Optimizing Seed Germination of Acacia crassicarpa by Scarification and Plasma Fine Bubbles Treatments
Abstract
The availability of appropriate methods to increase germination capacity (GC), thereby increasing the growth and vigor of seedlings in the nursery, plays an important role in the success of tree planting in industrial plantation forests. The aim of this research was to determine the effect of scarification and plasma fine bubbles (PFBs) treatments on the GC, viability, and vigor of Acacia crassicarpa A. Cunn. Ex Benth. seeds, one of the important acacias trees in industrial plantation forests. A completely randomized design with two factors, namely, scarification (soaking in 80°C to cold water for 24 h and 96% H2SO4 for 15, 20, and 25 min) and PFBs water treatment with ozone concentrations of 2 and 3 ppm for 5 and 10 min was used. Our study showed that the combination of scarification with H2SO4 solution for 25 min and 3 ppm PFBs water for 10 min gave the highest GC (90.75%), germination rate (GR) (20.28% days−1), and germination value (GV) (1.41). This combination also provided better results compared with methods widely used in industrial plantation forests by increasing GC 76.2%, GR 103.1%, and GV 291.7% and increasing hypocotyl length from 3.76 cm to 6.15 cm, radicle length from 2.24 cm to 6.31 cm, normal seedling length from 6.11 cm to 12.20 cm, and vigor index (VI) from 1.2 to 11.07 compared with control. This combination has been proven to increase germination, growth, and vigor of A. crassicarpa seeds; therefore, this treatment combination is recommended for use at the operational level of industrial plantation forests.
1. Introduction
The availability of appropriate methods to increase germination capacity (GC) thereby increasing the growth and vigor of seedlings in the nursery plays an important role in the success of tree planting in industrial plantation forests. Acacia crassicarpa A. Cunn. Ex Benth., which has excellent wood quality for the pulp and paper industry in industrial forest plantations [1, 2], and is widely used for building construction, furniture, ships, firewood as well as preventing erosion, as a shade tree and can fix nitrogen in the air [3]. The species, which can grow fast and has high adaptability, has been planted on an area of around 700,000 ha, mostly in Indonesia [4] both on dry (mineral) land and wetland, such as swamps and peatlands [1, 5, 6]. As one of the acacias that are easily adapted to peat soils [7], the potential for planting A. crassicarpa in peatlands is increasing because Indonesia has around 14 million ha of peatlands which have great potential to be developed to support national development, especially related to economic strengthening and in enhancing employment opportunities [8]. This great potential, however, has not been accompanied by an efficient germination method to support its planting in large-scale industrial plantation forests.
A. crassicarpa seeds, which are categorized as orthodox with hard and thick seed coats, require pregermination treatment, such as scarification treatments [9–11]. Seed scarification is a method to remove or erode the seed coat or outer layer of the seed which will directly affect the physical and physiological traits of the seed by breaking dormancy and increasing seed viability and vigor [11–13]. A. crassicarpa seeds have very strong dormancy, which limits the absorption of oxygen and water and prevents embryo development and shoots emergence [11]. Seed scarification techniques are quite diverse, such as mechanical scarification [14], physical scarification such as soaking in hot water [12], and chemical scarification such as soaking in sulfuric acid [15, 16]. Several scarification treatments produced varying levels of germination. The most popular scarification treatment for A. crassicarpa is soaking them in hot water (80°C) to cold for 24 h, resulting in the germination of 39%–70% [9, 10, 17] while tearing the seed coat can produce 58%–72% [18]. Another way of scarification is by soaking the thick and hard-coated seeds in chemical solutions, such as sulfuric acid (H2SO4), hydrochloric acid (HCl), and hydrogen peroxide (H2O2) [19]. This has been tested by soaking them in H2SO4 for 30 min and with a GC of 68% [17]. Meanwhile, at the operational level, several industrial plantation forests that have applied the 98% H2SO4 soaking method for 15 min produced many seeds that did not germinate [20]. To increase germination, the authors in [21, 22] recommend soaking seeds in H2SO4 for 5 min, which are then tested using a paper test method in a germinator and resulted in a GC of 70% [23]. This technique can break the hard seed coat, thereby increasing the permeability of the seed to water and air [11, 12]. These differences in method and soaking time, however, need to be validated and tested to obtain the optimal scarification method with minimal seed damage so that it can be used on a large scale, especially at the operational level of industrial plantation forests.
Soaking seeds in H2SO4 can soften the seed coat so that water can more easily enter the seed tissue, but H2SO4 itself has no direct effect in increasing seed germination [24]. For this reason, other treatments are needed to stimulate seed germination such as seed invigoration or priming techniques. One of the new seed priming technologies is plasma fine bubbles (PFBs) technology, which produces ultrafine bubbles containing ozone and reactive oxygen species (ROS). Ozone is a bluish-colored molecule that evaporates easily at room temperature. The use of high-voltage electricity and ultraviolet light can produce ozone from oxygen reactions [25] by plasma discharges [26]. Plasma-generated ozone gas and the gas ozone were injected into the water through a fine bubble nozzle which produces ROS [27]. ROS produced by fine bubbles has an important role in seed germination by providing facilities in the form of cell walls needed for cell extension. ROS in the right amount will improve the physiological processes of living organisms [27, 28].
PFBs induces mechanical stresses in the seed coat, resulting in cracks and defects [29]. Increased seed coat permeability in plasma-treated seeds can increase water imbibition, which might be due to an increase in the concentration of oxygen- and nitrogen-containing groups at the surface of the plasma-treated seeds [30, 31]. PFBs technology can produce ROS by irradiating water using plasma. Recent studies on seed physiology prove that ROS has an important role in cell signals that support seed germination and dormancy [32, 33]. ROS plays a very important role in increasing gibberellin levels, which are needed to break seed dormancy which causes seed germination [28, 32]. The oxidation reaction of ozone molecules into ROS also can increase the permeability of the seed coat [31, 34]. Several previous studies reported the effectiveness of ozone treatment in accelerating seed germination, such as on Lycopersicon esculentum [35], Lens culinaris [36], Achillea clavennae, Plantago alpina, and Silene suecica [37]. Ozone treatment will also inactivate pathogens (such as bacteria and fungi) of seeds so that the seeds are free from seed diseases [38] without causing a negative effect on germination [39].
The combination of appropriate scarification treatment with invigoration using PFBs are expected to increase the germination and seedling growth of A. crassicarpa more efficiently. Optimal scarification treatment is needed because inappropriate treatment can damage the seeds, while the ozone PFBs treatment, which is mainly used on agricultural species, needs to be tested on tropical tree species. Therefore, the aim of this research is to analyze the combination of scarification treatment and PFBs technology to increase the viability and vigor of A. crassicarpa seeds. The results can be applied not only at the operational level of industrial plantation forest companies but can also be used for other purposes such as conservation and rehabilitation. The optimal combination of scarification and priming using PFBs water treatment is believed to be able to break dormancy and improve the viability and vigor of A. crassicarpa seeds because previous research shows that scarification treatment with H2SO4 can increase seed germination [12, 15, 16] and PFBs treatment also can increase seed germination and vigor, such as in Oryza sativa [31] and Solanum lycopersicum [40] seeds.
2. Materials and Methods
2.1. Materials
A. crassicarpa seeds were collected from the seed production area in Mandi Angin Village, Siak, Riau Province (0.82811 S, 101.46875 E, 8 m asl) managed by PT. Arara Abadi (Sinar Mas Forestry Group). The seeds were processed at the Research and Development Division of PT. Arara Abadi and packed in airtight plastic with a seed moisture content of 6.7%. The seed was stored in a dry cool storage room (temperature: 4°C–8°C and relative humidity: 40%–50%) at the Seed Testing Laboratory, Forestry and Environment Standard and Instrument Implementation Institute, Bogor, West Java for 2 years.
2.2. PFBs Preparation
PFBs water production was carried out at the Biosystems and Environmental Engineering Laboratory, Bogor Agricultural University (IPB University). PFBs water was produced using an ozone generator and an oxygen concentrator by installing a hose between the two devices and placing the other hose in a container filled with water so that the ozone generator can produce ozone in the form of PFBs water. This system works where the ozone generator produces plasma which forms ozone gas [26]. The ozone gas from plasma was dissolved into the water through a fine bubble nozzle, which is then called a PFBs. The process of making PFBs water was carried out for 2–5 min; if the water has changed color to milky white, it means the ozone has flowed. The ozone content was measured using a dissolved ozone detector. The ozone concentration in the PFBs water used in this research was 2 ppm and 3 ppm.
2.3. Experimental Design and Seed Sowing
A factorial completely randomized design (CRD) was used to test the effectiveness of two (2) seed treatment factors, namely, seed scarification (5 treatments) and application of seed priming using PFBs water (5 treatments). A total of 10,000 A. crassicarpa seeds were divided into 25 combination treatments and each treatment was repeated with four (4) replications with each replication consisting of 100 seeds. The first factor was seed scarification consisted of control (seeds without treatment) (S0), soaking the seeds in hot water (80°C) which was allowed to cool for 24 h (S1), soaking the seeds in 96% H2SO4 for 15 min (S2), soaking the seeds in 96% H2SO4 for 20 min (S3), and soaking the seeds in 96% H2SO4 for 25 min (S4). Determination of the soaking time for scarification treatment was based on previous studies on A. crassicarpa seeds [17, 18, 20]. The second factor was seed priming using PFBs water consisting of control (seed without treatment) (P0), priming the seeds in 2 ppm ozone concentration of PFBs water for 5 min (P1), priming the seeds in 2 ppm ozone concentration of PFBs water for 10 min (P2), priming the seeds in 3 ppm ozone concentration of PFBs water for 5 min (P3), and priming the seeds in 3 ppm ozone concentration of PFBs water for 10 min (P4). The duration of priming in PFBs water was determined based on the preliminary experiment.
Seeds were sown using the top paper test method in Petri dishes with straw paper media and germinated in Germinator Type IPB 73-2A/B. The temperature and humidity inside the germinator were set at 24°C–30°C and 90%–95%, respectively, with a photoperiod of 8 h of light and 12 h of darkness. Previously, the Petri dishes and straw paper were sterilized using an oven at 103°C for 2 h. Each Petri dish was filled with 3 straw papers soaked in distilled water and then 100 seeds were sown on top. The humidity of the straw paper media is maintained so that the germination process can run optimally.
2.4. Seed Viability and Vigor Measurement
2.5. Data Analysis
The data obtained were processed using a factorial CRD. Each treatment was analyzed to test the effect of seed treatment on A. crassicarpa seed germination parameters using analysis of variance with SAS 9 for Windows software. If the analysis of variance finds significant results on several germination parameters with a p value < a (0.05), then data analysis continues with the Duncan multiple range test (DMRT).
3. Result
Seed scarification treatment using hot water and H2SO4 affected all germination parameters of A. crassicarpa seeds, while PFB water treatment only had a significant effect on GC, GR, HL, RL, NSL, and VI. The interaction between scarification and PFBs water treatments had a significant effect on all seed germination parameters (Table 1). This significant treatment interaction effect indicates that this combination of treatments has the potential to produce the best treatment to increase the seed viability and vigor of A. crassicarpa.
| Parameters | Source of variation | Degree of freedom | F-test |
|---|---|---|---|
| GC (%) | Scarification (S) | 4 | 563.641 |
| Ozone plasma fine bubbles water (P) | 4 | 3.712 | |
| Interaction S ∗ P | 16 | 3.282 | |
| GR (% days−1) | Scarification (S) | 4 | 385.051 |
| Ozone plasma fine bubbles water (P) | 4 | 6.911 | |
| Interaction S ∗ P | 16 | 4.231 | |
| GMT (days) | Scarification (S) | 4 | 53.971 |
| Ozone plasma fine bubbles water (P) | 4 | 0.70ns | |
| Interaction S ∗ P | 16 | 2.852 | |
| GV | Scarification (S) | 4 | 77.871 |
| Ozone plasma fine bubbles water (P) | 4 | 2.21ns | |
| Interaction S ∗ P | 16 | 2.262 | |
| HL (cm) | Scarification (S) | 4 | 16.561 |
| Ozone plasma fine bubbles water (P) | 4 | 7.161 | |
| Interaction S ∗ P | 16 | 4.751 | |
| RL (cm) | Scarification (S) | 4 | 35.431 |
| Ozone plasma fine bubbles water (P) | 4 | 4.392 | |
| Interaction S ∗ P | 16 | 2.332 | |
| NSL (cm) | Scarification (S) | 4 | 33.061 |
| Ozone plasma fine bubbles water (P) | 4 | 7.831 | |
| Interaction S ∗ P | 16 | 2.072 | |
| VI | Scarification (S) | 4 | 33.061 |
| Ozone plasma fine bubbles water (P) | 4 | 7.831 | |
| Interaction S ∗ P | 16 | 2.072 | |
- Note: ns = not significant at the 95% confidence level.
- 1Significant at the 99% confidence level.
- 2Significant at the 95% confidence level.
S4P4 treatment gave the best results for GC, GR, and GV of A. crassicarpa seeds (Figure 1). This treatment was able to increase the GC, GR, and GV of the control, respectively, from 19.5% to 90.75%, 3.2% days−1–20.3% days−1, and 0.22 to 1.4. When compared with the operational treatment applied in industrial plantation forest companies, namely, soaking seeds in 96% H2SO4 for 15 min (S2P0), the S4P4 treatment gave an increase in GC, GR, and GV of 76.2%, 103.1%, and 291.7%, respectively. The germination process was faster with the MGT in the control (S0P0), S2P0, and S4P4, respectively, 7.14 days, 7.98 days, and 5.77 days. These results prove that the S4P4 treatment provided the best A. crassicarpa seed germination results with the largest number of germinated seeds and a shorter germination process.
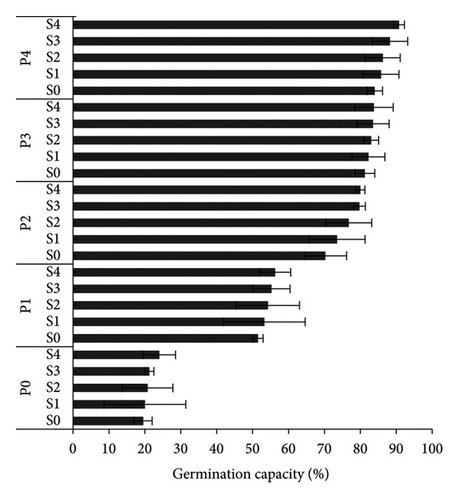
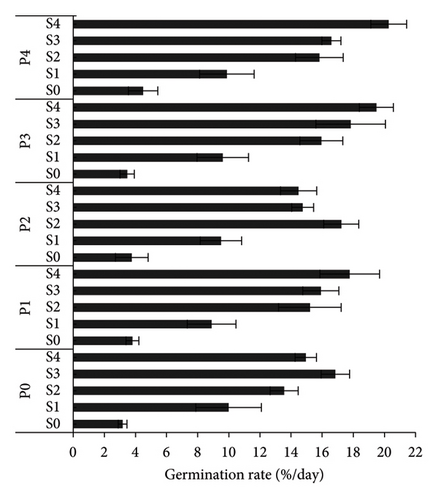
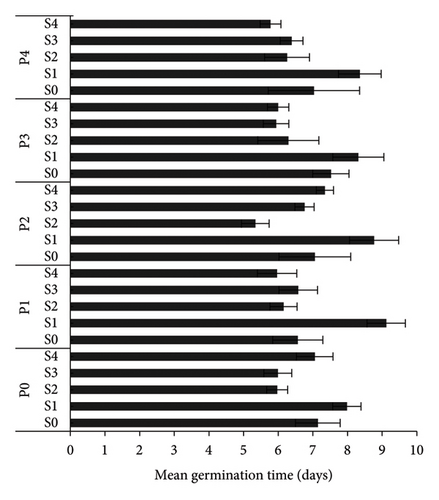

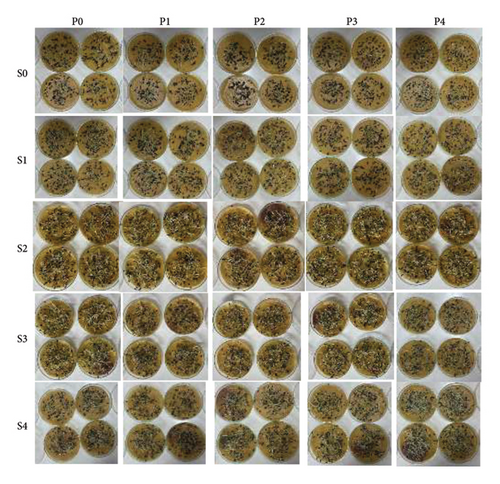
The S4P4 treatment was also able to increase seedling growth, with an HL of 6.15 cm (an increase of 182.9% compared with the control), an RL of 6.31 cm (an increase of 63.5% compared with the control), and an NSL of 12.20 cm (99.7% increase compared with control) (Table 2). The results of this treatment also increased the growth of A. crassicarpa seedlings when compared with the treatment that is usually operationally carried out in industrial forest plantation companies (S2P0), i.e., increase in the length of the HL, RL, and NSL by 112.4%, 23%, and 50.4%, respectively. The VI also increased significantly (Figure 2). The S4P4 gave the highest VI (11.07) or an increase of 807.3% from the control and 162.3% from the treatment that is widely used operationally (S2P0). Figure 3 shows the germination performance of A. crassicarpa seeds in each treatment. These results illustrate that seeds with high vigor that germinate more quickly will have higher seedling growth and VI as well. In general, in addition to increasing the germination of A. crassicarpa seeds, the S4P4 treatment also provided better seedling growth with stronger seedling vigor.
| Seed treatments | Hypocotyl length (cm) | Radicle length (cm) | Normal seedling length (cm) |
|---|---|---|---|
| S0 ∗ P0 | 3.76i | 2.23e | 6.11f |
| S0 ∗ P1 | 4.47gh | 3.10cd | 7.69e |
| S0 ∗ P2 | 4.82efgh | 3.03d | 7.71e |
| S0 ∗ P3 | 4.36hi | 3.80bcd | 8.01de |
| S0 ∗ P4 | 5.54bcdef | 3.48bcd | 8.99cde |
| S1 ∗ P0 | 5.00defgh | 2.98d | 8.11de |
| S1 ∗ P1 | 4.92defgh | 3.45bcd | 8.16de |
| S1 ∗ P2 | 4.91defgh | 3.50bcd | 8.19de |
| S1 ∗ P3 | 4.93defgh | 3.40bcd | 8.19de |
| S1 ∗ P4 | 4.84efgh | 3.12cd | 8.24de |
| S2 ∗ P0 | 4.89defgh | 3.48bcd | 8.33de |
| S2 ∗ P1 | 5.24defg | 3.26bcd | 8.41de |
| S2 ∗ P2 | 5.25def | 3.43bcd | 8.74cde |
| S2 ∗ P3 | 5.54bcdef | 3.49bcd | 9.03cde |
| S2 ∗ P4 | 5.35cdef | 3.55bcd | 8.85cde |
| S3 ∗ P0 | 5.55bcdef | 3.33bcd | 8.73cde |
| S3 ∗ P1 | 5.53bcdef | 3.33bcd | 8.76cde |
| S3 ∗ P2 | 5.29def | 3.68bcd | 8.88cde |
| S3 ∗ P3 | 5.66bcd | 3.43bcd | 8.94cde |
| S3 ∗ P4 | 5.58bcde | 3.50bcd | 9.00cde |
| S4 ∗ P0 | 5.49bcdef | 3.70bcd | 9.13cd |
| S4 ∗ P1 | 6.70a | 4.04b | 10.43b |
| S4 ∗ P2 | 6.06abc | 3.95bc | 10.01bc |
| S4 ∗ P3 | 6.57a | 3.65bcd | 10.03bc |
| S4 ∗ P4 | 6.15ab | 6.31a | 12.20a |
- Note: See Figure 1 for information of the seed treatment, the same letters behind the numbers in the same column indicate that they are not significantly different at the 95% confidence level.

4. Discussion
In general, H2SO4 treatments can soften the thick and hard seed coat of A. crassicarpa seeds so that the inhibition process can run optimally, while PFBs water treatment is able to increase the metabolic process of seeds so that the seeds get energy to germinate more quickly and grow normally. Scarification treatment by soaking the seeds in 96% H2SO4 is quite effective in breaking seed dormancy. The seeds used in this study have been stored for 2 years and have deteriorated [46], requiring invigoration treatment to increase their viability and vigor. In this study, PFBs water treatment was able to increase the germination and vigor of A. crassicarpa seeds. The interaction (combination) of seed scarification treatment and soaking seeds in PFBs water had a significant effect on all seed germination parameters (GC, GR, MGT, GV, HL, RL, NSL, and VI).
The combination treatment of 96% H2SO4 scarification for 25 min and 3 ppm ozone concentration of PFBs water for 10 min gave the best results on GC (90.75%), GR (20.28% days−1), GV (1.41), RL (6.31 mm), NSL (12.20 mm), and VI (11.07) of A. crassicarpa seeds. This treatment was able to increase GC up to 365.3% compared with the control (without seed treatment) and 76.2% compared with operational seed sowing treatment in industrial forest plantation companies (S2P0). Increasing GC is very important to increase the success of seedling production in the nursery [13, 36]. Likewise, the GR increased 5 times compared with the control and 2 times compared with the operational treatment of sowing seeds in industrial forest plantation companies (S2P0). GR is an important indicator of the vigor of seed growth capacity. Seeds that have the ability to germinate quickly can be more resistant to suboptimal environments [44, 47]. The higher the GR, the better the seed vigor. A similar trend was also obtained in the S4P4 treatment.
The use of H2SO4 to break seed dormancy has long been used in testing the germination of seeds with strong physical dormancy [21, 41, 48, 49]. Scarification treatment can soften the thick seed coat, thereby opening the way for water and oxygen to enter the seed easily for inhibition and germination process so that the seed can germinate more quickly [24, 50, 51]. The application of H2SO4 in seed scarification treatment must be handled carefully because H2SO4 is a dangerous material, and it is necessary to determine the optimal time required [52, 53]. The effectiveness of seed scarification with H2SO4 varies depending on the characteristics of the seeds of each species. It is recommended to soak in 50% H2SO4 for 15 min in Zanthoxylum armatum seeds [48], 98% H2SO4 for 30 min in Crotalaria senegalensis seeds [54], 98% H2SO4 for 45 min in Capparis spinosa seeds [49], and 96% H2SO4 for 30 min in Acacia tortillas seeds [55]. Soaking seeds for too long in the H2SO4 solution will result in damage to important parts of the seed embryo.
The use of ozone in PFBs water to increase seed viability and vigor, especially tropical forest tree seeds, is still very limited. Several positive responses of ozone on seed germination have been shown on agricultural plants, such as Lycopersicon esculentum [35], Lens culinaris [36], Achillea clavennae, Plantago alpina, and Silene suecica [37]. In this study, ozone in PFBs water was able to significantly increase the germination of A. crassicarpa seeds. The presence of PFBs-containing ROS can function as secondary signaling molecules to increase seed germination and growth [26, 29]. PFBs technology contains ROS which are formed from the use of high voltage electricity so that the temperature of the PFBs water will be higher. ROS is a result of metabolism originating from incomplete or partial reduction of oxygen which will form radical anion superoxide (O2−), H2O2, and hydroxyl radicals (HO) [27]. This component is believed to encourage seeds to germinate in the direct plasma exposure process [29]. The role of ROS is very important for growth by providing cell wall requirements to elongate cells [56]. The components contained in ROS are the main factors that encourage seed germination and growth. Plasma that produces water causes physiochemical changes and plasma water provides signals and encourages seed germination and root and vegetative growth, as well as plant reproduction [53].
ROS plays a very important role in increasing gibberellin levels which are needed to break seed dormancy and provide shorter seed germination times. ROS can increase gibberellin levels in seeds [35]. Gibberellin is a hormone found in plants to stimulate plant growth and development. Gibberellins stimulate seed germination, vegetative to flowering, and seed development with the interaction of environmental factors [57]. The accumulation of H2O2 contained in ROS can affect hormonal balance by increasing gibberellin [32]. Furthermore, ROS also functions to provide seed protection from pathogen attacks. H2O2 produced by ROS will inhibit the entry of pathogens and fight cell infection in seeds [32]. Ozone treatment will also inactivate pathogens (bacteria and fungi) of seeds so that the seeds are free from seed diseases [38] without having a negative effect on germination [39].
5. Conclusions
The most effective treatment to increase the viability and vigor of A. crassicarpa seeds was a combination of scarification with 96% H2SO4 solution for 25 min and a priming technique using PFBs water containing 3 ppm ozone for 10 min. Therefore, this combination treatment is recommended for use on an operational scale to improve seed germination and vigor of A. crassicarpa.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this study. This research was funded by Join Collaboration Research Rumah Program, Biological and Environmental Research Organization.
Acknowledgments
All authors would like to thank PT. Arara Abadi (Sinar Mas Forestry) for providing Acacia crassicarpa seed samples and the Biosystems Environmental Engineering Laboratory, IPB University, and the Tree Seed Testing Laboratory, Forestry and Environment Standard and Instrument Implementation Institute, Bogor, for facilitating this research.
Open Research
Data Availability Statement
Data are contained within the article.




