Research Into the Differences and Clinical Diagnostic Importance of Different Ct Values in COVID-19 Patients of Different Age, Albumin, Lactate Dehydrogenase, and Lymphocyte Percentages
Abstract
Introduction: This study aims to investigate the differences and clinical diagnostic importance of different Ct (cycle threshold) values in COVID-19 patients of different age, albumin, lactate dehydrogenase (LDH), and lymphocyte percentage (LYM%).
Methods: A total of 2836 patients confirmed with COVID-19 in our Hospital between January 1 and May 30, 2023 were divided into the high Ct value (34.21–40.00), the medium Ct value (26.51–34.21), and the low Ct value groups (0.00–26.51) using the interquartile method against the negative (control group).
Results: Significant differences were observed in clinical indicators between the middle Ct group and the control group; however, no significant differences were noted between the middle and high Ct groups. In the high Ct group, notable disparities in albumin and LDH were found compared to the control. The low Ct group exhibited significant differences from the high Ct group regarding age and LYM%. Furthermore, age was significantly different within the low Ct group when compared to the middle one. Nonetheless, no significant variations in LDH were detected between the low Ct group and the control.
Conclusion: People over 59 years old are at an elevated risk for higher viral loads and increased susceptibility to COVID-19 infection. Albumin and LYM% serve as protective factors against this disease. Age, albumin, and LYM% demonstrate favorable predictive values for distinguishing individuals within the low Ct category from other groups.
1. Introduction
The novel coronavirus (SARS-CoV-2), first identified on December 12, 2019, is classified within the Betacoronavirus genus of the Coronaviridae family, exhibiting a genome sequence that closely resembles that of SARS-CoV. This virus utilizes the receptor-binding domain of its spike protein and the host receptor ACE2 to facilitate entry into host cells. ACE2 receptors are extensively distributed across anatomical sites, including alveoli, trachea, and myocardium. The primary factors contributing to the disease mechanism involve interactions between the spike protein and ACE2 receptor with 2019-nCoV, resulting in atrophy, fibrosis, inflammation, and subsequent damage to host tissues [1, 2]. However, it is crucial to remain vigilant regarding the highly variable nature of SARS-CoV-2 necessitating timely detection and preventive measures.
The objective aim of measuring cycle threshold (Ct) values for SARS-CoV-2 is to evaluate virus presence in samples. Ct values indicate the number of PCR cycles required for a sample to yield a positive result; they are semi-quantitative and inversely proportional to viral nucleic acid quantity present in the sample. Existing research demonstrates a robust correlation between Ct values and viral infectivity; especially, an odds ratio (OR) reduction of 0.67 occurs with each unit increase in Ct values [3]. Furthermore, Ct values among COVID-19 patients correlate significantly with intubation risk as well as in-hospital mortality rates, providing critical insights for patient management strategies [4]. According to Umakanthan’s research findings, the median age among COVID-19 patients stands at 56 years (range: 55–65 years) [5]. Additionally, older individuals exhibit heightened susceptibility requiring more complex treatment interventions [5].
Albumin is directly associated with both inflammation responses and nutritional status; however, its predictive role concerning outcomes related to COVID-19 infection remains inadequately defined thus far. Nonetheless, studies suggest that a decreased albumin may lead to the upregulation of ACE2 receptors, thereby heightening vulnerability toward SARS-CoV-2 infection [6].
Lactate dehydrogenase (LDH), an enzyme prevalent within all cellular cytoplasm compartments, can leak into serum following tissue damage or pathological alterations leading to elevated LDH activity levels observed primarily during diagnoses such as myocardial infarction, leukemia, malignant tumors, and etcetera. Elevated serum LDH activity has been positively correlated with early pulmonary lesions of COVID-19, thus reflecting the severity of the disease [7].
Moreover, reduced lymphocytes have been associated with the pathogenesis of SARS-CoV-2. Lymphocytes serve as important biomarkers for the early identification of severe patients and for predicting the clinical progression of COVID-19 infection [8].
Previous investigations have focused on the age distribution of affected populations, changes in common laboratory indicators before and after infection with SARS-CoV-2, and the extent of variations in indicators among patients with severe, moderate, mild, and asymptomatic infections. However, limited studies on affected populations′ viral load (Ct values) exist. This study aims to principally explore differential analyses of various viral loads, common clinical indicators, and the associated clinical values.
2. Methods
2.1. General Information
A total of 2836 patients who visited our hospital between January 1, 2023, and June 15, 2023, were included in this study. Data on Ct values with age, albumin, LDH, and LYM% were collected for correlation analysis. The diagnostic criteria for COVID-19 patients were based on the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection (Trial Version 10)” issued by the National Health Commission of the People’s Republic of China involved in clinical manifestations related to COVID-19 infection; one or more of the following pathogenic or serological test results include (1) positive nucleic acid test for the coronavirus; (2) positive antigen test for the coronavirus; (3) positive isolation and culture of the coronavirus; and (4) the level of specific IgG antibodies to the coronavirus in the recovery period is four times or more higher than in the acute phase. This diagnostic criterion also complies with WHO standards [9]. Inclusion criteria: patients aged over 18 years old; the inclusion criteria for COVID-19 patients were positive as per the nucleic acid amplification test, and the negative group did not meet the diagnostic criteria of “Diagnosis and Treatment Protocol for New Coronavirus Infection (Trial Version 10).” The positive diagnostic criteria comply with the WHO guideline diagnostic criteria A, while the negative population fails to meet WHO diagnostic criteria A and B. This diagnostic criterion also adheres to WHO standards (two options, A through B) 1:A. A person with a positive nucleic acid amplification test (NAAT), regardless of clinical criteria or epidemiological criteria. B. A person meeting clinical criteria AND/OR epidemiological criteria (see suspect case A) or with a positive professional use or self-test SARS-CoV-2 antigen-RDT. Selection criteria: Age above 18 years old; the selection criteria for new crown patients are as follows: meet the positive nucleic acid amplification test, and the negative group does not meet the diagnostic criteria of “Diagnosis and Treatment Protocol for Novel Coronavirus Infection (Trial Version 10).” The positive diagnostic criteria meet the WHO guideline diagnostic criteria A, and the negative group does not meet the WHO diagnostic criteria A and B.
2.2. Instruments and Reagents
The Japanese Sysmex XN9000 fully automatic hematology analyzer, Beckman Coulter AU5800, and ABI Q5 fluorescence quantitative analyzer were used. Albumin and LDH assay kits were from Beckman Coulter. The COVID-19 nucleic acid test kit was from Shanghai Bio-Engineering Co., Ltd.
2.3. COVID-19 Nucleic Acid Ct Value Detection
PCR amplification was performed at 50°C for 10 min ⟶ 97°C for 1 min ⟶ 45 cycles: 97°C for 3 s ⟶ 60°C for 15 s. Fluorescence was collected at 60°C for 15 s, with the fluorescence channels set as follows: detection targets ORF1ab and N were in the FAM and VIC/HEX channels, respectively, and the internal reference control RNase P was in the ROX channel. The result was considered positive when the negative control had no Ct value, and the positive control had FAM and VIC/HEX channel Ct values ≤ 30 or FAM and VIC/HEX channel Ct values ≤ 40. A sample was considered negative if it had no Ct value or Ct value > 40. If the Ct value of a single target was ≤ 40, a retest was conducted. The sample was considered positive if, after the retest, the Ct value of both (or single) targets was ≤ 40 and was considered negative if both targets were > 40. For human-derived samples, the Ct value of RNase P detected on the real-time PCR system should be < 45, In high-concentration 2019-nCoV samples where both the ORF1ab and N genes show strong positive signals, competitive inhibition in the reaction system may lead to undetectable Ct values for the RNase P internal control due to insufficient copies. If both the ORF1ab and N genes are negative in human-derived samples and the RNase P internal control shows no Ct value, it is recommended to retest the sample or collect a new sample for retesting.
2.4. LYM%, Albumin, and LDH Assessment
LYM% was detected using a semiconductor flow cytometer. Albumin was determined using the bromocresol green method. LDH was measured using the lactate substrate method.
2.5. Observation Indices
A retrospective analysis entailed querying patient records from our hospital’s electronic medical record system encompassing COVID-19 nucleic acid test results alongside age albumin, LDH, LYM%, as well as underlying health conditions such as hypertension, diabetes, and coronary heart disease presence or absence. Given the higher viral load sensitivity of the N gene (median Ct: 30.28 vs. ORF1ab: 31.00, p < 0.001), all analyses were based on N gene Ct values unless otherwise specified.
2.6. Statistical Analysis
SPSS 25.0 and GraphPad Prism 8 software were used for data processing and statistical analysis. The Kolmogorov–Smirnov test was used to test the data normality. Based on the data characteristics, normally distributed data are presented as mean ± standard deviation (SD), while non-normally distributed data are expressed as median (interquartile range, IQR), and categorical data were presented as frequencies and rates. The Mann–Whitney U test was used to analyze differences between groups, and the Bonferroni correction was used for pairwise comparisons within groups. p < 0.05 represented statistical significance. The agreement between ORF1ab and N genes was validated using Spearman’s rank correlation, Wilcoxon signed-rank test, and Bland–Altman analysis. Binary logistic regression analysis was used to determine independent predictors of disease severity, calculating the ORs of significantly correlated variables. The effectiveness of disease severity diagnosis was assessed using receiver operating characteristic (ROC) curve analysis.
3. Results
3.1. Clinical Characteristics
This study encompassed a total cohort comprising 2836 individuals who sought treatment at our hospital between January 1, 2023, and June 15, 2023, meeting specified inclusion and exclusion criteria forming our analytical sample set. Among these participants: 2133 tested negative for COVID-19 presenting median ages recorded at 62 years (range: 51–72 years) inclusive of subgroups categorized into 954 individuals (43.17%) aged between 18 and 59 years, 921 individuals (12.09%) aged spanning 59–79 years, and 258 individuals (37.10%) falling within age brackets ranging from 79 to 97 years. Additionally, 703 subjects yielded positive COVID-19 diagnosis reflecting median ages documented around 70 (ranging from 59 to 78.75). This subset included 202 individuals (47.08%) aged between 18 and 59, 331 individuals (24.18%) aged between 59 and 79, and 170 cases (34.00%) represented those above 79 but below 97.
Laboratory examinations accounted comprehensively involving albumin assessment on 2525 samples, LYM% evaluation across 2788 specimens, and LDH quantification derived from analyzing 1456 samples. The COVID-19 negative group had median values of 37.10 for albumin (range: 32.40–41.40), 13.60 for LYM% (range: 6.30–25.20), and 218.30 for LDH (range: 182.90–307.00). Conversely, the COVID-19 positive group had median values of 34.00 for albumin (range: 30.50–37.50), 8.5 for LYM% (range: 5.2–16.00), and 279.10 for LDH (range: 212.41–371.25). In totality, 485 confirmed COVID-19-positive individuals had available Ct values, with a median of 30.28 (range: 26.51–34.21).
Ct values were categorized into three groups based on quantile distribution: 0.00–26.51 (119 cases, 24.53%), 26.51–34.21 (245 cases, 50.51%), and 34.21–40.00 (121 cases, 24.94%). This grouping aligns with previous studies by La Scola B and Junna Oba, which identified low viral load Ct ranges (27–34) and Ct ranges (> 35) associated with the inability to isolate live virus or extremely low infectiousness [10–12]. This study utilized these thresholds to analyze correlations between Ct values and clinical indicators. Detailed information is shown in Table 1 and Figure S1.
| Variable | Total | COVID-19 negative individuals | COVID-19 positive individuals |
|---|---|---|---|
| Age in years, median (Q1–Q3) | 2836 | 62.00 (51.00–72.00) | 70.00 (59.00–78.75) |
| 18–59 [N (%)] | 1156 | 954 (43.17) | 202 (47.08) |
| 59–79 [N (%)] | 1252 | 921 (12.09) | 331 (24.18) |
| 79–97 [N (%)] | 428 | 258 (37.10) | 170 (34.00) |
| Albumin (g/L) | 2525 | 37.10 (32.40–41.40) | 34.00 (30.50–37.50) |
| Lactate dehydrogenase (U/L) | 1456 | 218.30 (182.90–307.00) | 279.10 (212.41–371.25) |
| Lymphocyte percentage (%) | 2788 | 13.60 (6.30–25.20) | 8.50 (5.20–16.00) |
| Ct value | 485 | — | 30.28 (26.51–34.21) |
| 0.00∼26.51 [N (%)] | 119 | — | 119 (24.53) |
| 26.51∼34.21 [N (%)] | 245 | — | 245 (50.51) |
| 34.21∼40 [N (%)] | 121 | — | 121 (24.94) |
- Note: Q1–Q3: First and third quartiles; percentage share relative to N; N represents the number of cases with corresponding characteristics in the respective group. Diagnostic cutoffs of 26.51 and 34.21 Ct were derived from the interquartile range (IQR) analysis of 485 PCR-confirmed cases, consistent with clinical infectivity thresholds where viral loads with Ct > 34 correlate with non-transmissible infection status. Reference ranges for healthy adults: Albumin (40–55 g/L), lactate dehydrogenase (120–250 U/L), and lymphocyte percentage (20%–50%).
3.2. Differences in Clinical Indicators Between COVID-19 Positive and Negative Individuals
Significant differences (p < 0.05) were found in the distribution of lab indicators (albumin, LDH, LYM%) and age between COVID-19 positive and negative individuals. However, no significant differences (p > 0.05) were observed in the 18–59 age group after data stratification. Significant differences (p < 0.05) were noted in the 59–79 and 79–97 age groups. Detailed results are shown in Table 2.
| Mann–Whitney U | Z value | p value | |
|---|---|---|---|
| Albumin | 464,639.5 | −9.797 | ≤ 0.001 |
| Lactate dehydrogenase | 111,222.5 | −6.185 | ≤ 0.001 |
| Lymphocyte percentage | 581,159 | −8.845 | ≤ 0.001 |
| Age | 522,119 | −10.664 | ≤ 0.001 |
| 18–59 | 72,978 | −0.079 | 0.937 |
| 59–79 | 522,119 | −10.664 | ≤ 0.001 |
| 79–97 | 522,119 | −10.664 | ≤ 0.001 |
3.3. Consistency Analysis of Ct Values Between ORF1ab and N Genes
The Ct values of ORF1ab and N genes demonstrated an extremely strong monotonic correlation (Spearman’s r = 0.945, p < 0.001) but with significant systematic bias (Wilcoxon signed-rank p < 0.001). The Bland–Altman analysis showed that 95% of the differences ranged from −4.148 to 2.904 Ct. The Ct values of ORF1ab and N genes were non-normally distributed (Kolmogorov–Smirnov p < 0.001), whereas RNase P Ct values (mean ± SD: 29.18 ± 0.10; range: 20.21–35.99) followed a normal distribution (p = 0.102) and confirmed specimen integrity, as no samples exceeded the 45 Ct quality control threshold. Wilcoxon test results revealed significant differences between the two genes: N gene Ct values (median: 30.28; IQR: 26.51–34.21) were consistently lower than those of ORF1ab (median: 31.00; IQR: 26.75–35.42) (p < 0.001). Detailed results are shown in Figure S4. In this study, the lower Ct values of the N gene (indicating higher viral loads) were prioritized for analysis to reflect maximal infectious risk. Previous studies by Vogels CBF have validated the diagnostic utility of the N gene in COVID-19 [13].
3.4. Differences in Clinical Indicator Distribution by Ct Value Quartiles
3.4.1. The Kruskal–Wallis Test Was Conducted on Clinical Indicators Under Different Ct Value Groups
Kruskal–Wallis tests revealed significant differences in the distribution of LYM%, LDH, and albumin among different Ct value quartiles. Age distribution also varied significantly across Ct value quartiles (p < 0.05). After data stratification, significant differences were observed in the 18–59 and 59–79 age groups among different Ct value quartiles (p < 0.05) but not in the 79–97 age group (p > 0.05). Detailed results are shown in Table 3.
| Kruskal–Wallis test | |
|---|---|
| Albumin | ≤ 0.001 |
| Lactate dehydrogenase | ≤ 0.001 |
| Lymphocyte percentage | ≤ 0.001 |
| Age | ≤ 0.001 |
| 18–59 | 0.002 |
| 59–79 | ≤ 0.001 |
| 79–97 | 0.814 |
3.4.2. Comparison of Age, Albumin, LDH, and LYM% Between Ct Value Groups
Significant differences were found in albumin and LDH distribution between the Ct (34.21–40.00) group and the control group (p < 0.05) but not in age and LYM% (p > 0.05). The Ct (26.51–34.21) group differed significantly from the control group in age, albumin, LDH, and LYM% distribution (p < 0.05). The Ct (0.00–26.51) group differed from the control group in age, albumin, and LYM% (p < 0.05) but not LDH (p > 0.05). No significant differences were found between the Ct (26.51–34.21) and Ct (34.21–40.00) groups (p > 0.05). The Ct (0.00–26.51) group differed from the Ct (34.21–40.00) group in age and LYM% (p < 0.05), but not albumin and LDH (p > 0.05). The Ct (0.00–26.51) group differed from the Ct (26.51–34.21) group in age (p < 0.05), but not albumin, LDH, and LYM% (p > 0.05). Details are shown in Table 4.
| Group | Age | Albumin | Lactate dehydrogenase | Lymphocyte percentage | ||||
|---|---|---|---|---|---|---|---|---|
| z value | p value | Z value | p value | Z value | p value | Z value | p value | |
| Control group-Ct (34.21–40) | −1.739 | 0.492 | 4.099 | ≤ 0.001 | −3.177 | 0.009 | 1.706 | 0.528 |
| Control group-Ct (26.51–34.21) | −4.153 | ≤ 0.001 | 4.799 | ≤ 0.001 | −4.146 | ≤ 0.001 | 5.459 | ≤ 0.001 |
| Control group-Ct (0.00–26.51) | −8.337 | ≤ 0.001 | 4.971 | ≤ 0.001 | −2.166 | 0.182 | 5.803 | ≤ 0.001 |
| Ct (34.21–40)-Ct (26.51–34.21) | −1.070 | 1.000 | −0.471 | 1.000 | −0.219 | 1.000 | 1.917 | 0.331 |
| Ct (34.21–40)-Ct (0.00–26.51) | −5.002 | ≤ 0.001 | 0.717 | 1.000 | 0.369 | 1.000 | 3.009 | 0.016 |
| Ct (26.51–34.21)-Ct (0.00–26.51) | −4.691 | ≤ 0.001 | 1.283 | 1.000 | 0.589 | 1.000 | 1.554 | 0.721 |
- Note: The Bonferroni correction has been applied to adjust the significance values for multiple tests.
3.4.3. Description of Median and Interquartile Range for Age, Albumin, LDH, and LYM%
The positive group had a higher median age and lower median albumin and LYM% than the negative group but a higher median LDH. Within the Ct value range < 34.21, the median age decreased with decreasing Ct values, but no significant difference was observed > 34.21. No significant difference was found in the internal distribution of albumin and LDH within the positive group. However, within the positive group, the median LYM% in Ct (34.21–40.00) was significantly higher than in Ct (0.00–26.51). Details are shown in Figure S2.
3.4.4. After Stratifying the Participants by Age, Pairwise Comparisons Were Conducted Between the Age Groups and Ct Value Groups
No significant differences were found in the distribution of individuals aged 18–59 and 59–79 in the Ct (34.21–40.00) group compared to the control (p > 0.05), in the 18–59 age group in the Ct (26.51–34.21) group compared to the control as the same (p > 0.05). However, significant differences were observed for individuals aged 59–79 in the Ct (26.51–34.21) group (p < 0.05) and for individuals aged 18–59 and 59–79 in the Ct (0.00∼26.51) group compared to the control (p < 0.05). No significant differences were found between the Ct (26.51–34.21) and Ct (34.21–40.00) groups, but significant differences were observed between the Ct (0.00–26.51) group and both the Ct (34.21–40.00) and Ct (26.51–34.21) groups for individuals aged 18–59 and 59–79 (p < 0.05), as shown in Table S1.
3.4.5. Comparison of Ct Values Between Different Age Groups and Distribution of Ct Values
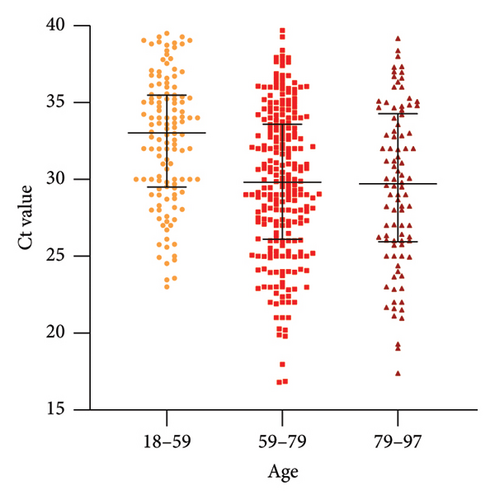
The distribution of Ct values varied among different age groups in the positive individuals. After grouping the ages (18–59, 59–79, 79–97), the Kruskal–Wallis test was conducted, revealing a significant difference (p = 0.003), which indicated that at least one group showed a distinct distribution. As shown in Table S2, the results of the intra-group comparisons showed that Ct values between the 18–59 and 59–79 year groups, as well as between the 18–59 and 79–97 year groups, exhibited significant differences (p < 0.05). However, there was no significant difference in Ct values between the 59–79 and 79–97 years and within the higher age group (> 59 years). The Ct values were significantly lower compared to the lower age group (< 18 years), with no difference in distribution within the higher age group. Visual representation is shown in Figure 1.
3.5. Multifactorial Analysis of Ct Values
3.5.1. Binary Logistic Regression Analysis Was Performed to Assess the Impact of Age, Albumin, LDH, and LYM% on the Ct Values
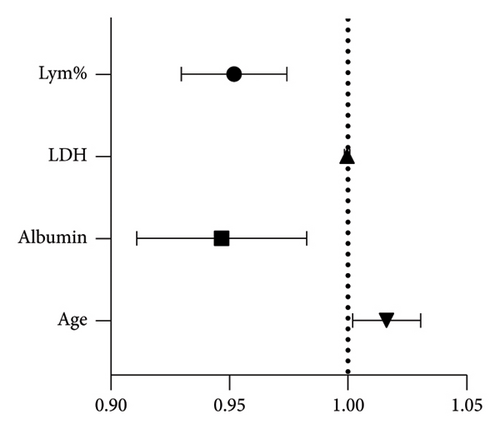
Following the recommended stratification of SARS-CoV-2 infectivity by Oba et al. [11], the Ct values were divided into two groups, that is, the control group (negative individuals and Ct > 34.21) and the experimental group (Ct < 34.21). The binary logistic regression analysis included age, albumin, LDH, and LYM%. The predicted ORs for age, albumin, LDH, and LYM% were 1.016 (p < 0.05), 0.946 (p < 0.05), 1.000 (p > 0.05), and 0.952 (p < 0.05), respectively (Figure 2).
3.5.2. ROC Analysis Was Performed for Age, Albumin, and LYM%
The area under the curve (AUC) for age, albumin, and LYM% were 0.6280 (95% CI: 0.5970–0.6590), 0.6058 (95% CI: 0.5966–0.6510), and 0.6238 (95% CI: 0.572–0.683), respectively. Youden’s index values were 0.2039, 0.2007, and 0.2172, respectively. The optimal cutoff values were 69.50 for age, 38.85 for albumin, and 17.95% for LYM%. The specificity values were 68.79%, 39.65%, and 39.06%; the sensitivity values were 51.6%, 80.42%, and 82.66%, respectively. The detailed results are presented in Table S3 and Figure S3.
4. Discussion
As a type of RNA virus, SARS-CoV-2 possesses an inherent high mutation rate. Although effective public health policies have slowed its spread in China and other regions, it is of utmost importance to address the potential impact of the virus on organs such as the lungs, heart, liver, and immune system in the post-emergence era [14]. Available data suggest that XBB infection has gradually increased since April 16, 2023 and is replacing other variants, gradually becoming the dominant strain [15, 16]. It is important to note that the variant pathogenicity of XBB1.5 is similar to other omicron variants based on its Gibbs energy of biosynthesis; however, it exhibits stronger immune evasion and weaker ACE2 affinity compared to BQ.1.1, XBB, and XBB.1, thus making it more transmissible [17].
Considering the significant relationship between Ct values and the positivity rate in virus culture, patients with Ct values ≥ 34 do not shed infectious viral particles, and blind passage culture between 27 and 34 is consistent with low viral load [10]. In this study, Ct values were used to determine the SARS-CoV-2 viral load, and the quartiles 26.51 and 34.21 were chosen as critical values to differentiate between high and low infectivity indicators; meanwhile, RNase P data (Figure S4) further validate that sample collection quality was consistent across groups, minimizing potential bias from swab technique variability. The Ct threshold selected in this study is supported by multiple prior studies. La Scola et al. demonstrated that samples with Ct > 34 failed to yield culturable virus [10], which aligns with Oba et al.’s clinical recommendation that patients with Ct > 34 may not require isolation [11]. Nonetheless, as the risk of early infection with low viral load cannot be completely ruled out, Ct values should be combined with clinical observations and patient history (including recent exposure history) when selecting individuals for testing [11]. For participant selection in this study, individuals < 18 years old and those with tuberculosis, hematologic diseases, tissue diseases, and frequent use of immunosuppressive agents were excluded to avoid interference from immunodeficiency.
Considering age as a relevant factor, Levin et al. studied three age groups, that is, 0–34 years, 35–59 years, and > 60 years, pointing out that the virus poses a significant risk of death for middle-aged individuals and an even higher risk for the elderly [18]. Clinical characteristics of elderly COVID-19 patients suggest that those with underlying diseases are more likely to progress to severe and critical conditions. Family clusters of elderly patients are more common, and elderly patients are more prone to liver, kidney, and heart damage [19]. Previous studies have detected a higher mortality rate among participants aged ≥ 54 [20]. Wu and McGoogan conducted a large-scale retrospective study and found that the mortality rate for patients between 70 and 80 years old was 8%, while it was 15% for older patients [21]. Unfortunately, Ct values were not studied simultaneously. In the present study, we observed the following characteristics among infected individuals: when Ct values were < 34.21, the proportion of individuals aged > 59 years old increased as the Ct value decreased, while the proportion of individuals aged < 59 years old gradually decreased. On the other hand, individuals aged > 79 years old had mainly low Ct values. Regardless of age groups or different Ct value groups, the median Ct values of individuals aged > 59 were lower than those aged < 59. These features may indicate a correlation between the vulnerability of middle-aged and elderly individuals to COVID-19 and their susceptibility to higher viral loads. Focusing on the early risk stratification of the elderly population and implementing graded nursing interventions are particularly important, even though the case selection was limited to the hospital setting.
Previous studies have characterized immune dysregulation in SARS-CoV-2 infection. Lin et al. reported that severe COVID-19 cases exhibited elevated neutrophil-to-lymphocyte ratios compared to mild cases, though PSI scoring was not performed in our cohort [22]. At the cellular level, lymphocyte metabolic reprogramming during infections may influence effector functions, as evidenced by metabolic flux analyses in model systems [23, 24]. In this study, the Ct < 34.21 group showed significantly lower lymphocyte percentages than both the Ct ≥ 34.21 group and healthy controls, suggesting an association between lower LYM% and higher viral loads. However, no significant LYM% difference was detected between Ct ≥ 34.21 and healthy controls, possibly reflecting immune homeostasis restoration under lower viral loads, indicating limited discriminative power of LYM% for viral load stratification. These findings align with observations that lymphocyte dynamics reflect viral persistence [25, 26], though our cross-sectional design precludes causal inference.
The concentration of LDH in tissues is much higher than in serum. There is a significant relationship between LDH and cytokines. The down-regulation of various inflammatory mediators caused by low LDH activity and the lactic acid fluctuations that regulate macrophage inflammatory responses have anti-inflammatory effects [27]. When immune cells are triggered to produce an inflammatory response, they also undergo metabolic reprogramming. Tissues and organs that are in a state of hypercapnia and hypoxia for a long time can cause mitochondrial dysfunction and a decrease in adenosine triphosphate synthesis, which in turn leads to sodium pump dysfunction, cell swelling, necrosis, and an increase in the release of LDH. The damage to myocardial cells caused by inflammatory responses or severe infections can also lead to a large amount of LDH release, which in turn leads to an increase in serum LDH [28]. In this study, significant differences were found in LDH between the high and middle Ct value groups and the normal population, while there were no significant differences in the distribution of other categories. This discrepancy with previous studies may be due to the relatively small sample size and the focus on distinguishing Ct values without differentiating the severity of COVID-19 in this study. However, the median LDH of the positive group was higher than those of the normal population, which indicates the damage to the body caused by SARS-CoV-2. An increase in LDH is significantly related to the severity of the disease [29]. LDH can potentially reflect lung function and is a predictive factor for respiratory failure associated with COVID-19 [7]. Elevated LDHs have been highly correlated with an increased risk of severe COVID-19 [30]. LDH can serve as markers for the severity of COVID-19 and predict survival [31].
Albumin is the most important plasma nutritional protein and plasma carrier protein. Changes in plasma albumin or blood pH can enhance or weaken its physiological activity. Hypoalbuminemia can occur in many pathological conditions. Serum albumin determination can help evaluate liver function, infectious inflammatory diseases, etc., and assess disease status. Infection is the most common cause of hypoalbuminemia in the acute phase, and hypoalbuminemia may reflect the poor health status of the patient [32]. The physiology of albumin plays a key role in both the host’s effective immune response to pathogens and the destructive effects of immune dysregulation characterized by cytokine storms [33]. The leakage of serum albumin through capillaries is related to the severity of infection, and it increases the interstitial level of albumin [34]. Also, previous studies have found that the inverse correlation between albumin/LYM% and viral load may reflect immune-nutritional interplay: hypoalbuminemia upregulates ACE2 expression [6], while lymphopenia indicates impaired viral clearance. However, our study’s single-center design and lack of longitudinal data limit causal inference. Future multicenter cohorts should validate these biomarkers in dynamic infection stages. In COVID-19 infection, the virus binds to ACE2 surface receptors with high affinity, mediating the entry of the virus into host cells and enhancing the pathogenicity of SARS-CoV-2. Although the nature of the virus particle-albumin binding site remains unclear, the value of albumin in predicting the prognosis of COVID-19 patients is undeniable. In their experimental research on influenza viruses, Beck et al. showed that malnutrition not only inhibits the immune response to the virus but may also promote the emergence of new variants, which may exhibit stronger pathogenicity compared to the original viral strain [35]. This study found significant differences in albumin between the positive group and the normal population, while no significant differences were observed in the distribution among individuals with different Ct values. This suggests a certain relationship between the presence of SARS-CoV-2 and albumin; however, the magnitude of changes in albumin does not seem to be strongly associated with viral load. In many chronic progressive diseases, hypoalbuminemia worsens with the severity of inflammation and the risk of death [36]. Considering the negative impact of hypoalbuminemia on the outcome of COVID-19 patients, providing nutritional support during treatment is particularly important.
In this study, the ROC analysis was performed after dividing Ct values into two groups, and LYM%, albumin, and age showed a higher predictive value in distinguishing between Ct values < 34 and Ct values > 34.21, just as the negative group. Using a Ct value of 34.21 as a critical value can assist in distinguishing individuals with high viral load and high infectivity from those with low viral load and low infectivity, as well as the negative population. This approach can be applied to rapidly differentiate highly infectious patients and to distinguish individuals seeking medical treatment when nucleic acid information is lacking, thus improving treatment efficacy and reducing nosocomial infections. Age as a risk factor for high viral load, along with LYM% and albumin as protective factors, indicates individual differences in immune status and tolerance to viral load among different COVID-19 patients, leading to varying clinical presentations. Higher viral load patients are more likely to become severe, requiring early antiviral treatment [4, 20, 37].
This study disclosed the disparities in Ct values and their clinical significance among COVID-19 patients of diverse age groups, albumin, LDH, and LYM%. The research outcomes suggest that patients aged over 59 years, lower albumin, and reduced lymphocyte percentage are independent predictors of higher viral load (Ct < 34.21), highlighting their clinical utility in early risk stratification. Additionally, the percentage of albumin and lymphocytes is negatively correlated with viral load, signifying that they may play a protective role in resisting COVID-19 infection.
From a clinical perspective, our findings accentuate the importance of early risk stratification for patients aged over 59 years, and the implementation of graded nursing interventions. Furthermore, albumin and LYM% can serve as potential biomarkers for predicting viral load and disease severity in COVID-19 patients. These biomarkers facilitate the rapid identification of infected individuals, provide personalized and precise medical care for patients, reduce the risk of disease progression, and lower mortality rates.
Our research findings offer a novel perspective for future studies on COVID-19 viral load, biomarkers, and their relationship with disease severity. Particularly, our data endorse the utilization of Ct values, albumin, and lymphocyte percentages as crucial parameters for evaluating the infectivity and disease progression risk of COVID-19 patients. Future research can further explore the performance of these biomarkers in different populations, as well as how they combine with other clinical indicators (such as inflammatory markers), to validate the predictive value of the biomarkers identified in this study in larger multicenter samples, to study the changes of these biomarkers in different disease stages and different variant infections, and to explore the combination of biomarkers with other clinical parameters (such as inflammatory markers) to enhance the accuracy and clinical practicality of prediction models, diagnosis, and treatment strategies for COVID-19 patients.
Ethics Statement
The study was approved by the Biomedical Research Ethic Committee of Shandong Provincial Hospital (SWYX: NO.2023–416). The authors confirm that all methods were performed in accordance with the relevant guidelines. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent
The requirement for informed consent was waived by the Institutional Review Board of the Shandong Provincial Hospital because of the retrospective nature of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Han Su and Li Xu carried out the studies, participated in collecting data, and drafted the manuscript. Fulu Chu conducted literature search. Guolin Bao conducted data extraction and quality assessment. Shiyu Zhao and Shan Ding collected and analyzed the data. Yiqing Liu designed the study and edited the paper. All authors read and approved the final manuscript. Han Su and Li Xu contributed equally to this work and are co-first authors of the article.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (Grant/Award Number: No. ZR2020MH316, ZR2016HM52).
Acknowledgments
The authors have nothing to report.
Supporting Information
Figure S1 Proportion of different age groups in each Ct value group.
Figure S2 Median and interquartile range of age, albumin, LDH, and Lym% in each Ct value group.
Figure S3 ROC curves and distribution of cutoff values for age, albumin, and Lym%.
Figure S4 Consistency analysis of Ct values among ORF1ab, N genes, and Rnase P.
Table S1 Pairwise comparison of age groups among different Ct value groups.
Table S2 Pairwise comparison of Ct values between different age segments.
Table S3 ROC analysis results for age, albumin, and lymphocyte percentage.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




