Comparison of 3D Printing Technology and Artificial Intelligence Assisted in Total Knee Arthroplasty
Abstract
This study aimed to evaluate the efficacy of personalized 3D printing osteotomy guides and artificial intelligence (AI)-assisted surgical planning in total knee arthroplasty (TKA). A cohort of 60 cases was randomly allocated into two groups: one receiving assistance from personalized 3D printing osteotomy guides and the other benefiting from AI-assisted surgical planning for the procedure. We compared surgical duration, intraoperative bleeding, postoperative incision drainage, length of hospital stay, surgical accuracy, and postoperative visual analog scale (VAS) and Hospital for Special Surgery (HSS) outcomes between the two groups. Continuous data conforming to a normal distribution were analyzed using independent-samples t-tests, while categorical data were assessed with chi-square tests. The findings of our study indicated that the 3D printing-assisted TKA (3D-TKA) group experienced significant lower levels (p < 0.05) of bleeding and drainage (160.1 ± 24.3 mL, 199.5 ± 29.6 mL) compared to the AI-assisted TKA (AI-TKA) group (174.7 ± 25.7 mL, 223.8 ± 29.2 mL). Furthermore, the duration of surgery and hospital stay (81.4 ± 8.9 min, 7.7 ± 1.3 day) was significantly longer (p < 0.05) in the 3D-TKA group than the AI-TKA group (72.9 ± 10.0 min, 6.8 ± 1.6 day). No significant differences (p > 0.05) were observed in surgical accuracy between the two groups. On the first day postoperation, VAS scores were significantly lower (p = 0.001) in the 3D-TKA group. In summary, each surgical approach offers distinct benefits. 3D printing primarily enhances patient outcomes, whereas AI assistance tends to favor surgical efficiency.
1. Introduction
Knee osteoarthritis (OA) is a degenerative disease that predominantly occurs with advancing age. In China, where an aging population is on the rise, the cumulative incidence of knee OA exceeds 8%, with prevalence rates surpassing 10% in individuals over 55 years of age [1–3]. This incidence is projected to rise annually. Total knee arthroplasty (TKA) has been recognized as one of the most effective treatments for severe knee OA and is widely used in clinical practice [4–8]. Over the years, TKA techniques have evolved and matured, yet there remains room for improvement. The success of TKA heavily relies on two critical factors: the optimal alignment of the lower limb and the precise placement of the prosthesis [9–11]. Traditional preoperative planning for TKA typically involves assessing lower limb alignment deviations based on full-length standing X-rays of the lower extremities. However, due to the inherent limitations of two-dimensional imaging and X-ray conditions, it is challenging to guarantee standardized full-length standing X-rays for every patient [12, 13]. Consequently, manual angle measurements based on these X-rays can lack precision, leading to potential errors in preoperative planning [14–16]. Even experienced orthopedic surgeons face a risk of postoperative lower limb alignment deviation from the neutral position by more than 3° in over 10% of cases [15, 17].
Introduced in the 1990s to improve TKA precision, computer navigation technology has faced limited clinical adoption due to its expensive equipment and the extensive time required for intraoperative planning [18–20]. Recent advancements in digital orthopedics and artificial intelligence (AI) have facilitated the integration of patient-specific imaging data with AI-assisted three-dimensional surgical planning and the use of 3D-printed, patient-specific osteotomy guides in clinical practices [21–23]. Although both 3D-printed osteotomy guides and AI-assisted surgical planning have shown advantages over traditional surgery for TKA, a direct comparison and their specific effects on patients remain unclear. This study aims to compare and analyze the impacts of these two technologies on TKA’s postoperative outcomes. We hypothesize that AI-assisted surgical planning for knee OA treatment may lead to shorter surgery duration, reduced bleeding, and improved alignment accuracy compared to the use of 3D-printed osteotomy guides.
2. Methods
2.1. Design of Experiment
This research was a single-center, multisurgeon, double-blind, randomized controlled trial, adhering to the Declaration of Helsinki. Approval was granted by the Ethics Committee of Yantaishan Hospital (LL-2023-162-L). Participants were randomly assigned to the 3D-group or AI-group using a computer-generated sequence. This sequence was kept secret in opaque, sealed envelopes. Both the surgeons and evaluators were blinded to the group assignments. The surgical team learned about the specific procedure only right before the operation from a third-party staff member who was not involved in evaluations. Postoperative evaluators, responsible for assessing patient outcomes, were kept completely unaware of the group assignments throughout the study. All assessments were conducted in a blinded manner, with evaluators having no access to the randomization sequence or any patient-related information that could reveal the type of surgical intervention received.
The control group underwent TKA utilizing personalized, 3D-printed osteotomy guides, termed as 3D-group. Conversely, the experimental group received TKA assisted by AI-driven surgical planning, referred to as AI-group. Preoperative meetings between patients and surgeons were not conducted. Every participant provided written informed consent before inclusion in the study. In this trial, all participants who met the eligibility criteria were included, and data collection was thorough, with no instances of missing data during the hospitalization period or during postdischarge follow-up, which was conducted via telephone. As no data were lost during the study, the issue of missing data did not arise. The subject recruitment process is depicted in Figure 1.
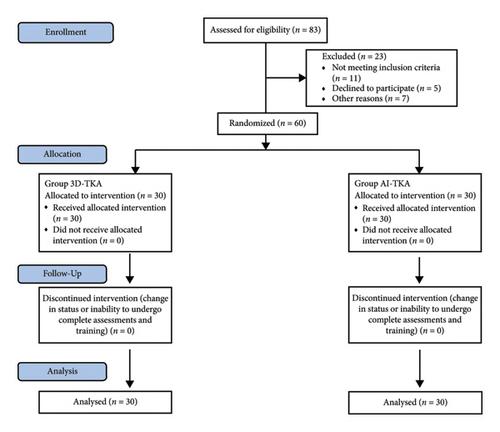
2.2. Patient Population
We recruited patients with Grade IV Kellgren–Lawrence primary OA of the knee from the Department of Arthropathy at Yantaishan Hospital between June 2023 and October 2023. Inclusion criteria included a diagnosis of primary OA, severe symptoms impacting quality of life, suitability for surgical treatment, and willingness to participate in the study. Exclusion criteria encompassed previous knee surgery, history of trauma or severe bone defects, osteoporosis, joint infection or tumor, severe knee deformities, extended hospitalization for medical issues, long-term oral anticoagulant use, abnormal coagulation, and intraoperative changes to conventional TKA surgery. Each group, 3D-group and AI-group, comprised 30 participants, totaling 60 individuals for the study eventually.
2.3. Preoperative Preparation
For our study, routine preoperative imaging encompassed comprehensive anteroposterior X-rays and detailed CT scans of the lower extremities, and weight-bearing lateral X-rays of the affected side. Imaging was conducted using a Philips Digital Diagnosis 4 X-ray machine and a GE 256-row spiral CT scanner. In the AI-group, the software by Beijing Changmugu Medical Science and Technology Co. processed full-length CT images of the lower limbs, facilitating the reconstruction of three-dimensional image data. Conversely, the 3D-group’s preoperative process involved importing CT images into a computer system to measure lengths and angles. This group employed a JS-6035 printer from Shenzhen Jinshi 3D Printing Technology Co. to fabricate tibial and femoral osteotomy guides, utilizing resin as the printing medium.
2.4. Surgical Procedures and Perioperative Management
Both groups in the study underwent TKA using either intralesional or general anesthesia. The surgeries were conducted by the same team of surgeons. In the 3D-group, the Accurate prosthesis from Xiamen Dabo Medical Technology Co. was used, while the AI-group received the ATTUNE prosthesis from Johnson and Johnson. Notably, patellar replacement was not performed in either group.
In the 3D-group, the surgery began with positioning the patient on their back. A medial parapatellar approach was used to access the joint. After flexing and everting the patella, osteophytes on the tibial plateau and distal femur were removed, followed by resection of the anterior cruciate ligament, posterior cruciate ligament, and menisci to fully expose the tibial plateau. Posteromedial soft tissue release was performed as necessary. The cartilage on the planned osteotomy surface was thoroughly scraped to reveal the underlying bone, enabling precise positioning of the predesigned cutting guide. Osteotomies of the distal femur and proximal tibia were then performed as per the guide’s specifications. After the cuts, soft tissue balancing was assessed, and additional soft tissue releases were performed as needed to achieve medial-lateral and flexion-extension gap balance before prosthesis placement. If significant discrepancies were observed between the cutting guide and the actual anatomical condition during surgery, the procedure was converted to standard TKA using conventional instruments.
In the AI-group, the surgical exposure technique was identical to that employed in the 3D-group. The femoral and tibial osteotomies were executed using traditional instruments, following the angles and plans devised by preoperative AI. The remainder of the operation conformed to the conventional standard TKA surgical method.
After surgery, all patients had their drainage tubes removed 24 h later, depending on the flow volume. Anticoagulation therapy started 12 h postsurgery with a standard dose of low molecular weight heparin (4000 units subcutaneously once daily), transitioning to oral rivaroxaban (10 mg daily) postdischarge. Rehabilitation started on the first day postsurgery with passive knee exercises and isometric quadriceps strengthening, progressing to active assisted exercises and walker-assisted walking on the second day. Routine examinations included weight-bearing anteroposterior and lateral knee X-rays, along with full-length anteroposterior X-rays of the lower extremities.
2.5. Outcome Measures
Primary outcome: various postoperative metrics were assessed and compared between the two groups, including operation time, bleeding, drainage, and average hospital stay. Postoperative X-rays of the lower limbs were analyzed to measure specific angles like hip knee ankle (HKA), frontal femoral component (FFC), and frontal tibial component (FTC). The precision of the surgery was evaluated by comparing the deviations of these angles from their ideal values (HKA: 180°, FFC: 90°, FTC: 90°).
Secondary outcome: effectiveness of the procedures was also assessed using the Hospital for Special Surgery (HSS) knee score and visual analog scale (VAS) from day 1–3 months postoperatively.
2.6. Statistical Analysis
Data analysis was conducted using SPSS 23.0 software. Normally distributed measurement data were presented as mean ± standard deviation and the t-test was utilized for intergroup comparisons. While count data were analyzed using the χ2 test or Fisher’s exact probability method. Statistical significance was deemed for values where the p value is less than 0.05.
3. Results
3.1. Basic Information
Thirty subjects from each group were included. The analysis revealed no significant differences between the two groups in terms of age, gender, affected side, or Body Mass Index (BMI). See Table 1 for details.
| Group | Gender (male/female) | Affected side (left/right) | Age (years) | BMI (kg/m2) |
|---|---|---|---|---|
| 3D-group | 12/18 | 16/14 | 68.3 ± 6.0 | 25.4 ± 2.2 |
| AI-group | 9/21 | 13/17 | 66.0 ± 6.4 | 25.5 ± 3.1 |
| χ2/t | 0.659 | 0.601 | 1.398 | −0.238 |
| p | 0.417 | 0.438 | 0.167 | 0.812 |
3.2. Comparison of Primary Outcome
In the 3D-group, compared to the AI-group, there was a significant increase in both operation time and duration of hospitalization (p < 0.05). However, this group experienced a significant reduction in both bleeding and drainage during the surgical process (p < 0.05) (Table 2). No statistically significant differences were found in the absolute values of HKA, FFC, and FTC angles between the two groups (p < 0.05).
| Group | Operation time (min) | Bleeding (mL) | Drainage (mL) | Duration of hospitalization (day) | HKA | FFC | FTC |
|---|---|---|---|---|---|---|---|
| 3D-group | 81.4 ± 8.9 | 160.1 ± 24.3 | 199.5 ± 29.6 | 7.7 ± 1.3 | 1.8 ± 0.7 | 1.0 ± 0.6 | 1.2 ± 0.5 |
| AI-group | 72.9 ± 10.0 | 174.7 ± 25.7 | 223.8 ± 29.2 | 6.8 ± 1.6 | 1.6 ± 0.6 | 1.2 ± 0.9 | 1.3 ± 0.6 |
| t | 3.482 | −2.264 | −3.201 | 2.436 | 1.212 | −1.142 | −0.898 |
| p | 0.001 | 0.027 | 0.002 | 0.018 | 0.231 | 0.258 | 0.373 |
3.3. Comparison of Secondary Outcome
The VAS scores were significantly lower in the 3D-group than in the AI-group one day after surgery (p = 0.001). However, at other time points, there were no significant differences in VAS scores between the two groups (p > 0.05) (Table 3). The comparison of postoperative HSS scores between the two groups showed no significant difference (p > 0.05) (Table 4).
| Group | VAS | ||||||
|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative-1 d | Postoperative-3 d | Postoperative-7 d | Postoperative-14 d | Postoperative-1 month | Postoperative-3 months | |
| 3D-group | 7.4 ± 1.2 | 5.4 ± 0.6 | 4.8 ± 1.1 | 5.4 ± 1.0 | 3.2 ± 1.3 | 3.3 ± 1.5 | 1.4 ± 1.0 |
| AI-group | 8.0 ± 1.3 | 6.2 ± 1.2 | 4.9 ± 0.9 | 5.3 ± 1.1 | 3.3 ± 1.2 | 3.0 ± 1.6 | 1.9 ± 0.9 |
| t | −1.803 | −3.484 | −0.382 | 0.501 | −0.407 | 0.754 | −1.916 |
| p | 0.077 | 0.001 | 0.704 | 0.618 | 0.686 | 0.454 | 0.06 |
| Group | HSS | ||||
|---|---|---|---|---|---|
| Preoperative | Postoperative-7 d | Postoperative-14 d | Postoperative-1 month | Postoperative-3 months | |
| 3D-group | 43.9 ± 4.0 | 63.5 ± 3.9 | 73.4 ± 7.1 | 79.3 ± 7.7 | 85.8 ± 7.1 |
| AI-group | 44.1 ± 3.3 | 65.4 ± 4.8 | 72.5 ± 6.2 | 80.4 ± 8.2 | 84.6 ± 8.1 |
| t | −0.21 | −1.676 | 0.543 | −0.501 | 0.631 |
| p | 0.834 | 0.099 | 0.589 | 0.618 | 0.531 |
4. Discussion
Knee OA predominantly impacts the elderly, frequently as a result of prolonged weight-bearing stress [24, 25]. TKA stands as the most effective treatment for knee OA, where the prosthesis’s precise placement and postoperative lower limb force line recovery are vital for the knee joint’s function, prosthesis longevity, and patient satisfaction [9, 26–29]. It is noted that a coronal alignment shift over 3° in the lower limb can increase prosthesis wear and TKA failure rates [30]. Current surgical instruments in China, designed based on knee structures of Caucasian races and ignoring anatomical differences across races, may cause osteotomy inaccuracies [31, 32]. Traditional surgery, which relies on average anatomical parameters, faces challenges in attaining precise osteotomies and personalized treatment, owing to the complex biomechanics of the knee joint and the variability in individual anatomy.
The advancement of computer technology has led to the integration of intelligent assistive systems in clinical practice, notably in TKA. These technologies include preoperative AI for surgical planning, computer-assisted navigation, and 3D-printing personalized osteotomy guides [33–35]. While computer navigation is costly and complex, AI planning and 3D printing promise improved prosthesis placement accuracy and operation efficiency [36, 37]. However, the superiority of these intelligent systems over traditional methods remains debated [38–41]. This study aims to evaluate the effectiveness of AI surgical planning and 3D printing technology in TKA through a prospective, randomized controlled trial.
Our study found significant differences in operation time between the 3D-group and AI-groups, with the 3D-group experiencing longer surgeries. This may be attributed to the necessity of exposing subchondral bone for precise guide placement and the additional time needed to verify guide accuracy [42]. Conversely, the AI-group’s process was streamlined by preoperative AI planning. Despite theoretical advantages of the 3D printing technology in reducing surgery time [43], practical application challenges led to longer durations. Additionally, the 3D-group showed less intraoperative and postoperative bleeding, which may be attributed to not opening the femoral marrow cavity. The 3D-group experienced longer hospital stays than the AI-group, primarily due to the additional steps involved in preparing osteotomy guides, including their production, sterilization, and qualification for use in surgery.
In terms of surgical accuracy, the 3D-group and AI-groups both demonstrated precise osteotomy capabilities and effectively restored the lower limb’s mechanical axis. The postoperative angle deviations in the HKA, FFC, and FTC were less than 3° in both groups, with no significant differences observed. Data analysis, however, indicated a slight trend where postoperative variations in the FTC and FFC angles were marginally reduced in the 3D-group in comparison to the AI-group, suggesting a minor advantage in coronal plane alignment accuracy for the 3D-group. Regarding surgical outcomes, our comparative analysis indicated that the VAS scores were lower in the 3D-group than in the AI-group on the first day postoperatively. This difference may be attributed to the absence of femoral canal reaming in the 3D-group procedure, resulting in reduced intraoperative blood loss, postoperative drainage, and a milder inflammatory response [44, 45]. Similar study on TKA has demonstrated that the application of 3D printing-assisted technology significantly mitigates postoperative pain compared to conventional surgical approaches [46]. Both methods yielded significant improvements from preoperative baselines, indicating that both adjunctive techniques are effective in achieving early positive outcomes. It is important to note that all postoperative patients underwent rehabilitation therapy under the guidance of professional therapists, who provided individualized training plans. While the rehabilitation programs were standardized across participants, they were administered by different therapists, which could potentially influence the results.
ZHANG et al. highlighted several advantages of 3D printing technology [47], including enhanced understanding of injury mechanisms, which improves patient communication; more effective preoperative planning; increased treatment precision; and a lower likelihood of complications. We evaluated two surgical approaches regarding their surgical outcomes and patient recovery. 3D-TKA proved more effective than AI-TKA in reducing perioperative hemorrhage and alleviating early postoperative pain. This advantage is attributed to the capacity of 3D printing technology to produce customized surgical guides, which more accurately conform to the patient’s unique anatomy [48]. For certain surgical procedures, particularly where anatomical fidelity and precision are paramount, such as orthopedic reconstructive surgery, 3D printing might offer a distinct advantage [49]. Despite its benefits, 3D printing technology presents several limitations, including the extended production time required for printed tools and the reduced intraoperative flexibility for surgeons to make adjustments. Furthermore, the longevity of implant materials postsurgery remains uncertain, which could affect long-term outcomes [49, 50].
AI-assisted technology also presents significant advantages, particularly in its ability to offer precise guidance that can narrow the experience gap between junior doctors and seasoned practitioners. This technology facilitates clearer and more efficient surgical planning, as evidenced by the reduction in surgical time observed in our study [51]. Additionally, by omitting the prosthesis alignment process, AI-assisted surgery can minimize damage to surrounding tissues and expedite patient recovery. However, AI technology has its limitations, such as the reliance on algorithms predominantly developed by programmers, which can limit clinician oversight and the ability to make timely corrections during surgery [52]. The current maturity level of AI algorithms is another concern, as both the quality and quantity of the data modeled need enhancement. Furthermore, the ambiguity in assigning responsibility for AI-related medical outcomes poses potential challenges, particularly in cases of medical negligence [53].
Overall, 3D printing technology enhances patient outcomes, providing better results and reduced pain, while AI-assisted technology benefits surgeons by facilitating more standardized and straightforward procedures with shorter operating times. The integration of AI with 3D printing could represent a transformative advance in surgical planning and execution. AI could enhance the design of 3D-printed models, optimizing them based on predictive analytics from previous surgeries, potentially leading to even greater accuracy and efficiency in operations [54]. Currently, efforts are underway to employ AI technology in the morphological design of 3D printed materials [55, 56]. However, this innovation has yet to gain significant traction in orthopedic surgery, a field that demands rigorous safety guarantees and reproducibility. Despite these challenges, the prospect of advancing new technologies in surgery remains promising.
Nevertheless, both technologies face several challenges that must be addressed to pave the way forward. Primarily, the substantial financial costs associated with these technologies, including expenses for software, prostheses, and additional surgical team training, are significant hurdles [57]. However, the cost-effectiveness of each technology must be considered: 3D printing, while initially expensive due to material costs and production time, can reduce long-term healthcare expenditures by lowering the rates of surgical complications and the need for revision surgeries. AI-assisted surgery, despite its high initial investment in software and training, offers cost savings through improved surgical efficiency, reduced operation times, and faster patient recovery, potentially leading to overall lower healthcare costs. Considering both the initial investment and long-term benefits, these technologies demonstrate promising cost-effectiveness in advancing surgical outcomes and patient care. Additionally, reducing the time doctors spend learning and operating AI-assisted technologies is crucial [58]. Developing a comprehensive software platform that integrates various data and provides direct results could streamline the learning process and eliminate the need for multiple software systems tailored to different data sets and requirements.
AI-assisted technology offers significant benefits in surgery, such as improving surgical planning and execution, reducing variability in outcomes, and enhancing training for surgeons [59, 60]. These advantages make AI particularly valuable in streamlining procedures and increasing consistency, especially in high-volume surgical settings or when training less experienced surgeons. 3D printing excels in providing customized surgical guides tailored to the patient’s anatomy, which is crucial for surgeries requiring high anatomical precision, such as orthopedic reconstructions. Given these complementary strengths, a balanced approach is recommended in clinical practice. For complex cases demanding high precision, 3D printing should be prioritized, while AI-assisted technology can be leveraged to enhance efficiency and standardization. Future research should focus on integrating AI-assisted technology with 3D printing to harness their combined potential. Efforts should include refining AI algorithms to optimize the design and functionality of 3D-printed surgical guides. Real-time AI navigation systems could complement 3D-printed tools, broadening their application across various surgical specialties. Additionally, exploring the cost-effectiveness and accessibility of these technologies in resource-limited settings is crucial for ensuring wider clinical adoption. Predictive modeling powered by AI can further enable personalized surgical strategies by forecasting patient-specific outcomes. Finally, addressing ethical, regulatory, and safety concerns is essential to ensure the responsible and sustainable deployment of these advanced technologies.
The findings of this study should be interpreted cautiously due to potential biases and confounding factors. First, the small sample size, influenced by the limited adoption of 3D printing and AI-assisted technologies, reduces statistical power and generalizability. Expanding the study to include larger, diverse populations across multiple centers would enhance validity. Second, the short follow-up period of 3 months may not capture long-term outcomes such as implant longevity or late complications, necessitating extended observation in future research. Third, differences in prosthesis types across groups could subtly affect functional outcomes, although the primary goal of mechanical axis realignment likely remains unaffected. Standardizing prostheses or stratifying outcomes based on their characteristics could address this limitation. Variability in surgeon expertise and familiarity with the technologies also introduces bias, as learning curves and skill levels may impact results such as operative time and surgical accuracy. Standardized training could help reduce this variability. Additionally, while all patients underwent rehabilitation, inconsistencies in therapist expertise and patient adherence to protocols may have influenced recovery, underscoring the need for uniform rehabilitation standards. Furthermore, the single-center design limits the study’s generalizability, as results may not reflect practices in other settings. Unmeasured confounders, such as socioeconomic status and comorbidities, could also influence outcomes. Finally, technology-specific considerations, such as deviations in preoperative 3D printing guides or the accuracy of AI-generated surgical plans, may impact results. Addressing these limitations in future multicenter, long-term studies with standardized protocols and broader data collection will refine understanding of the complementary roles of 3D printing and AI-assisted technologies in optimizing patient outcomes.
5. Conclusion
In conclusion, both 3D printing osteotomy guides and AI-assisted surgical procedures in TKA offer distinct advantages for treating knee OA. AI-assisted techniques improve efficiency with shorter surgeries and hospital stays, while 3D printing minimizes perioperative bleeding and postoperative pain, emphasizing patient-centered care. Combining these technologies may optimize outcomes by balancing efficiency with personalization. Future studies should explore their synergy and long-term impact on patient outcomes and healthcare resources.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The research study was funded by the Shandong Province Medical Health Science and Technology Development Project (2016WS0699); Shandong Province Key Research and Development Project (2018GSF118220); and Yantai Science and Technology Project (2015WS056).
Open Research
Data Availability Statement
The raw data used to support the findings of this study are available from the corresponding author upon request.




