°C (Figure 1(a)). The icgA operon was reportedly transcribed from a promoter within an ORF of virR upstream of icgA (PicgA1, Figure 1(b)) [25]. We evaluated the mRNA level using a pair of primers that amplifies the downstream region of PicgA1 within virR. We found that the level of mRNA containing this region increased, but the change was ∼3-fold. Because the transcriptional changes of these regions are different (7- to 10-fold), we postulate that the icgA operon is transcribed from a promoter other than PicgA1. To examine this hypothesis, we constructed an R. equi strain that contains the virR–icgA intergenic region, followed by PCR-detectable t-tag in the chromosome (Figure 1(a)), and examined the level of transcription by real-time RT-PCR. The maximum expression of t-tag was observed when bacteria were cultured at 37°C and pH 6.5 and transcription was significantly reduced at pH 8.0 (Figure 1(c)), suggesting that the transcription of the icgA operon occurred from and was controlled by the virR–icgA intergenic region. We performed 5’-RACE to determine the transcription initiation site of the icgA operon. We found that the transcription initiation site was 1 bp upstream from the start codon of icgA. Sequences similar to TATAMT (−10) and TTGACW (−35), which are binding motifs for M. tuberculosis SigA [33], were found upstream of the transcription initiation site (TAGCGT and TGGACA, respectively) (Figure 1(d)). We designated this promoter PicgA2.
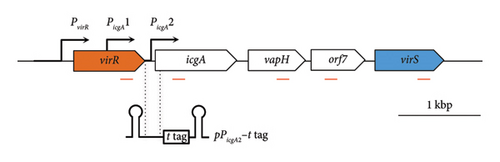
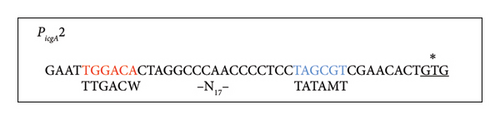
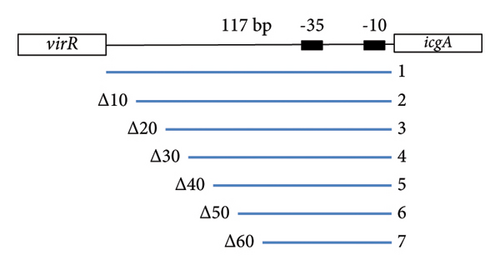
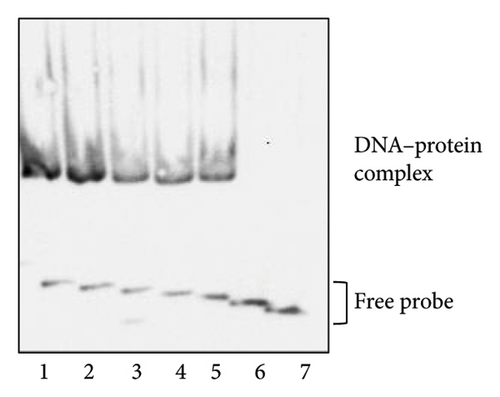
3.2. VirR Binds to the virR-icgA Intergenic Region
VirR has a positive effect on the transcription of the icgA operon [24], but whether this effect is directly mediated by VirR binding to the icgA promoter is unclear. We used EMSA to examine the binding between DNA upstream of icgA and VirR. When recombinant VirR was added, we detected a band shift (Figure 1(f)). When the probes were shortened by 10 bp from the 5’ end (Figure 1(e)), the shifted band disappeared in the probe shortened by 50 bp (Figure 1(f)). These results suggest that VirR binds to the virR–icgA intergenic region and that the binding region is located within 77 bp upstream of icgA.
3.3. The L98E Mutation in VirR Leads to a Constitutive Active State of the vapA Promoter
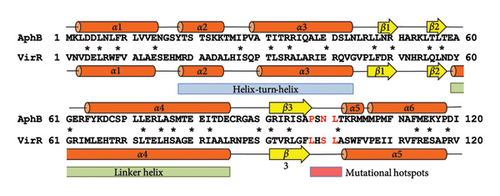
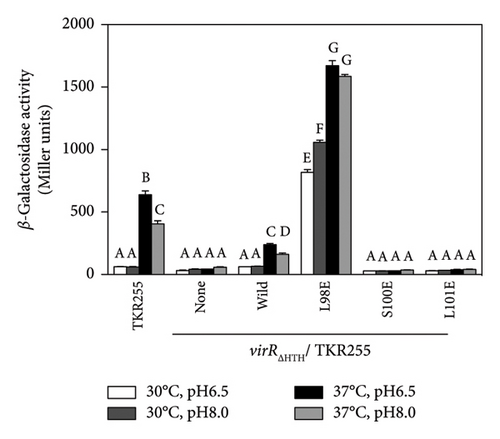
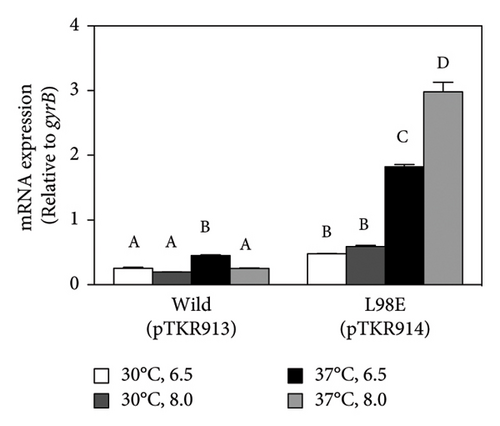
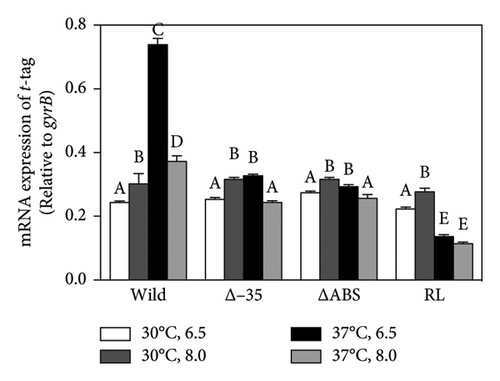
The N-terminal region (∼80 residues) of LTTR is relatively conserved across the LTTR family [27]. This region contains a helix-turn-helix domain (involved in DNA binding) and a linker helix (involved in protein intermolecular binding) [27]. The amino acid sequence of VirR did not show high similarity with that of Vibrio cholerae AphB, whose crystal structure is known [34]; however, a comparison of their secondary structures showed high similarity in the domain from the helix-turn-helix domain to α6 of AphB (Figure 2(a)). In LTTR family members, the region between β3 and α5 contains amino acids that form part of the coinducer-binding pocket involved in coinducer binding [27]. Because this region contains mutational hotspots, coinducer binding and responsiveness are altered [35–41]. P98, N100, and L101 mutations in AphB allow it to activate the promoter under nonpermissive culture conditions [34]. It is unclear if VirR requires coinducer binding for activation. To examine whether mutations in this region affect VirR function, we performed site-directed mutagenesis at positions corresponding to amino acid residues that lead to the formation of a constitutively active AphB. Wild-type and mutant VirR were expressed in a loss-of-function mutant of virR, and the activity of the vapA promoter was measured by the β-galactosidase reporter assay. High activity of the vapA promoter was observed in the strain expressing VirR with the L98E mutation even under noninducible conditions (30°C) (Figure 2(b)). The other mutations abolished the activity of the vapA promoter. To examine if the L98E mutation of VirR affects the transcription of the icgA operon, we determined the transcriptional levels of icgA and virS. The transcription of both genes markedly increased even under nonpermissive culture conditions when L98E VirR was expressed (Figure 2(c)). To further examine if PicgA2 is involved in this transcriptional control, PicgA2-t-tag was introduced in the virulence plasmid-cured strain with wild or mutant virR (Figure 2(d)). The transcription of t-tag significantly increased when L98E VirR was expressed (Figure 2(e)). These results suggest that the L98E mutation renders VirR constitutively active and could activate PicgA2 even under nonpermissive culture conditions.

3.4. Wild-Type and L98E VirR Bind to the icgA Promoter in the Same Manner
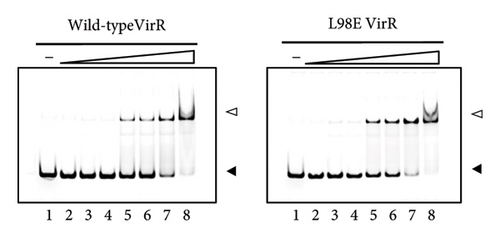
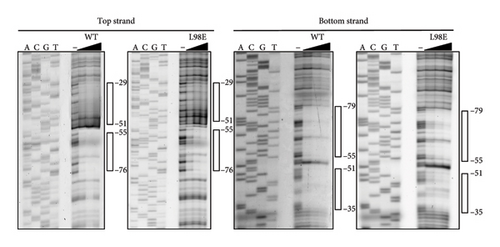
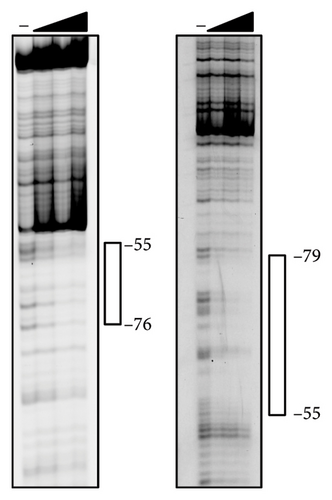
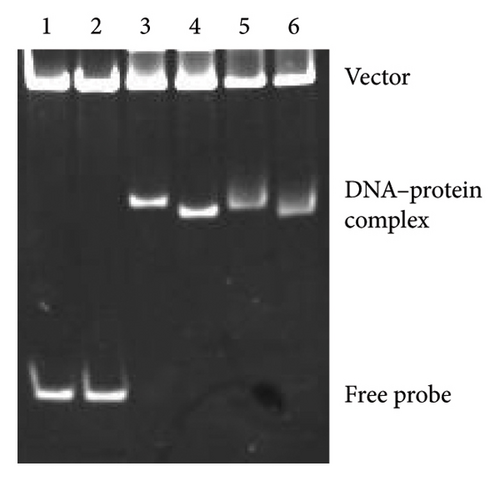
Because the vapA promoter was constitutively transcribed in the strain expressing L98E VirR, L98E VirR activates the icgA promoter even under noninducible conditions, leading to increased VirS levels and vapA promoter activity. If VirR binds to the coinducer and changes to the active form, the L98E mutation may mimic the conformational change of VirR due to coinducer binding. In other LTTRs, complexes of genes and bound transcription factors show different mobilities in EMSA from genes bound to transcription factor without coinducer [42]. To examine this hypothesis, EMSA was performed using wild-type VirR and L98E VirR. No difference was observed in complex mobility between mutant and wild-type VirR (Figure 3(a)). We performed DNase I footprinting to examine the binding site of wild-type and L98E VirR in detail. Wild-type VirR protected two regions of the top strand (−76 to −55 and −51 to −29) relative to the transcriptional start site of the icgA operon (Figure 3(b)). The downstream region (−51 to −29) was more weakly protected than the upstream region (−76 to −55). Two hypersensitive sites (−53 and −54) were observed between these protected regions, where the intensity of the band increased. In the bottom strand, two regions (−79 to −55 and −51 to −35) were protected, with a hypersensitive site in between. Similar protected regions and hypersensitive sites were observed with L98E VirR. Thus, wild-type and L98E VirR bind identical sites in the upstream region of PicgA2.
3.5. Mutations in the LTTR-Binding Site Abolish VirR Binding
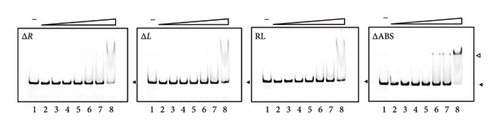
In the upstream region out of the two regions protected in DNase I footprinting, we identified a 17-bp region of partial dyad symmetry from position −74 to −58 in the top strand containing T–N11–A, which is characteristic of the LTTR-binding site (LTTR box) (Figure 3(c)). To examine whether this region is involved in VirR binding, gene fragments lacking the left (ΔL) or right (ΔR) half of the LTTR box or bearing substitutions from T–N11–A to A–N11–T (RL) were subjected to EMSA. Complex formation with VirR was not observed in all the cases tested (Figure 3(d)). These results suggest that this region is necessary for VirR binding. A decrease of free band and formation of a V-shaped complex were observed when the concentration of VirR was > 0.97 μM (also observed in Lane 8 of Figure 1(a), right). However, this binding was observed even with unrelated DNA fragments at this concentration (data not shown). Therefore, it was considered nonspecific binding.
3.6. The Downstream Binding Region Is Required for Maximum VirR Binding and Promoter Activation
Although the region from −51 to −29 was protected by VirR, no LTTR box was found within this region. To examine the effect of this region on VirR binding, gene fragments lacking the region from −51 to −34 were subjected to EMSA. Complex formation was concentration-dependent; however, compared with the wild-type fragments (Figure 3(a)), complex formation required a higher concentration of VirR (Figure 3(d)). These results suggest that the sequences within this region have a small but significant effect on VirR binding. Next, we tested the effect of deleting this region by DNase I footprinting. Regions −76 to −55 and −79 to −55 were protected in the top and bottom strands, respectively, but the downstream region was unprotected (Figure 4(a)). This result suggests that VirR specifically binds to the downstream region, although no LTTR box is included. To examine the effect of the downstream region in VirR-mediated transcriptional activation, this region was deleted from PicgA2-t-tag and introduced into the wild-type strain. The transcriptional level of t-tag reduced to that of the −35 deletion and RL mutation (Figures 3(c) and 4(b)) when the downstream region was deleted (Figure 4(b)), suggesting that VirR binding to this region is necessary for PicgA2 activation.
3.7. The Bending Angle of DNA by Wild-Type VirR and L98E VirR Is Approximately 35°
The presence of hypersensitive sites between the two VirR-protected regions suggests that VirR bends the icgA promoter upon binding. In other LTTRs, changes in the bending angle in the promoter influence transcriptional regulation [27, 43]. To examine if VirR bends DNA, a standard circular permutation assay was performed. A 50-bp gene fragment containing the protected region was cloned into the XbaI and SalI sites of pBend2. When this plasmid is cleaved with EcoRV and BamHI, respectively, DNA fragments of the same size are excised from the vector. When the plasmid is cleaved with EcoRV or BamHI, the VirR-binding region is at the center or end of the excised DNA, respectively (Figure 5(a)). The DNA fragment with the highest or lowest mobility is expected to bind VirR at the end or middle, respectively. The bending angle is estimated from the relative electrophoretic mobility retardation caused by bending. The complex with wild-type VirR ran faster when BamHI was used (Figure 5(b)). The bending angle caused by wild-type VirR was estimated to be 35°. The mobility of the complex upon L98E VirR binding was similar to that upon wild-type VirR binding.
3.8. VirR Interacts Each Other and Binds to RpoA
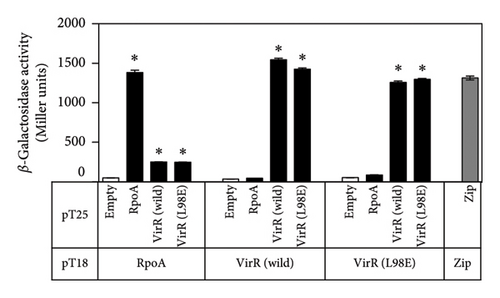
Most LTTRs bind DNA by forming homotetramers [44]. A bacterial two-hybrid system was used to analyze the interactions between VirR proteins [32]. In E. coli, where VirR was expressed as a fusion protein with T18 and T25, β-galactosidase activity was found to be similar to that of the positive control, the leucine zipper region of the yeast protein GCN4, which was also expressed as a fusion protein with T18 and T25 (Figure 6). This suggests that VirR proteins bind to each other with high affinity. VirR L98E had comparable β-galactosidase activity. When RpoA-T18 and T25-VirR were expressed, β-galactosidase activity was significantly higher than when T18 and T25 were expressed. Similar levels of β-galactosidase were detected in RpoA-T18 and T25-VirR L98E, suggesting that wild-type and L98E interact similarly with RpoA.
4. Discussion
VapA expression is regulated by temperature and pH [22, 23]. Two transcriptional regulators, VirS and VirR, are involved in this regulation [24–26]. The transcription of vapA is regulated by VirS, whose expression is transcriptionally regulated by VirR [24]. Additionally, virS transcription is regulated by temperature and pH, and the expression of VirS resulted in VapA expression in the absence of VirR even under noninducible conditions [24]. Therefore, quantitative changes in VirS may regulate VapA expression through these environmental stimuli. We found that PvapA was constitutively active in the strain that expressed mutant VirR (L98E). This result suggests that VirR is responsive to temperature and pH. A substitution at this residue renders VirR constitutive active as a transcriptional regulator.
Many LTTRs bind to a small molecule called the coinducer, which leads to structural changes, thereby controlling transcription [27]. It is unclear whether the coinducer is required for VirR-mediated transcriptional regulation, but in other LTTRs, amino acid residues in the region subjected to site-directed mutagenesis in this study are involved in coinducer binding [28, 45, 46]. Mutations in this region led to a coinducer-independent phenotype in many LTTRs [35–41]. Therefore, the coinducer may be involved in the regulatory function of VirR. Its cellular levels may change in response to temperature and pH, and by binding to VirR, it may change the amount of VirR that has undergone a structural change and regulate virS transcription.
The icgA operon is transcribed from the virR promoter (PvirR) and PicgA1 that is located within the coding region of VirR [25, 26]. PicgA1 is responsible for the transcriptional regulation of the icgA operon by temperature and/or pH [25]. However, this study indicated that PicgA2, between virR and icgA, is involved in VirR-mediated transcriptional regulation. Furthermore, 5’-RACE identified promoter sequences similar to the promoter consensus sequences of SigA and HrdB in M. tuberculosis and Streptomyces coelicolor, respectively. The virR and vapA promoters reportedly have similar sequences to the promoter consensus sequences of HrdB [25, 26]. SigA and HrdB are the principal sigma factors in each species, which are major components of the sigma factor during exponential cell growth. The features of their promoters indicate that VapA expression mainly occurs during the exponential growth phase [47]. IcgA is a transport protein belonging to the major facilitator superfamily. Deletion of icgA enhanced the bacterial growth within macrophages and significantly reduced macrophage viability [48]. The transcript of PicgA2 starts 1 bp upstream from the IcgA-coding ORF. Full-length IcgA is unlikely to be translated from this transcript because it lacks a ribosome binding sequence. Therefore, IcgA may be translated from mRNA transcribed from PvirR and PicgA1, because transcripts spanning virR and icgA were detected by an operon mapping experiment [25].
The results of EMSA and DNase I footprinting showed that VirR binds to the upstream region of PicgA2. Deletion of the HTH of VirR markedly reduced virS transcription [24]. This study showed temperature and pH regulate transcription from PicgA2, which is abolished in the virRΔHTH mutant (Figure 1(c)). Taken together, we conclude that VirR is the direct activator of PicgA2. DNase I footprinting showed VirR protected a wide region of ∼43 bp, which was divided into two regions interrupted by a hypersensitive site. An interrupted palindrome was found in the upstream protected region, which contained the T–N11–A motif, called the LTTR box [27]. No palindrome or LTTR box was found in the downstream protected region. Destruction of the palindrome and substitution of T–N11–A in the upstream protected region abolished VirR binding, although the downstream binding region was intact. Conversely, when the downstream protected region was deleted, VirR binding was concentration-dependent, but complex formation required a higher concentration of VirR, compared with the wild-type fragments (Figures 3(a), 3(d)). In the DNase I footprinting assay, the mutant probe displayed protection of the upstream region, whereas the downstream region was unprotected (Figure 4(a)). Transcription from this promoter was abolished when the downstream region was deleted (Figure 4(b)). These features in the nucleotide sequence of the binding site and the binding property of the transcriptional regulator are consistent with reports on other LTTRs [38, 49–51]. Therefore, we believe that the upstream and downstream regions correspond to the recognition and activation binding sites, respectively.
In the bacterial two-hybrid experiment, high β-galactosidase activity was detected when VirR-T18 and T25-VirR were coexpressed, suggesting that VirR monomers interact. Consistently, other LTTRs were reported to form homomers. With a few exceptions, such as CrgA and BenM, LTTR generally forms a complex with DNA as a homotetramer. LTTRs form a protomer through the linker helix domain, which connects the N-terminal helix-turn-helix domain with the C-terminal cofactor-binding domain. In addition, the protomers bind each other through their interface and form a tetramer (dimer of dimer). The high β-galactosidase activity of VirR-T18- and T25-VirR-coexpressed bacteria is presumed to reflect the stability of the coiled-coil structure formed between the linker helix domains of VirR. Considering the protected region in DNase I footprinting, VirR may form a tetramer and a complex with DNA, like other LTTRs.
In other LTTRs, conformational changes in the complex between regulator and DNA caused by the coinducer–regulator binding are detected as changes in mobility in EMSA and protected regions in DNase I footprinting. In AtzR of Pseudomonas sp. strain ADP, the presence of the coinducer, cyanuric acid, increased mobility in EMSA and decreased the protected region in DNase I footprinting [38]. Inducer-dependent shortening of the protected region in DNase I footprinting and relaxation of regulator-induced DNA bending are important features of the model proposed for LTTR activation, the so-called sliding dimer model [42, 52–56]. In this study, if the L98E substitution in VirR mimics structural changes due to coinducer binding, some differences in them might be observed. However, we did not detect differences in mobility and protected region between wild-type and mutant VirR. The reason for the discrepancy between in vivo and in vitro experiments is unclear. Crystal structure analysis of Klebsiella aerogenes CisB showed that the sulfate anion, and not the original coinducer, N-acetylserine, binds to the coinducer-binding site and is retained even after protein purification and crystallization [28, 57]. The ion-bound structure is believed to mimic the coinducer-bound active conformation. Consistently, in VirR, ions or small molecules might have bound VirR during the expression and/or purification of recombinant protein, resulting in the formation of an active conformation of wild-type VirR. In circular permutation analysis, no difference was found in the estimated bending angle between wild-type VirR and mutant VirR. The bending angle of DNA bound to wild-type VirR is ∼35°, which is considerably smaller than the bending angle of DNA in the absence of coinducer, reported in other LTTRs (range: 50°–100°) [27]. These results suggest that wild-type VirR is in an active conformation.
Another possible explanation for this discrepancy is that mutant VirR activates PicgA2 by a mechanism other than the sliding dimer model. Some LTTR family members become activated by coinducer binding, which changes their binding properties with RNA polymerase and promoter DNA [58]. Although DNA binding by wild-type VirR and L98E was similar, they may differ in their ability to attract RNA polymerase to the promoter and stabilize its DNA binding. In this study, RpoA binding was similar between wild-type and L98E. Studies must investigate their binding properties with other subunits of RNA polymerase, such as sigma factor.
In conclusion, this study revealed that VirR binds to the icgA operon promoter as a positive transcriptional regulator. The transcriptional response of the icgA operon to temperature and pH is mediated by VirR, although the mechanism of transcriptional activation by VirR is unclear and warrants further investigation.
Ethics Statement
This study does not contain any studies with human subjects or animals.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Tsutomu Kakuda: conceptualization, project administration, methodology, investigation, formal analysis, validation, writing–original draft preparation, writing–review and editing, and supervision.
Takashi Sato, Mari Takuhara, and Hirofumi Hagiuda: methodology and investigation.
Yasunori Suzuki: writing–review and editing.
Funding
No funds, grants, or other supports were received.
Acknowledgments
We thank Naotaka Iwagaki, Ryutaro Nakagawa, Yuuki Azami, Masato Sato, Ryo Sugioka, Madoka Ishigaki, and Yuusaku Nishiyori for their technical supports.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




