Diagnostic Value and Clinical Validation of Acute Thyrotoxic Myopathy Symptom Score in Acute Thyrotoxic Myopathy
Abstract
Objectives: Acute thyrotoxic myopathy (ATM) is characterized by bulbar paralysis and limb myasthenia on the basis of hyperthyroidism, which can cause respiratory failure, coma and death. However, the diagnosis of ATM is challenging. The purpose of this study was to develop a symptom rating scale named Acute Thyrotoxic Myopathy Symptom Score (ATMSS) to assist in the early diagnosis of ATM in clinical practice.
Methods: (1) The ATM reference cohort was constructed by searching for patients diagnosed with ATM or thyrotoxic bulbar palsy in the PubMed, Embase and Web of Science databases (ATM cohort); (2) the ATMSS was formulated based on the ATM cohort after multidisciplinary discussion; and (3) 38 patients with ATM and 40 patients with Graves’ disease (GD) were enrolled in the study; (4) receiver operating characteristic (ROC) curve analysis was applied to appraisal diagnostic value of the ATMSS for differentiating patients with ATM from patients with GD; (5) we also evaluated the correlation between ATMSS and age of onset, sex, thyroid function and thyroid-related antibody.
Results: The ATMSS showed better diagnostic value at an optimal cut-off value of 4.5 [area under the curve (AUC) = 0.978; 95% confidence interval (CI) = 0.949–1; p < 0.0001; sensitivity = 94.7%; specificity = 95%]. The ATMSS was positively correlated with free triiodothyronine (FT3) [Spearman correlation coefficient (rs) = 0.409, p < 0.001], free thyroxine (FT4) (rs = 0.486, p < 0.001) and thyrotrophin receptor antibody (TRAb) (rs = 0.332, p = 0.003), but negative correlation with the age of onset (rs = −0.349, p = 0.002).
Conclusions: We developed a symptom rating scale named the ATMSS, which is effective in diagnosing ATM from GD, and ATMSS is positively correlated with thyroid function and TRAb, but negative correlation with the age of onset. ATMSS can be used as an additional tool for diagnosing ATM.
1. Introduction
Acute thyrotoxic myopathy (ATM) is a serious and relatively rare clinical complication of hyperthyroidism that mainly manifests as bulbar paralysis and is also known as acute thyrotoxic bulbar palsy [1, 2]. Symptoms of bulbar paralysis, such as dysphagia, dysphonia, dysarthria, dyspnoea and muscle weakness of limbs, can quickly appear in this disease and are accompanied by aspiration pneumonia and hyperthyroid crisis, causing respiratory failure, drowsiness and coma until death [3, 4]. With early identification of ATM and timely administration of antithyroid drugs (ATDs), beta-blockers and other drugs, the disease can significantly recover in a short time [5]. At present, the understanding of this disease is based mainly on case reports. There are no clear diagnostic criteria or specific related tests in domestic or foreign guidelines, and the diagnosis is not easy and usually based on the combination of clinical symptoms and retrospective diagnosis after follow-up. Some patients with ATM may not have typical symptoms of bulbar paralysis [6, 7], and some patients with hyperthyroidism may also present with muscle weakness [1] or with atypical hyperthyroidism symptoms at the same time [8]; in addition, there may be insufficient clinical understanding about ATM, and all of these conditions can make the diagnosis of ATM difficult. Therefore, it is necessary to develop an effective method to assist in the diagnosis of ATM.
In this study, by reviewing the clinical cases of ATM publicly reported before 2020 and summarizing the clinical characteristics of the patients, we developed a symptom score called Acute Thyrotoxic Myopathy Symptom Score (ATMSS) to assist in the diagnosis of ATM. The diagnostic efficacy of ATMSS was evaluated in clinical patients with ATM and Graves’ disease (GD). The ATMSS is simple to administer in the form of questionnaires and physical examinations, which is expected to help clinicians better identify ATM, reduce misdiagnosis and provide help for the early treatment of ATM. We also evaluated the correlation between ATMSS and thyroid-related markers, providing a clinical basis for further research on the pathogenesis of ATM.
2. Methods and Materials
2.1. Determination of Reference Cohorts and Formulation of ATMSS
Case reports of patients with ATM or thyrotoxic bulbar palsy were screened by searching electronic databases (PubMed, Web of Science and Embase) with the following text terms: ‘thyrotoxic myopathy’, ‘thyrotoxic bulbar palsy’ and ‘thyrotoxicosis and dysphagia’. The search time frame was from the establishment of the database to 31 December 2019, and a total of 35 patients considered to be ATM or thyrotoxic bulbar palsy were included in the ATM reference cohort. By analysing the clinical characteristics of the reference cohort, combined with the diagnostic features of bulbar palsy, it was found that ATM patients most commonly had seven clinical features, namely, nasal reflux, dysphagia, hoarseness, dysarthria, dyspnoea, muscle weakness and abnormal gag reflex. The selection of items included in the ATMSS was based on the above clinical features and designed by the available literature [9–11], expert opinion, preliminary experiments, the clinical experience of the authors’ team and discussion with neurologists from the Department of Neurology of the First Affiliated Hospital of Guangxi Medical University. A total of 19 related items were designed in the ATMSS. Each clinical symptom is assigned a total score of eight points (hoarseness is considered to be a manifestation of pitch and volume changes in dysarthria, combined as dysarthria [12]), except for gag reflex assignment, which is assigned for two points as a single test item. The total score ranges from 0 to 42 consisting of the sum of the scores for each item, with higher scores predicting more significant clinical presentation. The ATMSS was previously evaluated in 10 newly diagnosed ATM patients to confirm that their ATM-related symptoms were adequately detected.
2.2. Subjects and Verification of the Application of ATMSS
The ATM group included 38 patients with ATM who were diagnosed and treated at the Department of Endocrinology of the First Affiliated Hospital of Guangxi Medical University from January 2020 to February 2024. The diagnosis was based on the following criteria: (1) a definitive diagnosis of GD which was confirmed by symptoms of hyperthyroidism, increased free triiodothyronine (FT3), free thyroxine (FT4) and thyrotrophin receptor antibody (TRAb), diffuse goitre on ultrasound; (2) one or more symptoms of bulbar palsy, such as nasal reflux, hoarseness, dysarthria, dysphagia or dyspnoea, were diagnosed through the research team; and (3) other diseases that could cause bulbar paralysis need to be ruled out, such as (i) retrosternal goitre was detected via thyroid ultrasound; (ii) a diagnosis of myasthenia gravis via neostigmine test; (iii) results of brain magnetic resonance imaging (MRI) and other methods of examination showing central nervous system diseases, such as acute medullary vascular occlusive cerebral infarction, brainstem inflammation, acute myelitis (brainstem type), multiple sclerosis, botulism and trauma; (iv) a diagnosis of hypokalaemia or hypercalcaemia by serum electrolyte examination; and (v) the presence of throat diseases, such as chronic pharyngitis, pharyngeal tumour revealed on laryngoscopy or other methods. Patients who met the above three criteria were considered patients with suspected ATM. (4) If the above symptoms were significantly relieved after treatment for ATDs or combined with steroid, β-blockers and energy support by follow-up for 1 week to 2 months, the patient was considered to have ATM.
In addition, we recruited 40 patients with GD as a control group. GD was confirmed by symptoms of hyperthyroidism only, and increased thyroid hormones and TRAb diffuse goitre on ultrasound. Patients with any complication of hyperthyroidism were not included. All subjects were at the stage of newly diagnosed hyperthyroidism or relapse after medication withdrawal at the time of inclusion and were in a drug-free state. Exclusion criteria for all subjects were as follows: (i) aged < 14 years; (ii) pregnant; (iii) suffering from other endocrine diseases, such as diabetes, pituitary and adrenal diseases; (iv) those with severe acute or chronic diseases, such as heart failure, renal failure and respiratory failure; and (v) those with other autoimmune diseases, such as rheumatic diseases, systemic lupus erythematosus and neuromuscular diseases.
All patients with suspected ATM and GD underwent the detection of thyroid function such as FT3, FT4, thyroid-stimulating hormone (TSH) and thyroid-related antibody, such as TRAb, thyroglobulin antibody (TGAb), thyroid peroxidase antibody (TPOAb) and ATMSS evaluation. FT3, FT4 and TSH were tested by electrochemiluminescence (Beckman Coulter Inc.). TRAb, TGAb and TPOAb were measured by radioimmunoassay. The ATMSS is implemented by clinicians within the study group through training.
2.3. Protocol Approval and Patient Consent
This study was performed in line with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. All participants or their legal guardians provided informed consent.
2.4. Statistical Analysis
SPSS 26.0 was used for the statistical analysis. Normally distributed data are expressed as the mean ± standard deviation ( ± s). Independent sample t tests and one-way analysis of variance (ANOVA) were used for comparisons. Non-normally distributed measurements are presented as the median (M), 25th to 75th percentiles (P25-P75) and were compared using the Mann—Whitney U test. Spearman correlation analysis was used to analyse the correlation between non-normally distributed variables. Receiver operating characteristic (ROC) curve was used to analyse the sensitivity and specificity of the ATMSS for the diagnosis of ATM. The optimal cut-off was determined as the value corresponding to the maximum Youden index (sensitivity + specificity-1). p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Reference Cohort of Patients With ATM
Thirty-five patients screened out in the databases with the diagnosis of ATM or thyrotoxic bulbar palsy were selected for the ATM cohort. In the cohort, the male-to-female ratio was 1 : 1.06, the mean age at first manifestation was 50.77 (±18.05) years, all patients underwent thyroid function testing and were diagnosed with hyperthyroidism in the ATM cohort, 33/35 patients had dysphagia, 21/35 had varying degrees of hoarseness, 4/35 had dysarthria, 14/35 had nasal reflux, 28/35 had muscle weakness predominantly proximal limb weakness, 6/35 had dyspnoea, and another 4/35 had abnormal gag reflex. The above clinical manifestations were significantly relieved after treatment with ATDs or combined beta-blockers, steroids or iodine. The median recovery time was 4 (1–8) weeks. Patients with other disorders that cause bulbar paralysis symptoms, such as central nervous system lesions, myasthenia gravis, throat and oesophagus imbalances, were excluded from the diagnosis. The ATM cohort data, such as sex, age at onset, bulbar palsy-related symptoms, response to treatment, therapeutic medications and disease duration, are displayed in Table 1.
| Age of onset (years) | Sex (M : F) | Hoarseness (N/T) | Dysarthria (N/T) | Nasal reflux (N/T) | Dysphagia (N/T) | Muscle weakness (N/T) | Dyspnoea (N/T) | Abnormal gag reflex (N/T) | Treatment | Time to resolution (weeks) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATM reference cohort of 35 patients | 50.77 (±18.05) | 1 : 1.06 | 21/35 | 4/35 | 14/35 | 33/35 | 28/35 | 6/35 | 4/35 | ATDs or combined BBs, steroid, iodine | 4 (1, 8) |
- Note: The data are expressed as the mean ± standard deviation and 25th–75th percentile (P25–P75). F, female; M, male; N, number of people with symptoms; T, total number of people.
- Abbreviations: ATDs, antithyroid drugs; BBs, beta-blockers.
3.2. Diagnostic Score Scale ATMSS
- i.
The use of this scale should be based on a clear diagnosis of hyperthyroidism;
- ii.
ATMSS should be performed on the basis of exclusion of other diseases causing bulbar palsy;
- iii.
Patients with positive limb strength assessment alone are not suitable for this scale.
-
Algorithm 1: Symptom scores of patients with ATM.
-
ATMSS
- A.
Nasal reflux
- 1.
Do you have a cough or feel like coughing after drinking water? 0: None; 1: Only choking; 2: Cough after drinking water.
- 2.
Is there regurgitation from the nasal cavity? 0: No; 1: Yes, but not frequently (no more than 3 times a day); 2 frequently (more than 3 times a day).
- 3.
In the drinking water test, the patient sits upright, drinks 30 mL of warm water and observes (if the patient chokes severely, the test will be stopped).
-
0: Swallowed water smoothly once; 1: Swallowed more than 2 times without choking; 2: Swallow it once, but choke; 3: Swallow more than 2 times, but choke; 4: Frequent choking and not being able to swallow it all.
- B.
Dysphagia
- 1.
Is it difficult to swallow when eating? 0: No; 1: Yes.
- 2.
Is it more strenuous to eat solid foods than to eat semiliquid (or liquid) foods? 0: No; 1: Yes.
- 3.
Is there a situation where medicine hangs in the throat and is difficult to swallow when taking medicine? 0: No; 1: Yes, but on continuous medication; 2: Present at the beginning of medication.
- 4.
Do you vomit after swallowing food or medicine? 0: No; 1: Yes.
- 5.
Repetitive saliva swallowing test: The patient swallows as quickly as possible within 30 s, and the number of completed swallows is recorded as 0: ≥ 3 times; 1: 2 times; 2: 1 time; 3: 0.
- C.
Dysarthria
- 1.
Whether there is a hoarseness or slurred pronunciation that is unconsciously or noticed by family members? 0: No; 1: Yes.
- 2.
Which of the following is the case?
-
0: None of the following cases exist; 1: Pitch becomes low or pronunciation is not fluent; 2: nasal or slurred articulation (usually none); 3: inability to complete long sentences or fatigue after normal speaking for a long time (can be observed during examination to distinguish); 4: Inability to speak.
- 3.
Patients were asked to sit and count from 1 to 100 at a constant rate.
-
0: can be counted smoothly; 1: can be counted, but it requires multiple pauses or slurred pronunciation; 2: can count over 50; 3: cannot count past 50.
- D.
Dyspnoea
- 1.
Is cough laborious? 0: No; 1: Yes (It is recommended that the patient cough forcefully and observe whether cough is strong).
- 2.
Whether there is any sputum that is difficult to cough up. 0: No; 1: Yes.
- 3.
Is there chest tightness or difficulty breathing? 0: No; 1: Yes.
- 4.
Can a decrease in respiratory mobility be observed under calm conditions? 0: No; 1: Yes.
- 5.
Is it possible to take 5 deep breaths in a row? 0: can be performed; 1: can be performed less than five times; 2: can be performed less than four times; 3: can be done fewer than three times; 4: can be done fewer than two times.
- E.
Gag reflex
-
Gag reflex. 0: sensitive; 1: sluggish; 2: not elicited.
- F.
Muscle weakness
- 1.
The induced upper limb fatigue time was recorded with the arms raised horizontally, and the angle between the upper limb and body was 90° for 120 s.
-
0: Can be completed; 1: can maintain ≥ 90 but < 120 s; 2: can maintain ≥ 60 but < 90 s; 3: can maintain ≥ 10 but < 60 s; 4: can maintain < 10 s.
- 2.
The patient attempted to complete 10 squats in a row, with both arms raised as flat as possible. 0: can be done almost without support; 1: can complete 10 squats but need to be supported by arms; 2: can complete 5 reps or more and less than 10 reps of squats with support of arms; 3: cannot complete 5 squats with arm support; 4: cannot do once.
3.3. Evaluation of the Diagnostic Efficacy of ATMSS in ATM Patients
Table 2 displays the demographic and laboratory data of all the subjects, including 38 patients in the ATM group and 40 patients in the GD group. Patients with ATM had higher FT3 (p < 0.001), FT4 (p < 0.001) and TRAb (p = 0.007) levels but lower TSH levels (p = 0.042) than those in GD group. Females were heavily overrepresented in both the ATM (F/M: 34/4) and GD (F/M: 36/4) groups.
| ATM (n = 38) | GD (n = 40) | p value | |
|---|---|---|---|
| Gender (F/M) | 34/4 | 36/4 | 0.939 |
| Age of onset (years) | 30.5 (25,42) | 38.1 ± 12.12 | 0.056 |
| FT3 (pmol/L) | 22.81 (13.17, 38.67) | 10.86 (6.83, 21.04) | < 0.001 |
| FT4 (pmol/L) | 57.71 (35.58, 77.22) | 28.8 (16.7, 42.99) | < 0.001 |
| TSH (mIU/L) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.042 |
| TRAb (IU/L) | 14.9 (9.62, 28.33) | 7.98 (2.79, 17.28) | 0.007 |
| TGAb (%) | 35.73 (11.23, 52.32) | 24.97 (10.07, 53.34) | 0.447 |
| TPOAb (IU/mL) | 656 (401.55, 1000) | 437 (52.3, 896.25) | 0.088 |
- Note: The data are expressed as the mean ± standard deviation and 25th–75th percentile (P25–P75). p values are shown for differences between groups. F, female; M, male; FT3, free triiodothyronine; FT4, free thyroxine.
- Abbreviations: ATM, acute thyrotoxic myopathy; GD, Graves’ disease; TGAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TRAb, thyrotrophin receptor antibody; TSH, thyroid-stimulating hormone.
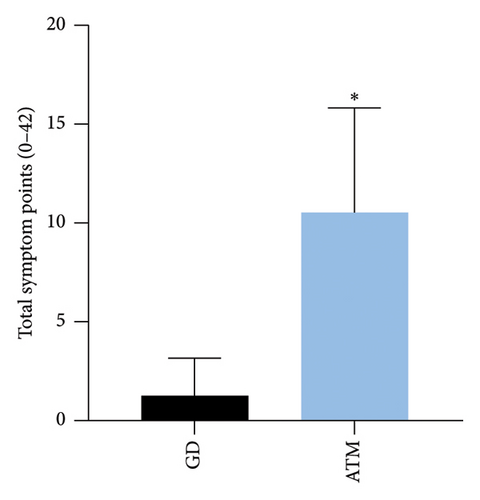
Compared with the GD group, the ATM group reported a greater symptom score [median total ATMSS [9 (6,15) vs. 0 (0, 2) p < 0.001] (Figures 1 and 2). The highest score reported by patients with ATM was dysarthria [4.5 (2, 6)] (Figure 2(b)). Each symptom score was greater in the ATM group than in the GD group (p < 0.001 and p < 0.01) (Figure 2(b)).
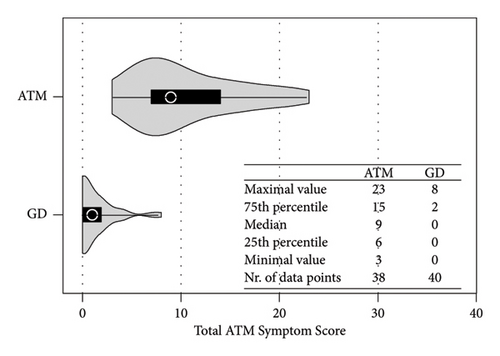

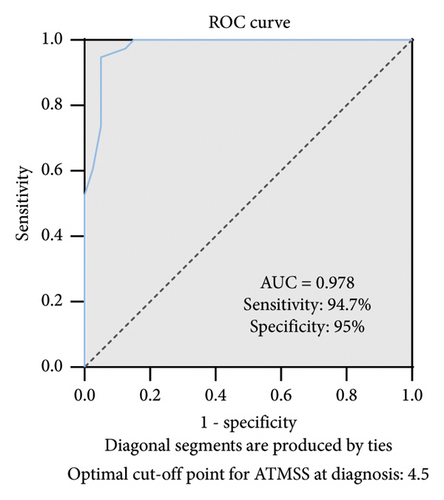
ROC curve analysis shows that ATMSS had an excellent diagnostic value in the distinguishing ATM group from GD group [area under the curve (AUC) = 0.978; 95% confidence interval (CI) = 0.949–1; p < 0.0001], and when the cut-off value was greater than 4.5, ATMSS demonstrated the highest sensitivity and specificity (sensitivity 94.7%, specificity 95%) with a positive predictive value (PPV) of 95% and a negative predictive value (NPV) of 95.2% at this threshold (Figure 3).
3.4. Correlation Between ATMSSs and ATM-Related Indicators
Spearman analysis was used to analyse 78 patients in the ATM group (38 patients) and the GD group (40 patients), and correlations between ATMSS and thyroid function-related indices were analysed.
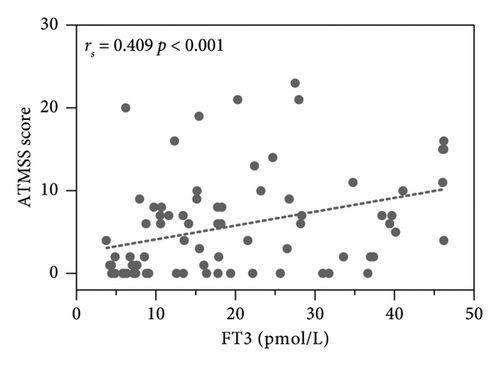
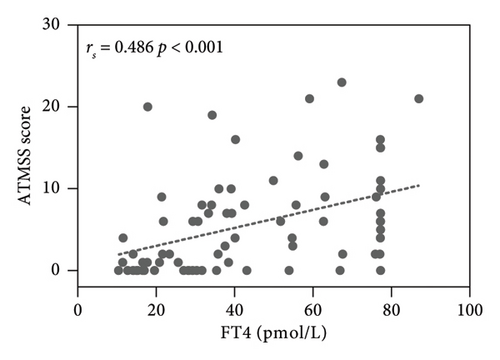
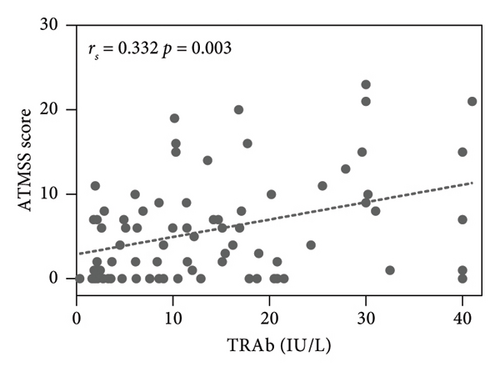
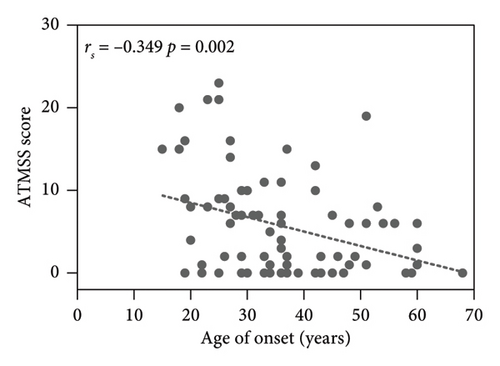
The results showed the ATMSS was positively correlated with FT3 [Spearman correlation coefficient (rs) = 0.409, p < 0.001], FT4 (rs = 0.486, p < 0.001) and TRAb (rs = 0.332, p = 0.003), negative correlation with the age of onset (rs = −0.349, p = 0.002) and no significant correlation with sex, TSH, TGAb or TPOAb (Table 3 and Figure 4).
| ATMSS | ||
|---|---|---|
| rs | p | |
| Gender | 0.068 | 0.552 |
| Age of onset | −0.349 | 0.002 |
| FT3 (pmol/L) | 0.409 | < 0.001 |
| FT4 (pmol/L) | 0.486 | < 0.001 |
| TSH (mIU/L) | −0.174 | 0.127 |
| TRAb (IU/L) | 0.332 | 0.003 |
| TGAb (%) | 0.165 | 0.148 |
| TPOAb (IU/mL) | 0.213 | 0.061 |
- Note: FT3, free triiodothyronine; FT4, free thyroxine.
- Abbreviations: ATMSS, acute thyrotoxic myopathy symptom score; TGAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; TRAb, thyrotrophin receptor antibody; TSH, thyroid-stimulating hormone.
4. Discussion
Hyperthyroidism complicated by bulbar weakness is rare and occurs in only 21% of patients with thyrotoxic myopathy [7]. Early on, it was believed that isolated bulbar paralysis would not occur without associated chronic thyrotoxic myopathy (CTM), but in recent years, several authors have reported the presence of acute bulbar paralysis in the absence of overt muscle atrophy, which is characterized by rapid progression and high mortality [2, 27]. Here, we hypothesized that a standardized and easy-to-apply ATM scoring table could be used to assist in early ATM diagnosis.
Due to the lack of a certain consensus on the diagnosis of ATM, we summarized the diagnostic characteristics of ATM in the established reference cohort and tried to set up the diagnostic principles of ATM. At the same time, we also excluded other causes of bulbar palsy as much as possible and specifically performed follow-up observations after the use of ATDs or combined with steroid, β-blockers and energy support for 1 week to 2 months to ensure the accuracy of ATM diagnosis in this study. After assessing ATMSSs for patients with ATM and GD, patients with ATM reported more bulbar paralysis symptom scores than did GD patients in all symptom categories [median total ATMSS9 (6,15) vs. 0 (0, 2)] (Figure 1), suggesting that ATM patients showed symptoms associated with bulbar paralysis [27], and ROC curve analysis showed that the ATMSS had good diagnostic sensitivity and specificity when the cut-off value was greater than 4.5 (AUC = 0.978, 95% CI: 0.949–1.000; p < 0.001, sensitivity 94.7%, specificity 95%) for distinguishing ATM from GD (Figure 3), and it could be used as an effective tool for ATM screening. In addition, the ATMSS is simple to operate, and clinicians are expected to use the ATMSS screening for ATM before conducting more difficult examinations to reduce the misdiagnosis and delay treatment of patients with ATM.
ATM patients were younger [average age of 30.5 (25,42) years] and predominantly female (F/M: 34/4) in our study (Table 2), which was inconsistent with the average onset age of 50.77 ± 18.05 years and the almost equal sex ratio in the reference cohort (M/F: 1/1.06) (Table 1). This may be because GD tends to occur in young women. When ATM screening is performed in all patients with hyperthyroidism like in our study, a large number of ATM patients with mild early symptoms can be detected. While almost all patients with ATM in our ATM reference cohort are hospitalized patients with severe conditions, elderly patients are more prone to have severe conditions. More clinical data are needed to support this view in the future.
There was one patient in the GD group had an ATMSS as high as eight points in our study. The increased score was mainly attributed to limb weakness, which is consistent with what was reported by Brennan et al. [28–30] that 80% of patients with hyperthyroidism commonly exhibit myasthenic symptoms, especially proximal muscle weakness. In addition, CTM mainly manifests as obvious myasthenia [2]. Therefore, before ATMSS is used, if the patient mainly presents with myasthenia and lacks significant bulbar involvement, ATM needs to be diagnosed with great caution, and it is necessary to exclude myasthenia caused by CTM and hyperthyroidism to avoid misdiagnosis.
In our study, dysarthria was most evident by assessing ATMSS in patients with ATM, but in the reference cohort, dysphagia was the most prevalent symptom (33/35) (Table 1 and Figure 2(b)). The possible reasons include that early mild manifestations of ATM may include mild abnormalities in pronunciation, such as pitch reduction and dysphasia, which can improve over time after related treatment or are easily overlooked, while dysphagia is more likely to predict the severity of disease and progressing to a serious complication. Chiu et al. [4] reported that ATM patients with dysphagia were prone to have aspiration pneumonia. In the ATM reference cohort, the prevalence of dysphagia was significantly increased, while gag reflex abnormalities were not prevalent. In addition to possibly being related to insufficient assessment of gag reflex in routine examinations, studies have also found that the presence or abnormality of gag reflex does not assess dysphagia, especially caused by neurological or neuromuscular causes [31, 32]. Therefore, gag reflex as a predictor of dysphagia in ATM is not advisable, but the correlation between them needs to be further elaborated in follow-up studies. Further studies with larger sample sizes are needed to clarify the relationships between symptoms of bulbar palsy, ATMSSs, severity, complications and prognosis.
The pathogenesis of ATM is still unclear. Possible mechanisms may include the direct toxic effects of excessive thyroid hormones on the neuromuscular system, leading to bulbar muscle weakness and causing oropharyngeal or oesophageal dysmotility, with patients presenting symptoms such as dysphagia, dysarthria and dysphonia [4, 33]. Additionally, the marked improvement in ATM with beta-blockers suggests that beta-adrenergic stimulation may contribute to the pathogenesis of this disease [34]. Historically, there have been reports, suggesting that ATM may manifest as an encephalopathy. In 1945, Waldenstrom described similar cases of acute bulbar palsy with encephalopathy associated with hyperthyroidism. These cases documented brain-related symptoms such as aphasia, acalculia and psychosis with hallucinations. Postmortem brain examinations in three nonsurviving patients revealed that in the medulla oblongata, degeneration of nerve fibres in the vagus nerve and haemorrhage surrounding the vagus nucleus at the floor of the fourth ventricle was observed [35], suggesting that ATM may represent brain injury induced by hyperthyroidism. Studies have found that T3 receptor mRNAs are predominantly located on neurons. Genetic studies in the rat striatum indicate that thyroid hormones regulate striatal physiology [36]. Whether thyroid hormones directly induce bulbar palsy through modulation of brain function remains to be further investigated. Li et al. [37] have shown that bulbar palsy in patients with ATM may be related to functional connectivity changes in the resting-state sensorimotor network and left frontoparietal network. Our study confirmed that the values of FT3, FT4 and TRAb were higher in ATM patients, and the difference was statistically significant (Table 2) and there were positive correlations between ATMSSs and the levels of FT3 (rs = 0.409, p < 0.001), FT4 (rs = 0.486, p < 0.001) and TRAb (rs = 0.332, p = 0.003) (Figure 4), which suggests that thyroid hormones and TRAb may have a direct effect on the occurrence of ATM from a clinical point of view. Our previous study suggested that receptor-stimulating antibody (TSAb) could cause proinflammatory polarization of microglia [38], and further studies are needed to determine whether the inflammatory response of microglia induced by TRAb plays a role in the pathogenesis of ATM.
In our study, the performance of the ATMSS in CTM patients did not be clarified. Second, future prospective studies should aim to test the utility of the ATMSS for evaluation of symptom progression or recovery over time, and patient outcomes in different ethnic and clinical settings. Finally, comparative studies with validated symptom scores, such as the Frenchay Aphasia Screening Test, are needed.
This article is one of the few that investigates the diagnosis of ATM and establishes a questionnaire-based scoring system to assist in the diagnosis of ATM. The ATMSS has excellent specificity and sensitivity for the diagnosis of ATM. In addition, the easy-to-administer nature of ATMSS could eliminate possible interviewer bias. The limitations of this study are its single centre, sample size, observational nature and referral bias.
Disclosure
A preprint has previously been published [39].
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Y.Z. and Z.L. provided the design and conception of the study. Y.Z., C.W., T.W., X.C., Q.L., X.L., H.Y. and Z.H. completed the selection of reference cases, and the collection and analysis of the cases studied. L.L., J.X., J.Z., D.L., Y.Q., D.L. and Z.L. contributed to the diagnosis of ATM and the formulation of ATMSS. Y.Z. wrote the first draft of the manuscript. Z.L. completed the final manuscript, and all authors participated in the revision and approved the final manuscript.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (Grant Nos. 82260159, 81860146, and 81660138).
Acknowledgments
The authors thank the experts from the Department of Neurology of the First Affiliated Hospital of Guangxi Medical University who provided professional advice for the development of the Acute Thyrotoxic Myopathy Symptom Score. This work was supported by research grants from the National Natural Science Foundation of China (Grant Nos. 82260159, 81860146, and 81660138).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




