Excessive Daytime Sleepiness and Oral Behaviors: A Pilot Investigation Among Patients With Temporomandibular Disorders
Abstract
Objective: To explore the relationship between excessive daytime sleepiness (EDS) and oral behaviors of patients with temporomandibular disorders (TMD).
Methods: A total of 567 TMD patients were included in the pilot study. The Oral Behavior Checklist (OBC) was employed to evaluate the oral behaviors of participants, and the Epworth Sleepiness Scale was utilized to assess EDS. Besides, the seven-item generalized anxiety disorder (GAD-7) and nine-item Patient Health Questionnaire (PHQ-9) were applied to assess the severity of anxiety and depression. All assessment tools were used in validated Chinese versions. Statistical analyses were conducted appropriately to detect the possible differences and correlations.
Results: Compared with non-EDS counterparts, TMD patients with EDS exhibited higher OBC scores and OBC level (p < 0.05). Binary logistic analyses demonstrated a significant correlation between EDS and OBC level (odds ratio (OR) = 1.780, p = 0.031). Specifically, “Awake grinding” (OR = 6.854, p = 0.020), “Awake clenching” (OR = 2.190, p = 0.012), and “Press tongue forcibly against teeth” (OR = 3.477, p = 0.032) were identified to be positively correlated with EDS in TMD patients.
Conclusions: Elevated OBC scores and oral behavior level were associated with the presence of EDS in TMD patients. Behaviors including awake grinding, awake clenching, and press tongue forcibly against teeth may serve as reliable predictors of EDS in individuals with TMD. Addressing these parafunctional oral behaviors could potentially yield therapeutic benefits in the comprehensive management of TMD patients. Longitudinal trials in the future are worthwhile for a further validation.
1. Introduction
Temporomandibular disorders (TMDs) represent a group of heterogeneous musculoskeletal disorders, typically characterized by orofacial pain condition or dysfunction of masticatory structures, such as temporomandibular joints (TMJs) noises, abnormal mandible movement, and masticatory muscle spasms [1]. The reported prevalence of TMD varied in different regions and populations, which was around 31% in adults and 11% in adolescents among the general population [2]. Another study reported a TMD prevalence of 38.4% in children and adolescents, with the risk ratio of females demonstrating a 2-fold increase approximately [3–6]. The great discrepancy among researches may be attributed to the iterative process of diagnostic criteria of TMDs. Since initially publicized in 1992, the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) have been broadly implemented in evaluating TMD till gradually replaced by a refined version labeled as the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) in 2014 [7, 8]. Nowadays, DC/TMD is considered as an evidence-based evaluation procedure that provides sufficient diagnostic information in the clinical setting. As described in DC/TMD, TMDs are categorized into two subtypes, the pain-related TMD (PT) and the intra-articular TMD (IT). The former exhibits pain-related symptoms including arthralgia, myalgia, myofascial pain, myofascial pain with referral, and headache ascribed to TMDs. The latter encompasses disc displacement disorders, degenerative joint disease, and TMJ subluxation. A combination of both is defined as combined TMD (CT) [7].
Although the causes or risk factors have not been utterly elucidated to date, TMDs are no longer believed to be local or individual conditions but rather are complicated disorders, of which the etiology is multifactorial and normally involves biological and psychosocial aspects functioning alone or in combination [9]. Thereinto, oral behaviors including oral parafunctional habits and postural habits have been proposed to be potential risk factors for the onset and progression of TMD [10]. Specifically, oral parafunctional habits refer to masticatory activities apart from normal functions of speaking and eating, such as diurnal grinding, clenching, and yawning, while postural habits consist of conscious or unconscious postures that break the body’s postural equilibrium and create imbalanced orofacial muscles, such as holding jaw in a rigid or tense position and sleeping in a face down position [11–13]. These oral behaviors are not uncommon and usually innocuous until exceeding individuals’ tolerance and thus begin to pose potential risks on TMD development [14].
On the other hand, some psychophysiological disorders, such as somnipathy and the concomitant anxiety or depression, are regarded as undisputed factors contributing to TMD with an intricate association [15, 16]. Recent studies have shown that TMD patients experienced reduced sleep quality compared to controls, while sleep disturbance could lead to increased risk of painful TMD in turn, suggesting a potential multidirectional interaction between sleep disturbance and TMD [17, 18]. In particular, among various sleep disorders, excessive daytime sleepiness (EDS), an inclination to nod off notwithstanding intentional attempts to maintain alertness of awake state, has been broadly validated to correlate with TMD [19–21]. It was reported by early publications that 14.79%–28.57% of TMD patients exhibited EDS and such patients were proved to present a significant higher level in the severity of TMD symptom, anxiety, and depression compared with non-EDS TMD controls [17, 22]. Another study by Lee et al. furtherly identified the prevalence of EDS in patients diagnosed with different TMD subtypes but found no significant difference despite of higher ESS scores found in the mixed TMD pain group than myalgia and arthralgia groups [23].
Taken above, although much effort has been devoted to reveal the relationship of oral behaviors–TMD and TMD–EDS, less is known in the direct interaction between oral behaviors and EDS among TMD patients. With such a purpose, this study carried out an investigation into the oral behaviors and EDS symptoms of patients diagnosed with TMD according to DC/TMD and explored the potential associations between. Besides, several characteristics of different TMD subtypes were verified again and reported as the secondary outcomes.
2. Materials and Methods
This study was approved by the ethics committee of West China Hospital of Stomatology (approval no. WCHSIRB-2020-378) and conducted following the Declaration of Helsinki. Fully informed consent was acquired from all participants when necessary.
2.1. Sample Size Calculation and Participants Inclusion
The sample size was calculated through G∗power (version 3.1.9, Germany). By using an alpha error of 0.05, a sampling ratio (the ratio of Non-EDS and EDS patients) of 5, and an effect size of 0.5, it was estimated that 378 participants were required to achieve a power of 80%.
As a consequence, patients seeking treatment at the Temporomandibular Joint Department of West China Hospital of Stomatology from December 2021 to January 2024 were enrolled and screened in accordance with the inclusion and exclusion criteria as follows. The inclusion criteria comprised as follows: 1. patients diagnosed with TMD according to the DC/TMD Axis I protocol; 2. patients with full sociodemographic information record and valid responses to OBC (Oral Behaviors Checklist), ESS (Epworth Sleepiness Scale), GAD-7 (7-item Generalized Anxiety Disorder), and PHQ-9 (9-item Patient Health Questionnaire), while the exclusion criteria included the following: 1. patients suffering from systemic diseases that affects TMJ or causes hypersomnia, such as rheumatoid arthritis and hypothyroidism; 2. patients with sleep disorder treatment history; and 3. patients with self-reported use of hypnotics, sedatives, or other antipsychotics.
2.2. Data Collection
A self-customed questionnaire composed of sociodemographic information, OBC, ESS, GAD-7, and PHQ-9 was delivered to each patient at the initial appointment, which was completed by participants with the assistance of two investigators (Jie Xiang and Jiaqi Liu). Any confusion about the questionnaire was further explained by a supervisor (Xin Xiong) throughout the whole process.
2.2.1. Demographic Information
The sociodemographic information, including age, sex, educational level, and family per capita monthly income, was collected, while the TMD diagnosis and other systemic disease history of patients were extracted from medical records.
2.2.2. OBC
The oral behaviors of participants were evaluated with a validated Chinese version of OBC, encompassing 21 questions in total where 19 for awake oral behaviors and 2 for asleep ones. Patients were required to rate their frequency of corresponding behaviors during the past month with 5 levels ranged from 0 (none of the time) to 4 (all of the time/four to seven nights per week). Based on previous reports [24], the OBC-total score was calculated and divided into two levels: no to low behavior (score 0–24) and high behavior (score 25–84). Besides, for each item of OBC, the frequency was determined as “Yes” when scoring 3 and 4; otherwise, it was classified as “No” [25].
2.2.3. EDS
Furthermore, the EDS burden was assessed via ESS in the Chinese version, which included eight individual items for participants to determine their possibility to fall asleep in corresponding scenarios. The total score of ESS could range from 0 to 24, and patients with a total score ≥ 10 were considered to suffer from EDS [26].
2.2.4. Anxiety and Depression
In addition, GAD-7 and PHQ-9, both with a score range from 0 to 21, were employed to assess the severity of anxiety and depression for participants, respectively. The total score of these two scales was interpreted as follows: normal (0–4), mild (5–9), moderate (10–14), and severe (15–21) anxiety or depression, accordingly [27, 28].
2.3. Statistical Analysis
Statistical analysis was carried out with SPSS (version 20.0; IBM Corporation). Descriptive statistics and normality test were initially applied to the collected data. Afterward, one-way analysis of variance (ANOVA) was employed for the comparison of normally distributed continuous variables, and Chi-square tests were used to compare proportions between groups. Nonparametric tests, including Mann–Whitney U test and Kruskal–Wallis test, were conducted as appropriate. In addition, multivariate binary logistic regression analyses were performed to detect the potential correlation between EDS and oral behaviors while having other confounding factors controlled. A probability value of p < 0.05 was recognized as significance.
3. Results
In total, 567 adult TMD patients were recruited in this study, including 133, 171, and 263 participants diagnosed with PT, IT, and CT, respectively. The sociodemographic characteristics of included patients are presented in Table 1, and statistically significant difference was not found among different TMD patients in the distribution of age, sex, and family per capita monthly income, but in educational level (p = 0.046).
| Items | TMD diagnosis | Total | p | |||
|---|---|---|---|---|---|---|
| PT | IT | CT | ||||
| n (%) | 133 (23.5%) | 171 (30.2%) | 263 (46.3%) | 567 | ||
| Age | Mean ± SD | 30.25 ± 12.66 | 27.22 ± 8.94 | 27.92 ± 11.91 | 28.25 ± 11.33 | 0.114 |
| Median | 27.00 | 26.00 | 25.00 | 26.00 | ||
| Sex | Male | 33 (24.8%) | 39 (22.8%) | 44 (16.7%) | 116 (20.5%) | 0.112 |
| Female | 100 (75.2%) | 132 (77.2%) | 219 (83.3%) | 451 (79.5%) | ||
| Edu | Secondary school and below | 37 (27.8%) | 35 (20.5%) | 89 (33.8%) | 161 (28.4%) | 0.046∗ |
| Undergraduate or junior college | 83 (62.4%) | 115 (67.3%) | 152 (57.8%) | 350 (61.7%) | ||
| Postgraduate and above | 13 (9.8%) | 21 (12.3%) | 22 (8.4%) | 56 (9.9%) | ||
| Income | < 3000 yuan | 18 (13.5%) | 20 (11.7%) | 45 (17.1%) | 83 (14.6%) | 0.272 |
| 3000–6000 yuan | 54 (40.6%) | 82 (48.0%) | 122 (46.4%) | 258 (45.5%) | ||
| > 6000 yuan | 61 (45.9%) | 69 (40.4%) | 96 (36.5%) | 226 (39.9%) | ||
- Note: Edu, educational level; Income, family per capita monthly income.
- Abbreviation: SD, standard deviation.
- ∗p < 0.05.
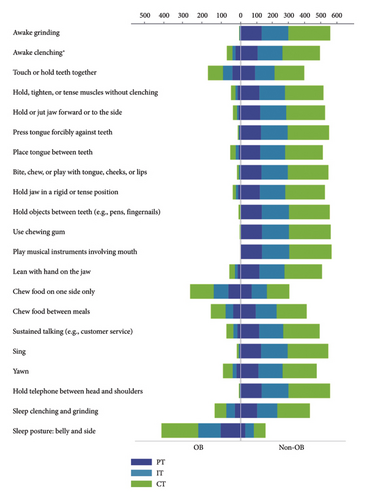
Additionally, the results of OBC, ESS, GAD-7, and PHQ-9 of different TMD patients were compared correspondingly. As displayed in Table 2, there was no significant difference in OBC-asleep score, OBC-total score, OBC level, ESS score, and EDS diagnosis among PT, IT, and CT patients. However, PT patients were found to score highest in OBC-awake (p = 0.029), GAD-7 (p < 0.001), and PHQ-9 (p = 0.002) and thus showed more severe levels in anxiety (p < 0.001) and depression (p < 0.001), while IT patients exhibited the least tendency. Furthermore, the frequencies of different TMD patients in each individual OBC item were compared separately and the result showed that significant difference was only recognized in “Awake clenching” (p = 0.009), as illustrated in Figure 1 (details are presented in SI Table 1).
| Items | TMD diagnosis | p | |||
|---|---|---|---|---|---|
| PT | IT | CT | |||
| OBC-awake | Mean ± SD | 21.20 ± 7.73 | 19.15 ± 7.92 | 20.75 ± 7.76 | 0.029∗ |
| Median | 21.00 | 19.00 | 21.00 | ||
| OBC-asleep | Mean ± SD | 4.39 ± 1.97 | 4.22 ± 2.45 | 4.19 ± 2.22 | 0.718 |
| Median | 4.00 | 4.00 | 4.00 | ||
| OBC-total | Mean ± SD | 25.59 ± 8.38 | 23.37 ± 9.44 | 24.94 ± 8.94 | 0.052 |
| Median | 25.00 | 24.00 | 25.00 | ||
| OBC level | No to low | 62 (46.6%) | 93 (54.4%) | 121 (46.0%) | 0.201 |
| High | 71 (53.4%) | 78 (45.6%) | 142 (54.0%) | ||
| ESS | Mean ± SD | 6.75 ± 3.60 | 5.98 ± 3.22 | 6.62 ± 3.34 | 0.054 |
| Median | 6.00 | 6.00 | 6.00 | ||
| EDS diagnosis | Non-EDS | 105 (78.9%) | 152 (88.9%) | 218 (82.9%) | 0.057 |
| EDS | 28 (21.1%) | 19 (11.1%) | 45 (17.1%) | ||
| GAD-7 | Mean ± SD | 5.41 ± 5.23 | 3.49 ± 4.31 | 4.89 ± 4.53 | < 0.001∗∗∗ |
| Median | 4.00 | 2.00 | 4.00 | ||
| Anxiety level | No | 68 (51.1%) | 122 (71.3%) | 144 (54.8%) | 0.002∗∗ |
| Mild | 45 (33.8%) | 32 (18.7%) | 80 (30.4%) | ||
| Moderate | 10 (7.5%) | 12 (7.0%) | 30 (11.4%) | ||
| Severe | 10 (7.5%) | 5 (2.9%) | 9 (3.4%) | ||
| PHQ-9 | Mean ± SD | 5.85 ± 5.73 | 3.75 ± 4.38 | 5.27 ± 4.60 | < 0.001∗∗∗ |
| Median | 5.00 | 3.00 | 5.00 | ||
| Depression level | No | 66 (49.6%) | 120 (70.2%) | 129 (49.0%) | < 0.001∗∗∗ |
| Mild | 41 (30.8%) | 35 (20.5%) | 89 (33.8%) | ||
| Moderate | 11 (8.3%) | 10 (5.8%) | 35 (13.3%) | ||
| Severe | 15 (11.3%) | 6 (3.5%) | 10 (3.8%) | ||
- ∗p < 0.05.
- ∗∗p < 0.005.
- ∗∗∗p < 0.001.
Based on these findings above, all the included patients with different TMD diagnoses were furtherly analyzed as a whole to detect the relationship between oral behaviors and EDS. Generally, no significant difference was found between patients with and without EDS in all the sociodemographic characteristics, except for age (p = 0.032, Table 3). Besides, the comparisons of OBC, GAD-7, and PHQ-9 related results between non-EDS and EDS patients revealed that the EDS group had significantly higher scores in OBC-awake, OBC-asleep, OBC-total, GAD-7, alongside PHQ-9, and showed higher OBC frequency as well as depression level (Table 4). Although the anxiety level of EDS patients seemed to be higher than non-EDS patients, the difference was not statistically significant (p = 0.226, Table 4).
| Items | Non-EDS | EDS | p | |
|---|---|---|---|---|
| n (%) | 475 (83.8%) | 92 (16.2%) | ||
| Age | Mean ± SD | 28.73 ± 11.58 | 25.82 ± 9.63 | 0.032∗ |
| Median | 26.00 | 24.00 | ||
| Sex | Male | 101 (21.3%) | 15 (16.3%) | 0.281 |
| Female | 374 (78.7%) | 77 (83.7%) | ||
| Edu | Secondary school and below | 137 (28.8%) | 24 (26.1%) | 0.841 |
| Undergraduate or junior college | 292 (61.5%) | 58 (63.0%) | ||
| Postgraduate and above | 46 (9.7%) | 10 (10.9%) | ||
| Income | < 3000 yuan | 72 (15.2%) | 11 (12.0%) | 0.572 |
| 3000–6000 yuan | 212 (44.6%) | 46 (50.0%) | ||
| > 6000 yuan | 191 (40.2%) | 35 (38.0%) | ||
| TMD diagnosis | PT | 105 (22.1%) | 28 (30.4%) | 0.057 |
| IT | 152 (32.0%) | 19 (20.7%) | ||
| CT | 218 (45.9%) | 45 (48.9%) | ||
- ∗p < 0.05.
| Items | Non-EDS | EDS | p | |
|---|---|---|---|---|
| OBC-awake | Mean ± SD | 19.67 ± 7.58 | 24.04 ± 8.11 | < 0.001∗∗∗ |
| Median | 19.00 | 23.00 | ||
| OBC-asleep | Mean ± SD | 4.12 ± 2.23 | 4.88 ± 2.18 | 0.001∗∗ |
| Median | 4.00 | 5.00 | ||
| OBC-total | Mean ± SD | 23.79 ± 8.80 | 28.92 ± 8.77 | < 0.001∗∗∗ |
| Median | 24.00 | 28.00 | ||
| OBC level | No to low | 248 (52.2%) | 28 (30.4%) | < 0.001∗∗∗ |
| High | 227 (47.8%) | 64 (69.6%) | ||
| GAD-7 | Mean ± SD | 4.40 ± 4.59 | 5.53 ± 5.12 | 0.030∗ |
| Median | 3.00 | 4.50 | ||
| Anxiety level | No | 288 (60.6%) | 46 (50.0%) | 0.226 |
| Mild | 128 (26.9%) | 29 (31.5%) | ||
| Moderate | 41 (8.6%) | 11 (12.0%) | ||
| Severe | 18 (3.8%) | 6 (6.5%) | ||
| PHQ-9 | Mean ± SD | 4.48 ± 4.50 | 7.36 ± 6.06 | < 0.001∗∗∗ |
| Median | 3.00 | 6.00 | ||
| Depression level | No | 283 (59.6%) | 32 (34.8%) | < 0.001∗∗∗ |
| Mild | 131 (27.6%) | 34 (37.0%) | ||
| Moderate | 43 (9.1%) | 13 (14.1%) | ||
| Severe | 18 (3.8%) | 13 (14.1%) | ||
- ∗p < 0.05.
- ∗∗p < 0.005.
- ∗∗∗p < 0.001.
Subsequently, binary logistic regression analyses were performed to determine the correlation between OBC level and EDS, with other confounding factors including age, sex, education, income, TMD diagnosis, anxiety level, and depression level adjusted consecutively in three models. As it was described in Table 5, significant correlations between OBC level and EDS were recognized in all three models (OR = 2.275, p = 0.001; OR = 2.176, p = 0.002; OR = 1.780, p = 0.031, respectively). To figure out the specific oral behaviors related to EDS, chi-square tests and binary logistic regressions similar to abovementioned ones were conducted for each OBC item in several. As Figure 2 illustrates, patients who had 10 particular oral behaviors were in higher prevalence of EDS (details are presented in SI Table 2), while the regression analyses showed that “Awake grinding,” “Awake clenching,” and “Press tongue forcibly against teeth” were significantly correlated with EDS in all three adjusted models. Details could be found in Table 6.
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| OBC level | 2.275 | (1.393, 3.714) | 0.001∗∗ | 2.176 | (1.328, 3.565) | 0.002∗∗ | 1.780 | (1.056, 3.002) | 0.031∗ |
| Constant | 0.119 | 0.007 | 0.183 | 0.037 | 0.113 | 0.011 | |||
| Age | 0.981 | (0.958, 1.006) | 0.130 | 0.979 | (0.955, 1.003) | 0.087 | 0.981 | (0.956, 1.007) | 0.155 |
| Sex (ref. male) | 1.239 | (0.672, 2.283) | 0.493 | 1.229 | (0.662, 2.282) | 0.514 | 1.256 | (0.663, 2.382) | 0.484 |
| Education (ref. secondary school and below) | 0.967 | 0.882 | 0.769 | ||||||
| Undergraduate or junior college | 1.044 | (0.609, 1.789) | 0.876 | 1.099 | (0.638, 1.895) | 0.734 | 1.125 | (0.641, 1.972) | 0.682 |
| Postgraduate and above | 1.117 | (0.481, 2.596) | 0.797 | 1.235 | (0.526, 2.899) | 0.628 | 1.377 | (0.577, 3.287) | 0.471 |
| Family per capita monthly income (ref. < 3000 yuan) | 0.871 | 0.791 | 0.921 | ||||||
| 3000–6000 yuan | 1.164 | (0.559, 2.422) | 0.685 | 1.195 | (0.572, 2.499) | 0.635 | 1.152 | (0.535, 2.478) | 0.718 |
| > 6000 yuan | 1.039 | (0.489, 2.209) | 0.920 | 1.020 | (0.477, 2.183) | 0.959 | 1.067 | (0.486, 2.345) | 0.871 |
| TMD diagnosis (ref. PT) | 0.056 | 0.234 | |||||||
| IT | 0.451 | (0.236, 0.864) | 0.016∗ | 0.555 | (0.282, 1.093) | 0.088 | |||
| CT | 0.708 | (0.411, 1.221) | 0.214 | 0.777 | (0.440, 1.372) | 0.384 | |||
| Anxiety (ref. no anxiety) | 0.312 | ||||||||
| Mild | 0.692 | (0.366, 1.308) | 0.257 | ||||||
| Moderate | 0.436 | (0.163, 1.161) | 0.097 | ||||||
| Severe | 0.372 | (0.097, 1.423) | 0.148 | ||||||
| Depression (ref. no depression) | 0.001∗∗ | ||||||||
| Mild | 2.463 | (1.313, 4.622) | 0.005∗ | ||||||
| Moderate | 3.207 | (1.306, 7.873) | 0.011∗ | ||||||
| Severe | 9.311 | (2.898, 29.912) | < 0.001∗∗∗ | ||||||
- Note: Model 1, adjusted for age, sex, education level, and income; Model 2, adjusted for age, sex, education level, income, and TMD diagnosis; Model 3, adjusted for age, sex, education level, income, TMD diagnosis, anxiety level, and depression level.
- ∗p < 0.05.
- ∗∗p < 0.005.
- ∗∗∗p < 0.001.
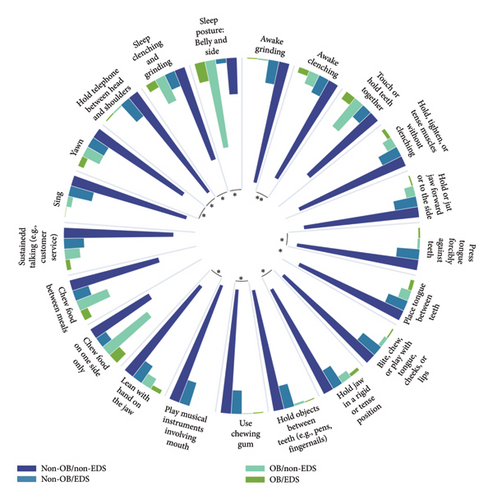
| OBC items | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95.0% CI | p | OR | 95.0% CI | p | OR | 95.0% CI | p | |
| Awake grinding | 5.003 | (1.083, 23.103) | 0.039∗ | 6.118 | (1.316, 28.442) | 0.021∗ | 6.854 | (1.360, 34.532) | 0.020∗ |
| Awake clenching | 2.495 | (1.405, 4.431) | 0.002∗∗ | 2.311 | (1.290, 4.142) | 0.005∗ | 2.190 | (1.186, 4.046) | 0.012∗ |
| Touch or hold teeth together | 1.331 | (0.828, 2.141) | 0.238 | 1.311 | (0.812, 2.116) | 0.268 | 1.361 | (0.829, 2.234) | 0.223 |
| Hold, tighten, or tense muscles without clenching | 1.988 | (1.004, 3.939) | 0.049∗ | 1.860 | (0.932, 3.709) | 0.078 | 1.812 | (0.875, 3.753) | 0.109 |
| Hold or jut jaw forward or to the side | 0.730 | (0.276, 1.929) | 0.525 | 0.682 | (0.257, 1.813) | 0.443 | 0.602 | (0.218, 1.658) | 0.326 |
| Press tongue forcibly against teeth | 4.290 | (1.445, 12.743) | 0.009∗ | 4.203 | (1.401, 12.610) | 0.010∗ | 3.477 | (1.113, 10.857) | 0.032∗ |
| Place tongue between teeth | 1.724 | (0.888, 3.349) | 0.108 | 1.720 | (0.881, 3.356) | 0.112 | 1.635 | (0.821, 3.255) | 0.162 |
| Bite, chew, or play with tongue, cheeks, or lips | 1.656 | (0.574, 4.776) | 0.351 | 1.719 | (0.590, 5.009) | 0.321 | 1.574 | (0.519, 4.772) | 0.423 |
| Hold jaw in a rigid or tense position | 1.915 | (0.918, 3.994) | 0.083 | 1.839 | (0.872, 3.877) | 0.109 | 1.582 | (0.722, 3.468) | 0.252 |
| Hold objects between teeth (e.g., pens, fingernails) | 1.696 | (0.430, 6.688) | 0.450 | 1.635 | (0.410, 6.526) | 0.486 | 2.264 | (0.545, 9.408) | 0.261 |
| Use chewing gum | 7.219 | (1.170, 44.531) | 0.033∗ | 8.709 | (1.379, 54.981) | 0.021∗ | 4.176 | (0.541, 32.241) | 0.170 |
| Play musical instrument that involves use of mouth or jaw | |||||||||
| Lean with hand on the jaw | 1.772 | (0.928, 3.385) | 0.083 | 1.671 | (0.869, 3.213) | 0.124 | 1.457 | (0.734, 2.893) | 0.282 |
| Chew food on one side only | 1.319 | (0.839, 2.073) | 0.230 | 1.290 | (0.819, 2.033) | 0.273 | 1.234 | (0.773, 1.971) | 0.378 |
| Chew food between meals | 1.064 | (0.642, 1.764) | 0.810 | 1.010 | (0.608, 1.680) | 0.969 | 0.901 | (0.531, 1.529) | 0.700 |
| Sustained talking (e.g., customer service) | 1.427 | (0.775, 2.628) | 0.254 | 1.405 | (0.759, 2.601) | 0.279 | 1.431 | (0.754, 2.716) | 0.273 |
| Sing | 0.217 | (0.028, 1.659) | 0.141 | 0.207 | (0.027, 1.587) | 0.130 | 0.235 | (0.030, 1.824) | 0.166 |
| Yawn | 1.646 | (0.944, 2.870) | 0.079 | 1.552 | (0.884, 2.727) | 0.126 | 1.409 | (0.780, 2.545) | 0.256 |
| Hold telephone between head and shoulders | 5.525 | (1.306, 23.382) | 0.020∗ | 5.763 | (1.326, 25.050) | 0.019∗ | 3.660 | (0.752, 17.823) | 0.108 |
| Sleep clenching and grinding | 1.666 | (1.018, 2.728) | 0.042∗ | 1.694 | (1.030, 2.787) | 0.038∗ | 1.551 | (0.929, 2.591) | 0.093 |
| Sleep posture: belly and side | 1.589 | (0.894, 2.826) | 0.115 | 1.493 | (0.836, 2.666) | 0.176 | 1.440 | (0.793, 2.613) | 0.231 |
- Note: Model 1, adjusted for age, sex, education level, and income; Model 2, adjusted for age, sex, education level, income, and TMD diagnosis; Model 3, adjusted for age, sex, education level, income, TMD diagnosis, anxiety level, and depression level.
- ∗p < 0.05.
- ∗∗p < 0.005.
4. Discussion
Within the scope of the present investigation, PT participants experienced the most pronounced symptoms of anxiety and depression compared to CT and IT participants. In addition, OBC level was reported to be positively associated with EDS in TMD patients, and more specifically, patients who exhibited 10 particular oral behaviors were more likely to experience EDS. Moreover, “Awake grinding,” “Awake clenching,” and “Press tongue forcibly against teeth” were significantly correlated with EDS in TMD patients after adjusting for confounders including age, sex, education level, income, TMD diagnosis, anxiety level, and depression level [29–32].
With regard to mental distress among TMD subtypes, patients with painful symptoms (PT and CT) displayed notably elevated levels in both anxiety and depression compared to IT patients, which is in accordance with prior research findings [18, 23, 33]. It has been reported that individuals with chronic pain are at a significantly increased risk of suffering from mental distress [34]. However, the relationship between mental distress and painful manifestations of TMD patients is perceived as bidirectional. On the one hand, PT/CT patients exhibit higher levels of depression and anxiety, potentially due to central sensitization, referring to an enhanced response of the central nervous system to peripheral nociception and sensory stimuli [33]. On the other hand, depressive or anxiety symptoms could escalate the risk of painful conditions by triggering muscular hyperactivity, leading to muscle pain, or initiating joint inflammation, resulting in joint pain [35, 36].
Our results showed that PT patients scored highest in OBC-awake and significant difference in frequency was found in “Awake clenching” among different TMD patients, which is congruent with several studies. A significant correlation between high levels of oral behaviors and chronic TMD pain was found by Keela et al. [24]. Michelotti et al. reported that sustained contact between teeth such as daytime tooth clenching/grinding was seen to be a significant risk factor for myofascial pain in TMD patients [37]. Miyake et al. revealed that the parafunction of tooth clenching was one of the risk factors for multiple TMD symptoms including TMJ pain [38]. According to previous researches, muscle’s capillary supply plays a vital role in the occurrence of muscle pain [39, 40]. Therefore, it is postulated that tooth clenching can be regarded as a form of microtrauma which may lead to muscle pain due to impaired capillary blood circulation, causing energy crisis within fibers, thereby resulting in muscle tissue damage and local pain.
Sleep disturbance could contribute to EDS, which is more common in TMD patients compared to healthy controls, and is thought to be a risk factor for more severe TMD symptoms such as pain and dysfunction [17, 41, 42]. In our study, 16.2% of TMD patients were found to have EDS, a debilitating condition that can significantly impact an individual’s overall quality of life through cognitive impairment and loss of physical or psychological functions, but without enough recognition [20]. While it is generally accepted that the elderly population is more susceptible to sleep disturbance and EDS [43], our study suggested that TMD may impose a greater effect on sleep quality in younger patients as evidenced by the significantly younger age of patients with EDS, which is in agreement with a previous study [22]. This is consistent with prior research suggesting that self-reported TMD symptoms tend to lessen with age [30, 44]. However, it contradicts previous research suggesting that an increase in age is associated with poor sleep in TMD patients [42].
TMD patients with EDS had higher scores in anxiety and depression, alongside exhibiting more pronounced depression levels as noted in our study, indicating that they experienced more anxiety and depression than the non-EDS TMD patients. Recent research has highlighted the intricate relationship between psychological factors and sleep quality, with anxiety and depression recognized as crucial factors influencing the onset and progression of sleep disturbances, particularly in conditions like TMD. Anxiety, for instance, not only lowers pain thresholds in TMD patients but also contributes to the development of sleep disturbances, creating a vicious cycle of anxiety and poor sleep [45]. Meanwhile, depression significantly correlates with sleep quality and an association between daytime sleepiness and moderate to severe depression has been revealed [20, 46]. Moreover, insomnia impairing daytime function is a significant factor in the onset of depression, and disturbances in the human circadian system can also lead to insomnia or EDS, which in turn are symptoms of depression [47, 48].
EDS group also had significantly higher scores in OBC-awake, OBC-asleep, OBC-total, and showed higher OBC frequency. Subsequent regression analyses revealed “Awake grinding,” “Awake clenching,” and “Press tongue forcibly against teeth” were significantly correlated with EDS with all other confounding factors adjusted. Oral parafunction behavior has been identified as a potential contributing factor to TMD. Ohrbach et al. have found that oral behaviors may increase the risk of TMD through hyperactive motor excitation, impaired motor inhibition, altered proprioception, and prolonged psychophysiological responses [49]. Excessive oral behaviors may function as both coping mechanisms for pain and perpetuating factors for pain, leading to increased muscle tonicity and stress on the TMJ, contributing to chronic symptoms [24]. It has been reported that oral parafunction behavior, such as bruxism, where individuals involuntarily grind or clench their teeth, is a potential contributing factor to painful TMD. However, the relationship between the two is complex and has been the subject of debate in the literature, with studies presenting varied results. Some studies have shown a significant association between tooth grinding or clenching and TMD pain, while others have not found a clear link [50, 51]. Tooth grinding or clenching, characterized by abnormal jaw muscle activity, can be influenced by a combination of psychosocial and pathophysiological factors and may lead to pain, muscle tenderness, and other TMD symptoms. Additionally, tooth grinding or clenching may adversely affect Oral Health-Related Quality of Life (OHRQoL), particularly in domains related to physical pain (pain and discomfort), psychological discomfort (self-conscious and tense), and psychological disability (difficult to relax and embarrassed), indicating that individuals with these conditions may face challenges related to oral function, pain management, and emotional well-being [52]. Thereby, tooth grinding or clenching may lead to EDS in TMD patients due to detrimental effects they impose on the abovementioned domains, which are in tight association with sleep quality. Mandibular “balance” is often used interchangeably with occlusal stability and is maintained by jaw-closing muscle contraction [53]. Certain jaw postures can lead to strain and microtrauma in the masticatory system, affecting the stability of the TMJ. “Hold or jut jaw forward or to the side” and “Hold jaw in rigid or tense position” have been found to be associated with painful TMD [24, 54]. Moreover, research suggests that “pressing the tongue forcibly against the teeth” can also contribute to chronic pain in TMD patients [24]. These oral behaviors may create uneven force distribution within the masticatory system, potentially leading to discomfort and dysfunction in the TMJ, thereby possibly causing impaired sleep quality of TMD patients.
In clinical practice, by recognizing these parafunctional behaviors as reliable predictors of EDS, healthcare providers can tailor their treatment plans to better manage the symptoms and improve life quality of TMD patients. This comprehensive approach to care is essential for addressing the complex and multifaceted nature of TMD.
This study bears certain limitations. First, the cross-sectional design of the study introduces potential biases, disabling it to establish definitive causal relationships between oral behaviors and EDS. Therefore, future studies with a prospective design and real randomization are required to validate the cause–effect association between the changes in oral behaviors and the occurrence of EDS among TMD patients. Second, the reliance on self-reported oral behaviors and the use of the ESS, a self-reported tool for screening EDS, may bring about recall biases. More objective measures like polysomnography could provide a more accurate assessment. Third, the sample was also a convenience sample from a single center, potentially limiting the generalizability of the findings. Besides, the heterogeneity within each TMD subtype, such as the symptom intensity and the disease duration, was not analyzed in this study due to a relatively small sample size when re-dividing subgroups of these subtypes. For a more comprehensive understanding about the complicated relationships between different TMD subtypes, oral behaviors, and EDS, it is advisable to recruit a larger number of participants from multicenters in future studies.
5. Conclusions
Despite the limitations, evidences found in this study indicated a significant association between oral behaviors and EDS of TMD patients. Compared with non-EDS counterparts, higher OBC scores (total, awake, and asleep) and OBC level were identified in EDS-bothered TMD patients. Additionally, the study also recognized “Awake grinding” (OR = 6.854), “Awake clenching” (OR = 2.190), and “Press tongue forcibly against teeth” (OR = 3.477) as reliable predictors of EDS in TMD patients. As a consequence, taking these parafunctional behaviors into consideration when making treatment plans is indispensable for the comprehensive management of TMD patients, especially those suffering from EDS. Future studies with prospective research design are recommended for a further validation.
Ethics Statement
Ethical approval of this study was sought and granted by the Institutional Review Board of West China Hospital of Stomatology (Approval no. WCHSIRB-2020-378).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jie Xiang: writing and review–editing, formal analysis, visualization, and investigation. Jiaqi Liu: writing – original draft, visualization, and investigation. Zihan Zhang: writing – original draft and formal analysis. Zejin Liu: writing – original draft and investigation. Yunhao Zheng: writing – original draft and visualization. Yating Yi: writing – original draft, project administration, and investigation. Xueman Zhou: writing and review–editing and investigation. Jun Wang: supervision and conceptualization. Fan Yang: supervision, project administration, and writing and review–editing. Xin Xiong: supervision, project administration, and conceptualization. Jie Xiang and Jiaqi Liu contributed equally.
Funding
This work was supported by the National Natural Science Foundation of China, 82301129; Clinical Research Project of West China Hospital of Stomatology, Sichuan University, LCYJ-2023-YY-2; Research and Develop Program of West China Hospital of Stomatology, Sichuan University, RCDWJS2023-13; Sichuan Science and Technology Program, No. 2023JDRC0096; Align Research Fund, No. AQKY22-3-1; and Key Science and Technology Program of Shaanxi Province, 2022ZDLSF03-08.
Acknowledgments
We express our gratitude to the patients who participated in this study.
Supporting Information
SI Table 1: Chi-square tests of TMD diagnosis and individual OBC items.
SI Table 2: Chi-square tests of EDS and individual OBC items.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding authors.




