Connection Between Oral Behaviors and Pain Intensity in Temporomandibular Disorder Patients Undergoing Orthodontic Treatment: A Cross-Sectional Study
Abstract
Proper management of painful temporomandibular disorders (PTMDs) is important for guaranteeing optimal orthodontic outcomes. Although correlations between oral behaviors and PTMDs have been confirmed in the general population, there is limited corresponding research within the orthodontic patients. The purpose of this cross-sectional study was to examine how pain intensity correlates with the frequency of various oral behaviors in orthodontic patients. Participants completed a questionnaire survey containing demographic details, an eight-item oral behaviors checklist, the five major temporomandibular disorders symptoms (5Ts) checklist, and a visual analog scale (VAS) to gauge pain intensity. Participants were divided into two groups: PTMD and without PTMD (NPT). The correlation between specific oral behaviors and pain intensity was assessed using both univariate and multivariate linear regression analyses, with adjustment for demographic variables such as age, gender, systemic diseases, and level of education. The study gathered 267 valid questionnaires, showing a prevalence of 59.93% for PTMD. In comparison to the NPT group, the PTMD group exhibited a higher frequency of oral behaviors. Significant correlations were identified between various oral behaviors and pain intensity. Univariate and multivariate linear regression analyses demonstrated positive relationships between pain and oral behaviors. After adjusting for demographic variables, these connections remained statistically significant in the multivariate analysis for sleep bruxism (β = 0.17; 95% CI 0.03, 0.31; p = 0.0175), sleep position pressuring jaw (β = 0.15; 95% CI 0.03, 0.26; p = 0.0118), awake bruxism (β = 0.57; 95% CI 0.26, 0.88; p = 0.0004), and holding, tightening, or tensing muscle without clenching (β = 0.18; 95% CI 0.01, 0.36; p = 0.0415). A significant association was identified between the frequency of oral behaviors and pain intensity in orthodontic patients. Screening and management of oral behaviors might be crucial for controlling TMDs during orthodontic treatment.
1. Introduction
Temporomandibular disorders (TMDs) cover various issues that impact masticatory muscles, temporomandibular joint (TMJ), and other structures [1]. These disorders are a notable public health issue, impacting approximately 60%–70% of the population [2]. Common symptoms of TMD include pain in the TMJs, joint noises, and restricted mouth opening [3, 4]. In the diagnostic criteria for temporomandibular disorders (DC/TMD), painful TMD (PTMD) is described as TMD characterized by pain symptoms [5]. PTMDs encompass various maxillofacial pains associated with TMD, including myalgia, headaches, and other symptoms [6]. The onset of TMD signs and symptoms is now understood to be multifactorial, involving a combination of biological, behavioral, environmental, social, emotional, and cognitive factors, either individually or collectively [7].
Oral behaviors, especially parafunctional ones, are actions of the masticatory muscles that go beyond regular functions such as talking and chewing [8]. These behaviors have been implicated in causing strain and microtrauma to the masticatory system [9] and thus identified as potential risk factors for TMD [10]. Oral behaviors have a significant association with PTMD, with individuals showing a higher likelihood of experiencing PTMD when exhibiting multiple oral behaviors [11]. Self-reports completed by patients, such as questionnaires or checklists, are frequently utilized to identify oral behaviors [12, 13]. Among these tools, the oral behaviors checklist (OBC) has been deemed a validated instrument specifically created to quantify the frequency of these behaviors [14].
The increasing demand for orthodontic treatment among adults, driven largely by esthetic concerns, is particularly notable among females aged 26–40 [15]. This demographic also shows a heightened incidence of TMD symptoms. While TMJ pain and dysfunction are not directly linked to orthodontic treatment [16], TMD symptoms can fluctuate and may arise during such treatment. The activation of orthodontic appliances applies forces to teeth, potentially causing transient discomfort or pain due to lowered pressure pain thresholds in muscles such as the masseter and temporalis [17]. These changes likely involve neuroplastic adaptations in brainstem neurons receiving inputs from trigeminal nerves [18, 19]. If a patient experiences pain during active orthodontic treatment, it is advisable to temporarily halt treatment to prevent exacerbation. Once the pain subsides, treatment can resume as planned or be adjusted based on the patient’s condition [20]. However, the emergence of pain would inevitably prolong orthodontic treatment, impacting patient compliance [21] and increasing the risk of side effects such as root resorption and enamel decalcification. Therefore, it is crucial to thoroughly evaluate factors contributing to PTMD during orthodontic treatment to mitigate such risks and potential legal issues.
Given the possible role of oral behaviors in the etiology of PTMD, effectively managing oral behaviors could offer a promising approach to prevent or alleviate PTMD. However, existing research has predominantly examined the general population [11], and the impact of oral behaviors on PTMD in orthodontic patients remains unvalidated. Thus, this study aims to explore the association between oral behaviors and PTMD specifically within orthodontic patients, considering various classifications of oral behaviors. Our null hypothesis posits that the severity of pain associates positively with the frequency of oral behaviors among orthodontic patients.
2. Materials and Methods
2.1. Sample Size Calculation
A medium effect size (0.15) using Cohen’s f2 was utilized to calculate the sample size with a significance level of 0.05 and a power of 0.8 [22], resulting in a minimum required sample size of 103. Ultimately, a total of 267 voluntary participants participated in the study, greatly surpassing the threshold.
2.2. Participants
In this cross-sectional study, patients who visited the Department of Orthodontics in West China Hospital of Stomatology between June 2023 and August 2023 were included.
The inclusion criteria comprised (a) patients undergoing orthodontic treatment for at least 1 month; (b) patients with TMD; and (c) capability to read and fill in questionnaires on electronic devices. Exclusion criteria encompassed (a) history of facial trauma or surgical procedures; (b) unmanaged autoimmune, metabolic, or psychiatric conditions; (c) cognitive impairment and/or illiteracy; (d) incomplete or incorrectly completed questionnaires; and (e) patients aged under 18 years.
2.3. Data Collection
Data were gathered from convenient questionnaires, which were structured into three parts (Supporting Table S1). The first part gathered demographic data including gender, age, education degree, and systemic diseases. Information regarding the type of orthodontic appliances was collected from clinical records.
The second part consisted of OBC as described in a previous study [11]. Each item was rated on a scale from 0 (none of the time) to 4 (all of the time). Individuals with oral behaviors scored 3 (most of the time) or 4 (all of the time) were defined as engaging in oral behaviors. The overall frequency was evaluated by the total points of all eight oral behaviors, referred to as OBC-8.
The third part examined the symptoms of TMDs, especially pain, of patients. To start with, the five major TMD symptoms (5Ts) was used because of its high sensitivity (at least 96.1%) and specificity (100%) in identifying potential TMD [23]. If either question was answered positively, it indicated that TMD might be present. The initial two symptoms of the 5Ts include (1) TMD pain, which encompasses pain in the jaw, temple, ear, or in front of the ear, and (2) headaches, specifically those in the temple area. Participants who reported experiencing either of these symptoms were classified into the PTMD group, while those without these symptoms were placed in the NPT group. In addition, the visual analog scale (VAS) was used for the assessment of pain severity [24] in TMJ or masticatory muscles. The VAS measures pain on a scale ranging from 0 (no pain) to 10 (the worst pain possible). This evaluation collected data on the most severe pain experienced in the last month (VAS highest).
2.4. Statistical Analysis
For descriptive statistics, the normality of data was examined with the Kolmogorov–Smirnov normality test. Nonnormally distributed continuous variables were analyzed with the Kruskal–Wallis test, while categorical variables were analyzed using both the chi-square test and Fisher’s exact test. Spearman’s correlation test was applied to determine the relationships between oral behaviors. Univariate linear regression was performed to assess the impact of demographic variables and each oral behavior on pain severity. Multivariate linear regression was conducted to identify specific oral behavior associated with pain and to account for potential biases, including gender, age, education, history of systemic diseases, and orthodontic appliances. Finally, interaction and stratified analyses based on age, gender, education, and systemic diseases were also carried out to comprehensively evaluate the association between the frequency of different oral behaviors and severity of pain in orthodontic patients. A significance level of 0.05 was used to determine statistical significance.
3. Results
A total of 267 valid questionnaires were collected, comprising 55 males and 212 females. The majority of participants had graduated from college (77.53%) and reported no systemic diseases (89.51%). Among all respondents, 40.07% (N = 107) were in the NPT group, while 59.93% (N = 160) were in the PTMD group (Table 1).
| Variables | NPT | PTMD | p value |
|---|---|---|---|
| N | 107 | 160 | |
| Age | 26.59 (5.28) 26.00 (22.00–29.50) | 26.46 (5.26) 26.00 (22.00–29.00) | 0.841a |
| OBC-8 | 7.21 (5.23) 7.00 (2.50–10.00) | 8.20 (5.73) 8.00 (4.00–11.00) | 0.151a |
| Gender | 0.212b | ||
| Male | 18 (16.82%) | 37 (23.12%) | |
| Female | 89 (83.18%) | 123 (76.88%) | |
| Education | 0.171c | ||
| Secondary school and below | 2 (1.87%) | 7 (4.38%) | |
| Undergraduate or junior college | 89 (83.18%) | 118 (73.75%) | |
| Postgraduate and above | 16 (14.95%) | 35 (21.88%) | |
| Systemic diseases | 0.189c | ||
| No | 99 (92.52%) | 140 (87.50%) | |
| Yes | 8 (7.48%) | 20 (12.50%) | |
| Orthodontic appliances | 0.841c | ||
| Clear aligner | 51 (47.66%) | 77 (48.12%) | |
| Fixed appliance | 56 (52.34%) | 83 (51.88%) |
- Note: Results presented as mean (SD) and median (Q1–Q3). NPT, nonpainful temporomandibular disorder.
- Abbreviations: OBC, oral behaviors checklist; PTMD, painful temporomandibular disorder.
- aKruskal–Wallis test for nonparametric data (normality test with the Kolmogorov–Smirnov normality test).
- bChi-square test.
- cFisher’s exact probability test.
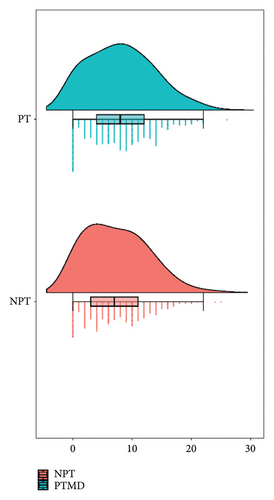
As illustrated in the rain cloud plot, orthodontic patients with PTMD showed a higher median value of the OBC-8 compared to those in the NPT group (Figure 1).
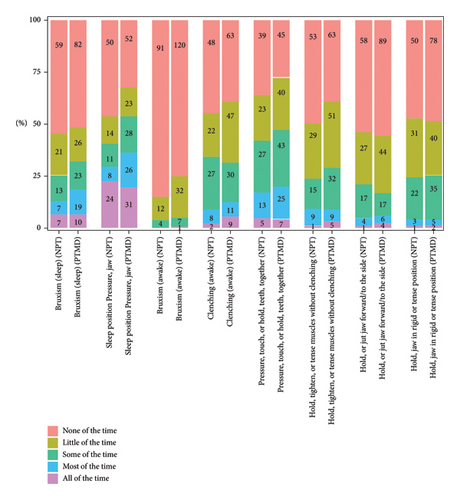
Among the eight oral behaviors, five were more common in the PTMD group than in the NPT group. However, the prevalence of awake bruxism, holding, tightening, or tensing muscles without clenching and holding jaw in rigid or tense position showed no obvious difference between the two groups. Within the PTMD group, the prevalence of various oral behaviors ranged from 0.006% for awake bruxism (the least common) to 35.63% for sleep position pressuring jaw (the most common) (Figure 2).
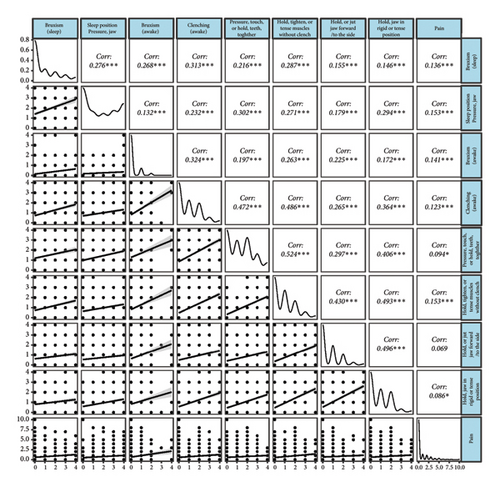
All oral behaviors were positively correlated with each other and pain. Pain showed weak correlations with sleep bruxism (r = 0.136, p < 0.001), sleep position pressuring jaw (r = 0.153, p < 0.001), awake bruxism (r = 0.141, p < 0.001), awake clenching (r = 0.123, p < 0.001), pressuring, touching, or holding teeth together (r = 0.094, p < 0.001), holding, tightening, or tensing muscles without clenching (r = 0.153, p < 0.001), holding or jutting jaw forward/to the side (r = 0.069, p < 0.001), and holding jaw in rigid or tense position (r = 0.086, p < 0.001) (Figure 3).
Further investigation into the potential contributions of oral behaviors to pain in orthodontic patients by univariate linear regression (Table 2) revealed a significant association between pain severity and the frequency of overall oral behaviors (OBC-8) (β = 0.05; 95% CI: 0.02, 0.08; p = 0.0013). Specifically, significant positive correlations were found for sleep bruxism (β = 0.17; 95% CI: 0.03, 0.31; p = 0.0157), sleep position pressuring on jaw (β = 0.16; 95% CI: 0.05, 0.27; p = 0.0059), awake bruxism (β = 0.60; 95% CI: 0.29, 0.91; p = 0.0002), and holding, tightening, or tensing muscles without clenching (β = 0.18; 95% CI: 0.01, 0.35; p = 0.0411). Additionally, no significant correlation was found between pain severity and demographic variables.
| Variables | Pain intensity |
|---|---|
| Gender | |
| Male | Reference |
| Female | 0.04 (−0.40, 0.49) 0.8443 |
| Age | −0.03 (−0.06, 0.01) 0.1007 |
| Education | |
| Secondary school and below | Reference |
| Undergraduate or junior college | −0.22 (−1.22, 0.77) 0.6616 |
| Postgraduate and above | 0.04 (−1.02, 1.09) 0.9420 |
| Systemic diseases | |
| No | Reference |
| Yes | −0.06 (−0.64, 0.53) 0.8528 |
| Orthodontic appliances | |
| Clear aligner | Reference |
| Fixed appliance | 0.27 (−0.09, 0.63) 0.1407 |
| OBC-8 | 0.05 (0.02, 0.08) 0.0013 |
| Bruxism (sleep) | 0.17 (0.03, 0.31) 0.0157 |
| Sleep position pressures jaw | 0.16 (0.05, 0.27) 0.0059 |
| Bruxism (awake) | 0.60 (0.29, 0.91) 0.0002 |
| Clenching (awake) | 0.13 (−0.03, 0.29) 0.1035 |
| Pressure, touch, or hold teeth together | 0.13 (−0.02, 0.28) 0.0830 |
| Hold, tighten, or tense muscles without clenching | 0.18 (0.01, 0.35) 0.0411 |
| Hold or jut jaw forward/to the side | 0.04 (−0.15, 0.23) 0.6627 |
| Hold jaw in rigid or tense position | 0.18 (−0.01, 0.37) 0.0634 |
- Note: Results presented as β (95% CI) p value. The bold values indicate statistically significant results.
- Abbreviation: OBC, oral behaviors checklist.
The results of multiple linear regression (Table 3) indicated significant positive associations between sleep bruxism (β = 0.17; 95% CI: 0.03, 0.31; p = 0.0157), sleep position pressuring on jaw (β = 0.16; 95% CI: 0.05, 0.27; p = 0.0059), awake bruxism (β = 0.60; 95% CI: 0.29, 0.91; p = 0.0002), and holding, tightening, or tensing muscles without clenching (β = 0.18; 95% CI: 0.01, 0.35; p = 0.0411) with the severity of pain. These significant positive associations between severity of pain and sleep bruxism (Model I: β = 0.17; 95% CI: 0.03, 0.31; p = 0.0169) (Model II:β = 0.16; 95% CI: 0.02, 0.30; p = 0.0257), sleep position pressuring on jaw (Model I: β = 0.15; 95% CI: 0.03, 0.26; p = 0.0113) (Model II: β = 0.14; 95% CI: 0.03, 0.26; p = 0.0146), awake bruxism (Model I: β = 0.58; 95% CI: 0.27, 0.89; p = 0.0003) (Model II: β = 0.56; 95% CI: 0.25, 0.87; p = 0.0005), and holding, tightening, or tensing muscles without clenching (Model I: β = 0.18; 95% CI: 0.01, 0.35; P = 0.0378) (Model II: β = 0.18; 95% CI: 0.00, 0.36; p = 0.0470) persisted after adjusting for demographic factors. In addition, there were positive associations between clenching (β = 0.13; 95% CI: −0.03, 0.29; p = 0.1035), pressuring, touching, or holding teeth together (β = 0.13; 95% CI: −0.02, 0.28; p = 0.0830), and holding jaw in rigid or tense position (β = 0.18; 95% CI: −0.01, 0.37; p = 0.0634) with pain intensity with borderline significance. These positive associations between severity of pain and positive associations between awake clenching (Model I: β = 0.12; 95% CI: −0.04, 0.28; p = 0.1352) (Model II: β = 0.13; 95% CI: −0.03, 0.30; p = 0.1033), pressuring, touching, or holding teeth together (Model I: β = 0.13; 95% CI: −0.02, 0.28; p = 0.0909) (Model II: β = 0.13; 95% CI: −0.02, 0.28; p = 0.0930), and holding jaw in rigid or tense position (Model I: β = 0.16; 95% CI: −0.03, 0.35; p = 0.0992) (Model II: β = 0.16; 95% CI: −0.04, 0.35; p = 0.1165) persisted after adjusting for demographic factors.
| Nonadjusted | Adjust I | Adjust II | |
|---|---|---|---|
| Bruxism (sleep) | 0.17 (0.03, 0.31) 0.0157 | 0.17 (0.03, 0.31) 0.0169 | 0.16 (0.02, 0.30) 0.0257 |
| Sleep position pressures jaw | 0.16 (0.05, 0.27) 0.0059 | 0.15 (0.03, 0.26) 0.0113 | 0.14 (0.03, 0.26) 0.0146 |
| Bruxism (awake) | 0.60 (0.29, 0.91) 0.0002 | 0.58 (0.27, 0.89) 0.0003 | 0.56 (0.25, 0.87) 0.0005 |
| Clenching (awake) | 0.13 (−0.03, 0.29) 0.1035 | 0.12 (−0.04, 0.28) 0.1352 | 0.13 (−0.03, 0.30) 0.1033 |
| Pressure, touch, or hold teeth together | 0.13 (−0.02, 0.28) 0.0830 | 0.13 (−0.02, 0.28) 0.0909 | 0.13 (−0.02, 0.28) 0.0930 |
| Hold, tighten, or tense muscles without clenching | 0.18 (0.01, 0.35) 0.0411 | 0.18 (0.01, 0.35) 0.0378 | 0.18 (0.00, 0.36) 0.0470 |
| Hold or jut jaw forward/to the side | 0.04 (−0.15, 0.23) 0.6627 | 0.04 (−0.15, 0.23) 0.6744 | 0.02 (−0.17, 0.21) 0.8121 |
| Hold jaw in rigid or tense position | 0.18 (−0.01, 0.37) 0.0634 | 0.16 (−0.03, 0.35) 0.0992 | 0.16 (−0.04, 0.35) 0.1165 |
- Note: Results presented as β (95% CI) p value. Adjust I model adjusted for age and gender. Adjust II model adjusted for age, gender, education, systemic disease, and orthodontic appliances. The bold values indicate statistically significant results.
Given that TMD prevalence was found to be higher among females and individuals aged 18–44 years, and considering that educational level may influence TMD pain [25], while systemic diseases could affect its development [26], multiple linear regression analyses stratified by demographic variables were further performed (Table 4). While only awake bruxism (β = 0.58; 95% CI: 0.06, 1.11; p = 0.0293) and pressuring, touching, or holding teeth together (β = 0.36; 95% CI: 0.03, 0.68; p = 0.0327) was significantly associated with pain in male orthodontic patients, up to four behaviors including sleep bruxism (β = 0.20; 95% CI: 0.04, 0.36; p = 0.0153), sleep position pressuring jaw (β = 0.15; 95% CI: 0.03, 0.28; p = 0.0161), awake bruxism (β = 0.61; 95% CI: 0.23, 1.00; p = 0.0018), and holding jaw in rigid or tense position (β = 0.22; 95% CI: 0.01, 0.43; p = 0.0390) were in significant positive association with pain in female orthodontic patients. As for age stratification, awake bruxism (β = 0.66; 95% CI: 0.09, 1.23; p = 0.0232), pressuring, touching, or holding teeth together (β = 0.26; 95% CI: 0.00, 0.51; p = 0.0473), holding tightening or tensing muscles without clenching (β = 0.30; 95% CI: 0.02, 0.59; p = 0.0354), and holding jaw in rigid or tense position (β = 0.36; 95% CI: 0.04, 0.67; p = 0.0265) were only significantly associated with pain in the population aged 24 to 28, while sleep (β = 0.23; 95% CI: 0.01, 0.46; p = 0.0440) and awake bruxism (β = 0.52; 95% CI: 0.08, 0.95; p = 0.0206) were significantly associated with pain in the population aged 18 to 23. Sleep bruxism (β = 0.20; 95% CI: 0.04, 0.35; p = 0.0143 and β = 0.17; 95% CI: 0.02, 0.32; p = 0.0233), sleep position pressuring jaw (β = 0.19; 95% CI: 0.06, 0.32; p = 0.0038 and β = 0.17; 95% CI: 0.05, 0.29; p = 0.0050), and awake bruxism (β = 0.65; 95% CI: 0.30, 1.00; p = 0.0003 and β = 0.58; 95% CI: 0.27, 0.90; p = 0.0004) were significantly associated with pain in orthodontic patients with undergraduate or junior college degree and without systemic diseases. Sleep bruxism (β = 0.29; 95% CI: 0.08, 0.51; p = 0.0080), awake bruxism and pressuring (β = 0.67; 95% CI: 0.21, 1.13; p = 0.0045), and touching or holding teeth together (β = 0.24; 95% CI: 0.04, 0.44; p = 0.0195) were significantly associated with pain in orthodontic patients treated with clear aligner, while sleep position pressuring jaw (β = 0.18; 95% CI: 0.02, 0.34; p = 0.0299) and awake bruxism (β = 0.51; 95% CI: 0.09, 0.93; p = 0.0174) were significantly associated with pain in orthodontic patients treated with fixed appliances. However, the interaction analyses yielded nonsignificant p values (p > 0.05), indicating that these stratifications did not significantly modify the relationships between oral behaviors and pain.
| Bruxism (sleep) | Sleep position pressures jaw | Bruxism (awake) | Clenching (awake) | Pressure, touch, or hold teeth together | Hold, tighten, or tense muscles without clenching | Hold or jut jaw forward/to the side | Hold jaw in rigid or tense position | |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 0.09 (−0.19, 0.37) 0.5372 | 0.18 (−0.09, 0.44) 0.1850 | 0.58 (0.06, 1.11) 0.0293 | 0.06 (−0.28, 0.40) 0.7175 | 0.36 (0.03, 0.68) 0.0327 | 0.19 (−0.19, 0.58) 0.3208 | −0.04 (−0.38, 0.30) 0.8181 | −0.01 (−0.46, 0.43) 0.9503 |
| Female | 0.20 (0.04, 0.36) 0.0153 | 0.15 (0.03, 0.28) 0.0161 | 0.61 (0.23, 1.00) 0.0018 | 0.15 (−0.03, 0.33) 0.1009 | 0.07 (−0.10, 0.24) 0.3989 | 0.17 (−0.02, 0.37) 0.0762 | 0.09 (−0.14, 0.32) 0.4659 | 0.22 (0.01, 0.43) 0.0390 |
| p interaction | 0.5001 | 0.8609 | 0.9286 | 0.6587 | 0.1267 | 0.9253 | 0.5436 | 0.3484 |
| Age | ||||||||
| 18–23 | 0.23 (0.01, 0.46) 0.0440 | 0.17 (−0.02, 0.35) 0.0732 | 0.52 (0.08, 0.95) 0.0206 | 0.03 (−0.24, 0.29) 0.8326 | −0.06 (−0.33, 0.21) 0.6597 | 0.10 (−0.19, 0.39) 0.4835 | 0.04 (−0.25, 0.34) 0.7707 | −0.02 (−0.33, 0.29) 0.8980 |
| 24–28 | 0.11 (−0.14, 0.35) 0.3974 | 0.16 (−0.03, 0.36) 0.1033 | 0.66 (0.09, 1.23) 0.0232 | 0.12 (−0.14, 0.39) 0.3614 | 0.26 (0.00, 0.51) 0.0473 | 0.30 (0.02, 0.59) 0.0354 | 0.13 (−0.22, 0.47) 0.4672 | 0.36 (0.04, 0.67) 0.0265 |
| 29–41 | 0.13 (−0.12, 0.39) 0.3149 | 0.12 (−0.09, 0.33) 0.2484 | 0.61 (0.12, 1.35) 0.1027 | 0.22 (−0.07, 0.51) 0.1461 | 0.15 (−0.10, 0.41) 0.2380 | 0.11 (−0.21, 0.43) 0.5087 | −0.08 (−0.42, 0.26) 0.6396 | 0.17 (−0.19, 0.53) 0.3569 |
| p interaction | 0.7148 | 0.9416 | 0.9174 | 0.6389 | 0.2216 | 0.5399 | 0.6893 | 0.2382 |
| Education | ||||||||
| Secondary school | −0.30 (−1.08, 0.49) 0.4629 | −0.10 (−0.63, 0.43) 0.7102 | 2.25 (−0.77, 5.27) 0.1455 | −0.47 (−1.89, 0.94) 0.5129 | −0.50 (−1.34, 0.34) 0.2431 | −0.45 (−2.40, 1.50) 0.6518 | −0.50 (−1.70, 0.70) 0.4135 | −0.47 (−1.89, 0.94) 0.5132 |
| Undergraduate or junior college | 0.20 (0.04, 0.35) 0.0143 | 0.19 (0.06, 0.32) 0.0038 | 0.65 (0.30, 1.00) 0.0003 | 0.13 (−0.04, 0.30) 0.1429 | 0.12 (−0.05, 0.29) 0.1581 | 0.15 (−0.05, 0.34) 0.1474 | 0.05 (−0.16, 0.27) 0.6241 | 0.19 (−0.02, 0.41) 0.0821 |
| Postgraduate and above | 0.14 (−0.20, 0.48) 0.4366 | 0.09 (−0.18, 0.36) 0.5297 | 0.32 (−0.37, 1.01) 0.3704 | 0.32 (−0.15, 0.80) 0.1837 | 0.31 (−0.05, 0.67) 0.0905 | 0.29 (−0.08, 0.67) 0.1286 | 0.02 (−0.38, 0.42) 0.9078 | 0.16 (−0.25, 0.58) 0.4404 |
| p interaction | 0.4682 | 0.4886 | 0.3855 | 0.5119 | 0.2005 | 0.6467 | 0.6640 | 0.6536 |
| Systemic diseases | ||||||||
| No | 0.17 (0.02, 0.32) 0.0233 | 0.17 (0.05, 0.29) 0.0050 | 0.58 (0.27, 0.90) 0.0004 | 0.14 (−0.03, 0.32) 0.1083 | 0.14 (−0.03, 0.30) 0.1038 | 0.16 (−0.03, 0.35) 0.0944 | 0.05 (−0.15, 0.25) 0.6388 | 0.15 (−0.05, 0.36) 0.1356 |
| Yes | 0.17 (−0.21, 0.56) 0.3798 | 0.07 (−0.28, 0.42) 0.7041 | 0.86 (−0.39, 2.10) 0.1785 | 0.11 (−0.28, 0.50) 0.5864 | 0.16 (−0.27, 0.58) 0.4696 | 0.35 (−0.11, 0.80) 0.1352 | −0.00 (−0.60, 0.59) 0.9922 | 0.42 (−0.15, 0.99) 0.14547 |
| p interaction | 0.9992 | 0.5761 | 0.6757 | 0.8656 | 0.9225 | 0.4572 | 0.8741 | 0.3790 |
| Orthodontic appliances | ||||||||
| Clear aligner | 0.29 (0.08, 0.51) 0.0080 | 0.12 (−0.03, 0.28) 0.1261 | 0.67 (0.21, 1.13) 0.0045 | 0.20 (−0.02, 0.43) 0.0726 | 0.24 (0.04, 0.44) 0.0195 | 0.17 (−0.06, 0.41) 0.1547 | 0.09 (−0.19, 0.37) 0.5200 | 0.17 (−0.09, 0.43) 0.1902 |
| Fixed appliance | 0.06 (−0.12, 0.25) 0.4938 | 0.18 (0.02, 0.34) 0.0299 | 0.51 (0.09, 0.93) 0.0174 | 0.04 (−0.18, 0.26) 0.7265 | −0.02 (−0.24, 0.21) 0.8872 | 0.18 (−0.07, 0.43) 0.1524 | −0.02 (−0.28, 0.23) 0.8584 | 0.17 (−0.10, 0.45) 0.2156 |
| p interaction | 0.1092 | 0.6390 | 0.6160 | 0.3014 | 0.0900 | 0.9550 | 0.5487 | 0.9950 |
- Note: Results presented as β (95% CI) p value or p value for interaction. The bold values indicate statistically significant results.
4. Discussion
This study investigated the correlation between oral behaviors and PTMD in orthodontic patients, specifically examining various oral behaviors. The findings indicated significant positive relationships between the overall frequency of oral behaviors and the severity of pain. Among the specific subcategories of oral behaviors, TMD pain intensity showed significant positive associations with sleep bruxism, sleep position pressuring jaw, awake bruxism, and holding, tightening, or tensing muscles without clenching, and borderline significant positive relationships between awake clenching, pressuring, touching or holding teeth together, and holding jaw in rigid or tense position. As a result, the null hypothesis was accepted.
To the best of our knowledge, our study was one of the few studies that had found an association between oral behaviors and PTMD in orthodontic patients, offering invaluable insights for orthodontists on managing PTMD by controlling oral behaviors. In comparison to previous research on oral behaviors and PTMD, our study still distinguishes itself through meticulous demographic stratification, thereby enhancing the delineation of indications for correcting parafunctional habits.
The majority of participants in this study were female, reflecting the common demographic of orthodontic clinic patients, who are often adolescents and young women [16]. It is generally believed that PTMD is more prevalent among females than among males [27]. However, a prospective study found no significant difference in the incidence of acute TMDs between males and females, with only chronic TMDs showing a higher prevalence in females [28]. This discrepancy suggests that the observed higher prevalence of PTMD in females in cross-sectional surveys may be attributed to the longer duration of TMDs in females. In this cross-sectional study, no significant difference in PTMD between males and females was observed. This result may be due to the preselection process, where the majority of PTMD patients were filtered out before receiving orthodontic treatment.
Orthodontic treatment typically does not significantly influence an individual’s likelihood of developing TMD through changes in occlusion or condyle position nor does it prevent future TMD [29, 30]. Therefore, the exacerbation of TMD pain during orthodontic treatment is likely due to other factors. Previous research has indicated that oral behaviors outside of normal physiological functions may contribute to the etiology or act as a risk factor for PTMD [31, 32]. Oral behaviors can cause microtrauma or mechanical overload of the TMJ, which can result in joint disc displacement, significantly contributing to PTMD [33]. Additionally, excessive joint activity deprives the orofacial muscles of necessary rest, resulting in pain. This pain can induce local muscle spasms, exacerbating the discomfort and perpetuating a vicious cycle. [34]. This suggests that even minor, persistent oral habits can cause joint degeneration over time [35, 36]. Several studies have confirmed the positive correlation between oral behaviors and PTMD [37, 38]. However, one study found different results, likely due to the focus on children aged 5 to 12 years, who have less awareness of their oral habits, leading parents to overlook behaviors without loud noise [39]. Consistent with the majority of studies, our analysis found that the frequency of overall and four specific oral behaviors were significantly associated with the intensity of pain in the orthodontic population.
Bruxism, a common oral behavior, is believed to cause microtrauma to the TMJ and surrounding muscles, potentially leading to inflammation and pain [33]. This can worsen PTMD by causing joint damage from the bite force during bruxism episodes. Additionally, patients with bruxism often have heightened pain sensitivity, leading to more frequent PTMD reports [40]. Bruxism is divided into awake bruxism and sleep bruxism, each with different mechanisms. Sleep bruxism is mainly linked to neurological disorders, while awake bruxism is associated with psychological factors [41]. Awake and sleep bruxism may impact PTMD differently, as sleep bruxism can indirectly affect PTMD by disrupting sleep quality and increasing psychological stress [42]. Some studies suggest TMD incidence is only linked to sleep bruxism [43, 44], while others connect TMD incidence to awake or mixed bruxism [45, 46]. Our multivariate regression analysis indicated that the frequencies of both sleep and awake bruxism were significantly related to the severity of pain in orthodontic patients, with awake bruxism having a greater impact on PTMD than sleep bruxism. However, further research is needed to evaluate the significance of this difference.
To ensure the undisrupted progress of orthodontic treatment, orthodontists should employ necessary approaches to control the symptoms of PTMD. Given the confirmed detrimental impacts of oral behaviors on pain aggravation in orthodontic patients in this study, the prompt diagnosis and management of these behaviors may serve as an effective method for controlling TMD pain during orthodontic treatment. Implementing a simple screening step, such as asking patients to complete an oral behavioral checklist before initiating orthodontic treatment, could be practical. Regarding treatment, clinicians should consider behavior modification strategies to address waking behaviors [47]. Habit reversal, a technique demonstrated to be successful in reducing TMD pain [48–50], enhances patient awareness of undesirable behaviors (e.g., parafunctions), develops alternatives (e.g., relaxation of masticatory muscles), and substitutes these alternatives for the unwanted behaviors [51]. In addition, cognitive behavior therapy (CBT) and biofeedback-based relaxation have also been reported to be successful in treating pain [47, 52]. However, these approaches may have limited effects on sleep-related behaviors. The efficacy of CBT, biofeedback strategies, and relaxation before bed for the management of sleep bruxism has been questioned [53, 54]. Occlusal appliances are also not applicable due to the consistently changing occlusion during orthodontic treatment. Moreover, drug therapy lacks sufficient supporting evidence, and the administration of botulinum toxin may induce side effects such as osteopenic changes in the condyles and muscle attachment sites [54]. Therefore, managing sleep-related oral behaviors, especially sleep bruxism, can be challenging. It is recommended that orthodontists fully inform patients of these challenges and the potential for pain exacerbation, for medicolegal purposes.
The current study had several limitations. Firstly, its cross-sectional design prevents the establishment of causal or temporal relationships between oral behaviors and PTMD in orthodontic patients. Future research should consider using a prospective longitudinal study design to better investigate these causal relationships and obtain more robust results. Secondly, the assessment of oral behaviors relied on self-report, a common method in epidemiological and clinical research due to its simplicity and cost-effectiveness. However, self-reporting may underestimate or overestimate the true prevalence of these behaviors due to lack of awareness [55]. Utilizing more objective measures, such as electromyography, could improve accuracy. Thirdly, participants were sampled from a single institution, which may introduce selection bias and limit the generalizability of our findings to broader populations. Future research should aim to include multiple institutions to enhance external validity. Fourthly, potential cofounders including psychological stress, anxiety, and sleep disorders, which could influence both oral behaviors and pain perception, were not assessed in our study. Lastly, our reliance on VAS to measure pain intensity may not fully capture the complexity of these dynamic pain experiences. More sophisticated pain assessment tools, such as the McGill Pain Questionnaire (MPQ) [56] and Brief Pain Inventory (BPI) [57], could provide a richer and more precise understanding of pain in TMD patients. Despite these limitations, the present study utilized a relatively large sample size and comprehensively analyzed it with consideration of multiple covariates, offering valuable epidemiological insights into the association between oral habits and TMD pain among orthodontic patients.
5. Conclusions
This study revealed significant associations between the intensity of TMD pain and the frequency of oral behaviors, as assessed by the OBC questionnaire, in orthodontic patients. Specifically, sleep bruxism, sleep position pressuring jaw, awake bruxism, and holding or tensing muscles without clenching were identified as significant factors associated with TMD pain. Early screening and management of these oral behaviors might be a promising approach to control TMD during orthodontic treatment, ensuring optimal orthodontic outcomes.
Ethics Statement
This study was reviewed and approved by the Ethical Committee of the State Key Laboratory of Oral Diseases at West China Hospital of Stomatology, Sichuan University (No. WCHSIRB-D-2022-118) and adhered to the ethical principles of the World Medical Association Declaration of Helsinki (Version 2002).
Consent
Participants provided informed consent after receiving a clear explanation of the purpose of study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Zihan Zhang and Li Zhang contributed equally to the study and they are co-first authors.
Funding
This work was supported by the National Natural Science Foundation of China (82301129); Clinical Research Project of West China Hospital of Stomatology, Sichuan University (LCYJ-2023-YY-2); Research and Develop Program of West China Hospital of Stomatology, Sichuan University (RCDWJS2023-13); Sichuan Science and Technology Program (No. 2023JDRC0096); and National Natural Science Foundation of China (82271019).
Supporting Information
Supporting Table S1: Convenient questionnaire used for data collection.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




