Prevalence of Hypertension and Blood Pressure Control in Familial Mediterranean Fever Patients
Abstract
Background and Objective: Familial Mediterranean fever (FMF) is characterized by inflammatory febrile attacks with polyserositis and is associated with an increased risk of cardiovascular disease. We aimed to determine the prevalence of hypertension (HT) in FMF patients and compare the frequency with that of the general population.
Methods: This was a retrospective cohort study. Patients diagnosed with FMF between 2000 and 2020 and participants in the PatenT 2 study were included. We used office blood pressure measurements taken with calibrated sphygmomanometers.
Results: We enrolled 528 patients with FMF and 1234 age- and gender-matched controls. The mean age of the study population was 35.48 ± 13.54, and 314 (59.5%) of the total patients were female. The prevalence of HT was lower in FMF patients compared to the control group [68 (12.9%) vs. 215 (17.4%), p = 0.02]. Patients without amyloidosis had significantly lower mean systolic blood pressure (SBP) and higher mean diastolic blood pressure (DBP) compared to the control group (113.4 ± 15.5 vs. 120.9 ± 15.94, p < 0.001, and 72.4 ± 11.03 vs. 71.07 ± 10.53, p = 0.05, respectively). Of the patients without amyloidosis, 294 (63.1%) had normal SBP and DBP, while 28 (45.2%) patients with amyloidosis and 616 (49.9%) control group participants had normal SBP and DBP. Regression analysis showed that GFR < 60 mL/min/1.73 m2 and increasing age were risk factors for the development of HT.
Conclusion: The prevalence of HT, mean blood pressure, and achievement of target blood pressure are better in FMF patients than in the general population. However, blood pressure control decreases with increasing age, especially when eGFR falls below 60 mL/min/1.73 m2.
1. Introduction
Familial Mediterranean fever (FMF) is an inherited disease characterized by recurrent fever and serosal inflammation. The disease is associated with pyrin dysfunction, which results from mutations in the Mediterranean fever (MEFV) gene and forms the basis of FMF pathogenesis [1, 2]. FMF is encountered more frequently in people from the Mediterranean region, with a prevalence in Turkey of approximately 1 in 1000 [2]. Clinically, FMF presents with fever, abdominal pain, chest pain, arthralgia/arthritis, and erysipelas-like skin lesions [3]. FMF is not solely a disease with inflammatory attacks; it also involves a chronic inflammatory process, with secondary amyloidosis being the most critical long-term complication and the leading cause of FMF-associated mortality [3, 4]. Therefore, the foremost objectives of FMF treatment encompass the prevention of acute attacks, the mitigation of subclinical inflammation between attacks, the prevention of the development of amyloidosis, and the deceleration of amyloidosis progression.
In the precolchicine era, secondary systemic amyloidosis was reported in approximately 50% of FMF patients, but with colchicine treatment, the incidence of amyloidosis has gradually decreased. Nevertheless, recent multicenter studies have reported a prevalence of amyloidosis of around 12.9% [4]. It is well known that elevated levels of proinflammatory cytokines—including IL-1, IL-6, IL-8, IL-12, and IL-18—persist not only during attacks but also during remission, contributing to endothelial injury [4–6]. This ongoing inflammatory state may increase cardiovascular disease risk in patients with FMF. Although there are conflicting findings in the literature, FMF has been associated with accelerated atherosclerosis, cardiac autonomic dysfunction, cardiovascular disease, and other inflammatory conditions [4, 7–9].
High systolic blood pressure (SBP) is the most prevalent modifiable cardiovascular risk factor and a leading cause of mortality worldwide, accounting for approximately 11 million deaths each year [10]. Additionally, hypertension (HT) affects approximately 1.15 billion adults globally [11]. There is substantial evidence indicating that low-grade chronic inflammation contributes to the development and maintenance of essential HT by impacting vascular and renal function [12]. Cytokines are a key component in the immune system and connect inflammation with vascular remodeling and HT development. However, in a disease such as FMF—where low-grade inflammation persists even during periods of disease control—data on the frequency of HT compared to the general population are limited.
The objective of this study is to determine the prevalence of HT in FMF patients and compare it to that of the general population using data from the PatenT 2 epidemiology study [13]. Additionally, we aim to identify FMF-associated risk factors for HT and assess blood pressure values among normotensive patients with FMF.
2. Materials and Methods
2.1. Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of Hacettepe University (May 22, 2020, GO 20/482), and exempted from informed consent due to its retrospective nature. Written permission to use the PatenT 2 data was obtained from the Board of Directors of the Turkish Hypertension and Kidney Disease Associations.
2.2. Study Population
This retrospective cohort study was conducted in the nephrology outpatient clinic. Patients diagnosed with FMF according to the Tel Hashomer criteria between 2000 and 2020 who were over 18 years of age were included in the study. Exclusion criteria for the patient population were determined as follows: (1) patients who required renal replacement therapy (hemodialysis, peritoneal dialysis, or kidney transplantation), (2) presence of a malignant disease, (3) diagnosis of diabetes mellitus, (4) presence of documented coronary artery disease or heart failure (preserved, borderline, or low ejection fraction), (5) diagnosis of nephrotic or nephritic syndrome other than amyloidosis (patients with proteinuria above 0.15 g/day whose diagnosis of amyloidosis was not confirmed by tissue biopsy were excluded), (6) patients who did not have regular clinic visits and did not use their medications regularly (as reported by the patients), and (7) documented cases of secondary HT. Additionally, exclusion criteria (1) through (5) were applied to the control group.
A power analysis was conducted to determine the minimum required sample size for detecting a 5% difference in HT prevalence between FMF patients and the general population, with an alpha level of 0.05 and a desired power of 80%. The analysis indicated that a sample size of approximately 480 FMF patients would be necessary to achieve sufficient statistical power for detecting this effect size.
Six hundred three patients with regular outpatient clinic visits were assessed for the study. Of these, 528 patients who met the inclusion criteria were selected. The control group was chosen from 5437 participants in the PatenT 2 study [13]. A total of 1234 control group participants, matched for age and gender, were randomly assigned to the 528 FMF patients in the study (blinded to BP measurements).
2.3. Data Collection
The hospital’s electronic medical records system was used for baseline information such as patient gender, age, comorbidities, treatment regimen, and genetic analysis results. Total follow-up time was determined for all patients. Data regarding serum creatinine, estimated glomerular filtration rate (eGFR), and 24-h urine protein levels were collected. We used the abbreviated Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) to estimate GFR [14]. Additional data included SBP and DBP measurements, HT diagnosis, and antihypertensive treatments for both the study population and the PatenT 2 control group.
2.4. Assessment of Blood Pressure
Blood pressure measurements were performed during clinical visits in a quiet room following a 10-min rest period. Participants were advised to refrain from smoking, drinking tea or coffee, and exercising for at least 30 min prior. Cuff size was chosen according to the circumference of the mid-upper arm, and the cuff was wrapped around the arm with the lower end positioned 2–3 cm above the cubital fossa. Measurements were performed with the arm at heart level using calibrated sphygmomanometers (mercury or automated). In the PatenT 2 study, blood pressure measurements were taken with an automatic sphygmomanometer, following similar recommendations [13]. At least three consecutive BP measurements were obtained, with an interval of at least 2 min between each measurement. The arithmetic mean of the BP measurements was noted.
2.5. Clinical Outcomes
HT was defined as a SBP of at least 140 mmHg, a diastolic blood pressure (DBP) of at least 90 mmHg, or a prior diagnosis of HT. Patients whose blood pressure was within normal limits but who were using angiotensin-converting enzyme inhibitors (ACEis) or angiotensinogen receptor blockers (ARBs) due to proteinuria were also identified. Target blood pressure was set at < 130/90 mmHg for all patients. Additional antihypertensive treatments were added to ACEi or ARB therapy until the target blood pressure was achieved.
2.6. Statistical Analysis
Continuous variables are reported as mean ± standard deviation or as median (interquartile range) according to data distribution, which was determined using the Kolmogorov–Smirnov test. Homogeneity of variance was assessed with the one-way ANOVA test. Student’s t-test or Mann–Whitney U test was used to compare continuous variables based on distribution. Categorical variables were reported as percentages. When comparing more than two groups, a one-way ANOVA test was applied for numerical variables, with post hoc analysis to determine differences between groups. The chi-square test was used for categorical variables. Logistic regression analysis was employed to assess the association between FMF and the development of HT, with a 95% confidence interval (CI). A p value ≤ 0.05 indicated statistical significance. Analyses were performed using SPSS Version 21.0.0.1 (SPSS, IBM, Armonk, NY, USA) for Windows.
3. Results
3.1. Demographic Characteristics
The mean age of the study population was 35.48 ± 13.54 years, with 314 (59.5%) female patients. The mean age of patients with amyloidosis was significantly higher than that of patients without amyloidosis (43.97 ± 14.9 vs. 34.35 ± 12.94, p = 0.04), while the proportion of female patients was lower in the amyloidosis group (21 (33.9%) vs. 293 (62.9%), p < 0.001). Additionally, median proteinuria, mean serum creatinine, and the number of patients with eGFR < 60 mL/min/1.73 m2 were significantly higher in patients with amyloidosis than those without (p < 0.001, p < 0.001, and p < 0.001, respectively). Genetic analysis was performed in 226 (42.8%) patients, with 159 (70.4%) having at least one M694V allele. Among those who developed amyloidosis, 12 (40%) were homozygous for the M694V mutation, and no mutation was found in 6 (9.7%). The frequency of the homozygous M694V mutation and the absence of any mutation were both significantly higher in patients with amyloidosis than in those without [12 (40%) vs. 47 (24%), p = 0.05, and 6 (9.7%) vs. 20 (4.3%), p = 0.01]. Demographic characteristics of the study population are demonstrated in Table 1.
| Total patients n: 528 | Patients without amyloidosis n: 466 (88%) | Patients with amyloidosis n: 62 (12%) | p value | |
|---|---|---|---|---|
| Age (mean ± SD) | 35.48 ± 13.54 | 34.35 ± 12.94 | 43.97 ± 14.9 | 0.04 |
| Gender (female), n (%) | 314 (59.5%) | 293 (62.9%) | 21 (33.9%) | < 0.001 |
| Disease duration (median, IQR) | 11 (5–17.8) | 11 (5–18) | 7 (1–15) | 0.007 |
| eGFR < 60 mL/min/1.73 m, n (%) | 41 (8%) | 11 (2.5%) | 30 (48.4%) | < 0.001 |
| Creatinine, mg/dL (mean ± SD) | 0.92 ± 0.98 | 0.77 ± 0.66 | 2 ± 1.9 | < 0.001 |
| Proteinuria g/day (median, IQR) | 0.14 (0.08–1.3) | 0.09 (0.06–0.14) | 55.03 (0.9–11.4) | < 0.001 |
| Comorbidities, n (%) | ||||
| SSpa | 37 (7%) | 30 (6.4%) | 7 (11.3%) | |
| IBD | 5 (0.9%) | 5 (1.1%) | 0 | |
| Nephrolithiasis | 5 (0.9%) | 5 (1.1%) | 0 | |
| Vesicoureteral reflux | 1 | 1 (0.2%) | 0 | |
| Hypertension | 68 (12.9%) | 48 (10.3%) | 20 (32.3%) | |
| Treatment, n (%) | ||||
| Colchicine | 466 (88.2%) | 427 (91.6%) | 39 (62.9%) | |
| Colchicine + IL-1 antagonist | 41 (7.8%) | 22 (4.7%) | 19 (30.7%) | |
| Colchicine + anti-TNF | 16 (3.1%) | 12 (2.6%) | 4 (6.4%) | |
| Colchicine + IL-6 antagonist | 5 (0.9%) | 5 (1.1%) | 0 | |
| Genetic analysis, n (%) | 226 (42.8%) | 196 (42.1%) | 30 (48.4%) | |
| M694V/M694V | 59 (26.1%) | 47 (24%) | 12 (40%) | 0.05 |
| One M694V allele | 100 (44.2%) | 92 (46.9%) | 8 (26.7%) | 0.03 |
| At least one M694V allele | 159 (70.4%) | 139 (70.9%) | 20 (66.7%) | 0.4 |
| −/− | 26 (4.9%) | 20 (4.3%) | 6 (9.7%) | 0.01 |
| M694V/M680I | 24 (10.6%) | 21 (10.7%) | 3 (10%) | 0.9 |
| M694V/V726A | 22 (9.7%) | 21 (10.7%) | 1 (3.3%) | 0.2 |
| M694V/E148Q | 16 (7.1%) | 15 (7.7%) | 1 (3.3%) | 0.4 |
| M680I/M680I | 16 (7.1%) | 14 (7.1%) | 2 (6.7%) | 0.7 |
- Note: Anti-TNF = antitumor necrosis factor, IL-1: interleukin-1, SSpa = seronegative spondyloarthropathies.
- Abbreviations: eGFR = estimated glomerular filtration rate, IBD = inflammatory bowel disease.
3.2. Blood Pressure Findings
The blood pressure–related findings for the FMF patients and their comparison with the control group are shown in Table 2. HT prevalence was significantly lower among FMF patients than in the control group [68 (12.9%) vs. 215 (17.4%), p = 0.02]. In patients using antihypertensive drugs, the mean antihypertensive pill burden was significantly higher in FMF patients compared to the control group [91 (17.2%) vs. 115 (9.3%), p < 0.001, and 1.58 ± 0.75 vs. 1.3 ± 0.46, p = 0.003]. FMF patients had lower SBP but higher DBP compared to the control group (113.9 ± 16.07 vs. 120.9 ± 15.94, p < 0.001, and 72.45 ± 11.17 vs. 71.07 ± 10.53, p = 0.02).
| FMF patients n: 528 | Control group n: 1234 | p value | |
|---|---|---|---|
| Age (mean ± SD) | 35.48 ± 13.54 | 35.67 ± 13.26 | 0.8 |
| Gender (female), n (%) | 314 (59.5%) | 700 (56.7%) | 0.3 |
| Hypertension, n (%) | 68 (12.9%) | 215 (17.4%) | 0.02 |
| Antihypertensive medication, n (%)∗ | 91 (17.2%) | 115 (9.3%) | < 0.001 |
| Antihypertensive pill burden (mean ± SD)∗∗ | 1.58 ± 0.75 | 1.3 ± 0.46 | 0.003 |
| Blood pressure | |||
| SBP mmHg (mean ± SD) | 113.9 ± 16.07 | 120.9 ± 15.94 | < 0.001 |
| DBP mmHg (mean ± SD) | 72.45 ± 11.17 | 71.07 ± 10.53 | 0.02 |
- Abbreviations: DBP = diastolic blood pressure, FMF = familial Mediterranean fever, SBP = systolic blood pressure.
- ∗Patients on angiotensin-converting enzyme inhibitors or angiotensinogen receptor blockers for proteinuria were also included.
- ∗∗Only patients with hypertension were analyzed.
3.2.1. Impact of Amyloidosis on Blood Pressure
In post hoc analysis, patients without amyloidosis had significantly lower mean SBP and higher mean DBP than the control group (113.4 ± 15.5 vs. 120.9 ± 15.94, p < 0.001, and 72.4 ± 11.03 vs. 71.07 ± 10.53, p = 0.05). However, both mean SBP and DBP were similar between patients with amyloidosis and the control group (117.9 ± 19.8 vs. 120.9 ± 15.94, p = 0.4, and 72.9 ± 12.3 vs. 71.07 ± 10.53, p = 0.4) (Table 3).
| Patients without amyloidosis n: 466 | Patients with amyloidosis n: 62 | Control group n: 1234 | p value | |
|---|---|---|---|---|
| Age (mean ± SD) | 34.35 ± 12.94 | 43.97 ± 14.9 | 35.67 ± 13.2 | < 0.001 |
| Gender (female), n (%) | 293 (62.9%) | 21 (33.9%) | 700 (56.7%) | |
| < 120 mmHg and/or < 80 mmH, n (%) | 294 (63.1%) | 28 (45.2%) | 616 (49.9%) | |
| 120–129 mmHg and/or 80–84 mmH, n (%) | 72 (15.5%) | 4 (6.5%) | 253 (20.5%) | |
| 130–139 mmHg and/or 85–89 mmH, n (%) | 52 (11.1%) | 10 (16.1%) | 150 (12.2%) | |
| Hypertension, n (%) | 48 (10.3%) | 20 (32.2%) | 215 (17.4%) | |
| Blood pressure | ||||
| SBP mmHg (mean ± SD) | 113.4 ± 15.5 | 117.9 ± 19.8 | 120.9 ± 15.9 | < 0.001 |
| DBP mmHg (mean ± SD) | 72.4 ± 11.03 | 72.9 ± 12.3 | 71.07 ± 10.5 | 0.04 |
- Abbreviations: DBP = diastolic blood pressure, SBP = systolic blood pressure.
3.2.2. Blood Pressure Control Status
While 294 (63.1%) patients without amyloidosis had normal SBP and DBP, this was true for only 28 (45.2%) patients with amyloidosis and 616 (49.9%) control group participants. Additionally, 124 (26.6%) patients without amyloidosis had normal-increased SBP and/or DBP, compared to 14 (22.6%) patients with amyloidosis and 403 (32.7%) control group participants with normal-increased SBP and/or DBP (Table 3).
3.2.3. Subgroup Analysis of Normotensive Population
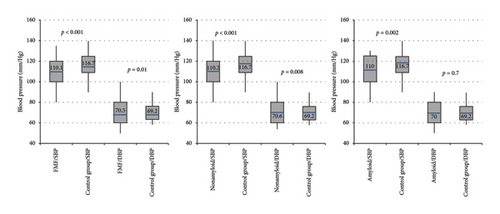
In normotensive patients, FMF patients had significantly lower mean SBP than the control group (p < 0.001). This difference persisted when comparing FMF patients with and without amyloidosis to the control group (p = 0.002 and p < 0.001, respectively) (Figure 1). Conversely, FMF patients had higher DBP than the control group (p = 0.01), which was also significant in patients without amyloidosis (p = 0.008).
3.2.4. FMF-Related Risk Factors for HT
Risk factors for HT development in FMF patients are shown in Table 4. Multivariate logistic regression analysis indicated that GFR < 60 mL/min/1.73 m2 and increasing age were independent risk factors for HT development [odds ratio (OR): 6.063 (95% CI: 2.223–16.538) and OR: 1.082 (1.059–1.106), respectively].
| Odds ratio (95% confidence interval) | p value | |
|---|---|---|
| Univariate analysis | ||
| Amyloidosis | 4.147 (2.252–7.636) | < 0.001 |
| eGFR < 60 mL/min/1.73 m | 11.101 (5.597–22.018) | < 0.001 |
| Age | 1.091 (1.068–1.114) | < 0.001 |
| Gender (male) | 1.556 (0.933–2.593) | 0.09 |
| Year of disease | 1.016 (0.988–1.045) | 0.2 |
| Gene allele | ||
| M694V/M694V | 0.937 (0.353–2.486) | 0.9 |
| One M694V allele | 0.598 (0.245–1.460) | 0.3 |
| At least one M694V allele | 0.550 (0.231–1.310) | 0.2 |
| M694V/M680I | 0.338 (0.044–2.625) | 0.3 |
| M694V/V726A | 0.375 (0.048–2.918) | 0.3 |
| M694V/E148Q | 0.542 (0.068–4.296) | 0.5 |
| M680I/M680I | 2.077 (0.547–7.883) | 0.3 |
| Multivariate analysis | ||
| Amyloidosis | 0.786 (0.334–2.297) | 0.8 |
| eGFR < 60 mL/min/1.73 m | 6.063 (2.223–16.538) | < 0.001 |
| Age | 1.082 (1.059–1.106) | < 0.001 |
| Gender (male) | 1.210 (0.650–2.251) | 0.5 |
- Abbreviation: eGFR = estimated glomerular filtration rate.
4. Discussion
FMF is a hereditary disease particularly prevalent in the Mediterranean region, with secondary amyloidosis and consequent end-stage renal failure as major contributors to long-term morbidity and cardiovascular mortality. Both chronic inflammation and chronic kidney disease progression in these patients are key factors that contribute to increased mortality. Consequently, the prevalence and management of comorbid conditions such as HT, which affects both renal and cardiovascular systems, are critical in this patient population. In our study, we found that the prevalence of HT in FMF patients was significantly lower than that in a control group of similar age. Additionally, normotensive FMF patients demonstrated better SBP and DBP control than the control group. To our knowledge, this is the first study to both reveal the prevalence of HT in FMF patients and evaluate blood pressure control within this population.
FMF is characterized by recurrent febrile episodes accompanied by polyserositis, lasting 1–3 days and typically resolving spontaneously [3, 15, 16]. However, the most serious complication of FMF is amyloidosis, which results from continuous overt or low-grade inflammation [17]. Inflammatory diseases like FMF may contribute to early atherosclerosis development, which is driven by chronic inflammation and the interaction between macrophages, T cells, modified lipoproteins, and arterial walls [18]. Studies have shown that FMF is associated with increased cardiovascular risk factors, such as atherogenic index, carotid intima–media thickness, arterial stiffness, and epicardial adipose tissue thickening [18]. Therefore, raising awareness of HT as a critical cardiovascular risk factor and ensuring adequate blood pressure control in FMF patients is essential. However, data on the prevalence of HT and blood pressure control in patients with FMF are limited, although an increase in the prevalence of HT would be expected in this population compared with the general population.
Blood pressure control in FMF patients is not only critical for individual health but also a public health concern. In 2012, the PatenT 2 study found that the prevalence of HT in Turkey was 30.3% [13]. In our study, the prevalence of HT in FMF patients was significantly lower than that in the age- and gender-matched general population. Possible explanations include regular clinical visits for FMF patients, during which blood pressure is monitored. Physician–patient discussions about the potential impact of high blood pressure on disease progression may further increase patient awareness. Furthermore, FMF patients’ health literacy may have improved over time, resulting in better adherence to lifestyle recommendations, such as salt restriction, regular exercise, and weight management, which are essential for blood pressure control.
Health literacy and disease awareness likely contribute to better blood pressure management in FMF patients. In fact, awareness of HT is often low among the general population. For example, in the PatenT 2 study, HT awareness was 54.7%, and only 47.4% of individuals received pharmacological treatment [13]. Similar findings were observed in the PatenT 2 subgroup in our study, where the prevalence of HT was 17.4%, and only 53.4% (115 of 215) of these individuals were on antihypertensive medication. In other cohort studies, awareness of HT ranged from 37% to 46.9% [19–21]. In our study, even among normotensive FMF patients, better mean SBP and DBP values were observed compared to controls, potentially due to greater awareness and the effects of previously mentioned factors.
One factor that may impact blood pressure control, though it has a weaker cause-and-effect relationship compared to other potential factors, is the use of colchicine. While its primary benefit lies in reducing FMF attacks and preventing amyloidosis, colchicine’s positive effects on endothelial function may aid in blood pressure control [22, 23]. Studies have also suggested a link between the pyrin domain–containing protein 3 inflammasome (NLRP3) and HT [24, 25]. By inhibiting NLRP3, colchicine may reduce renal inflammation and potentially lead to lower blood pressure in FMF patients.
Our findings suggest that blood pressure control and HT prevalence in FMF patients are better than those in the general population. However, this advantage disappears in patients with amyloidosis, with HT prevalence reaching nearly double that of the general population. The higher mean age of patients with amyloidosis may contribute to this result, as blood pressure control becomes more challenging with age, and HT prevalence rises accordingly. For instance, the PatenT 2 study found that the prevalence of HT was 11.5% for individuals aged 30–40, increasing to nearly 30% for those aged 40–49 [13]. In other cohort studies, HT prevalence in the 40–50 age group ranges from 17% to 41% [19, 21, 26]. Another possible explanation for the increased prevalence of HT in patients with amyloidosis is that nearly half of these patients have chronic kidney disease stage three or higher. As chronic kidney disease progresses, there is increased activation of the renin–angiotensin system due to decreased nephron mass and endothelial damage, sodium and salt retention, increased endothelin synthesis and sympathetic tone, and decreased nitric oxide levels [27]. All of these contribute to impaired blood pressure control and increased risk of HT.
Our study demonstrates that both advanced age and decreased glomerular filtration rate are independent risk factors for HT development in FMF patients. Thus, disease control, reduction of inflammatory activity, and preservation of renal function are crucial in maintaining blood pressure control and preventing HT in this patient population.
Another noteworthy result was that FMF patients in our study required more medications for blood pressure management than the control group. This may be due to the higher frequency of clinical visits among FMF patients, who benefit from regular monitoring, leading to a stricter approach to maintaining target blood pressure levels. As a result, FMF patients’ mean SBP may be lower than that of the control group.
This study has certain limitations. First, as a retrospective cohort study, it was not possible to standardize blood pressure measurements. For example, blood pressure in the PatenT 2 study was measured with automatic oscillometric devices, while both mercury and automatic oscillometric devices were used for FMF patients. Additionally, the study’s retrospective nature restricts our ability to establish causality. A prospective design with ambulatory blood pressure monitoring could more accurately assess the relationship between blood pressure, FMF, and amyloidosis. Furthermore, since the PatenT 2 control group represents the general population, the frequency of chronic kidney disease in this cohort is unknown. Finally, genetic analysis was performed in less than half of the patients, limiting our exploration of the effects of genetic variations on HT development. Despite these limitations, the study’s strengths include a large sample size and its status as the most comprehensive investigation on HT prevalence in FMF patients. Additionally, participants on antihypertensive drugs for non–blood pressure reasons, which could mask HT, were excluded, further ensuring accuracy.
5. Conclusion
The prevalence of HT in FMF patients is lower than that in the general population, with FMF patients also achieving better blood pressure control. However, this advantage diminishes with increasing age and declining kidney function, resulting in an elevated HT prevalence in older FMF patients, particularly those with advanced chronic kidney disease. Careful monitoring and proactive management of blood pressure in this population remain essential.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of Hacettepe University (May 22, 2020, GO 20/482), and exempted from informed consent due to its retrospective nature. Written permission to use the PatenT 2 data was obtained from the Board of Directors of the Turkish Hypertension and Kidney Disease Associations.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Gozde Kavgaci: data curation (equal), formal analysis (equal), investigation (equal), writing – original draft (equal). Haci Hasan Yeter: formal analysis (equal), investigation (equal). Muge Uzerk Kibar: data curation (equal). Tolga Yildirim: conceptualization (equal), supervision (equal). Banu Balci Peynircioglu: conceptualization (equal), supervision (equal). Seref Rahmi Yilmaz: conceptualization (equal), supervision (equal), writing – review and editing (equal). Yunus Erdem: conceptualization (equal), supervision (equal), writing – review and editing (equal).
All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the Turkish Hypertension and Kidney Diseases Association for allowing the use of PatenT 2 study data as the control group in our study.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




