Diagnostic Role of Plateletcrit in Pleural Effusion
Abstract
Objectives: Pleural effusion arises from either increased fluid production or reduced absorption within the pleural space. In instances where thoracentesis is contraindicated or unfeasible, alternative, less invasive diagnostic approaches are necessary. Thoracentesis with simultaneous venous blood sampling is pivotal for differentiating transudative from exudative pleural fluid, guided by the Light criteria In instances where thoracentesis is contraindicated or unfeasible, alternative, less invasive diagnostic approaches are necessary. Parameters such as platelet (PLT) count, plateletcrit (PCT), PLT distribution width (PDW), and mean PLT volume (MPV) have been proposed as adjuncts to other acute phase reactants in identifying inflammation activation. This study aims to investigate the feasibility of Plt activation markers in diagnosing pleural effusion.
Methods: This retrospective observational study involved patients diagnosed with pleural effusion who were admitted to hospital between 01 January 2021 and 31 May 2023. Cases where the effusion nature was determined through thoracentesis were included. Among the complete blood count parameters, neutrophil, lymphocyte, Plt count, PCT, PDW and MPV were calculated.
Results: The study encompassed a cohort of 155 cases, comprising 43.2% females and 56.8% males.Thoracentesis revealed 66.5% exudative effusions and 33.5% transudative effusions. PDW and MPV were significantly lower in the exudate group than in the transudate group. PCT values demonstrated significant effectiveness (area under the curve, 0.740 [0.653–0.828]) in distinguishing patients with exudates from those with transudates. A cut-off value of 0.275 for PCT yielded significant effectiveness with a sensitivity of 83.5%, and a specificity of 61.5%. For a PDW cut-off value of 10.5, the sensitivity was 49.5%, and specificity was 75.0%.
Conclusions: The sensitivity and specificity of Plt parameters in differentiating patients in transudate and exudate groups were found to be high.
1. Introduction
Pleural effusion (PE) arises from either increased fluid production or reduced absorption within the pleural space. It represents a clinically significant condition with diverse pathophysiological underpinnings [1]. While pulmonary and pleural causes predominate, extrapulmonary factors can also contribute to effusion development [2]. A comprehensive approach is crucial to ascertain its etiology [3], which may range from benign to life-threatening conditions [4]. Malignant PE (MPE) is a condition with morbidity and mortality which affects many individuals worldwide [5]. Although it is quite common, there has been no study identifying hospitalization rate of cases with MPE [6].
Evaluating a patient with PE necessitates a thorough history, including inquiries into potential causative factors such as asbestos exposure, tuberculosis, and liver or kidney failure [7]. Treatment strategies are tailored based on the varied etiopathogenesis [1]. Initial imaging typically involves anterior-posterior chest radiography, which can detect effusions exceeding 200 mL [8]. However, computed tomography (CT) offers superior diagnostic capabilities, particularly in cases where chest radiographs are inconclusive or when loculated effusions are present [9]. In recent years, thoracic ultrasound has been used to diagnose PE, make differential diagnosis, and guide sampling when necessary because it can be used at the bedside, is easy to apply, and does not expose either the patient or the physician to radiation. For these reasons, it has surpassed thoracic CT in PE follow-ups [10].
Thoracentesis with simultaneous venous blood sampling is pivotal for differentiating transudative from exudative pleural fluid, guided by the Light criteria [11]. Notably, a significant proportion of PEs (20%) remain undiagnosed [2, 12], prompting the exploration of novel diagnostic modalities [13]. In instances where thoracentesis is contraindicated or unfeasible, alternative, less invasive diagnostic approaches are necessary. The number of platelets (PLTs) in the blood can be measured rapidly using an automated hematological analyzer. PLT indicators are biomarkers of PLT activation. These allow for comprehensive clinical research, focusing on diagnostic and prognostic values in various studies at no additional cost. Among these PLT indices, plateletcrit (PCT), mean PLT volume (MPV) and PLT distribution width (PDW) are a group of PLT parameters available in an automated complete blood count [14]. Parameters such as PLT count, PCT, PDW, and MPV have been proposed as adjuncts to other acute phase reactants in identifying inflammation activation [15]. PLT and MPV/PLT have been used to predict mortality in transudative pleural fluid [16]. PCT represents the percentage of blood volume occupied by PLTs [14], while PDW serves as a marker of PLT function and activation, reflecting variability in PLT size [17]. Elevated PDW levels have been observed in conditions such as diabetes mellitus (DM), cancer, and cardiovascular diseases [18–20]. Likewise, a high PLT-lymphocyte ratio (PLR) among hematologic parameters has been associated with mortality in MPE [21]. This study aims to investigate the feasibility of PLT and PLT activation markers in diagnosing PE.
2. Materials and Methods
2.1. Study Design
This retrospective observational study involved patients diagnosed with PE who were either admitted to or referred to a tertiary care practice and research hospital, serving as a tertiary referral center, between 01 January 2021 and 31 May 2023. The Kahramanmaraş Sütçü İmam University Medical Research Ethics Committee approved the study protocol (Approval Date: 18.07.2023, Approval No: 02), and it adhered to the principles outlined in the Declaration of Helsinki. Since our study was retrospective, informed consent could not be obtained from the patients included in the study. Except for the routine examinations of the patients at the time of admission, the confidentiality of the other data of the patients was taken into consideration. This was communicated to the ethics committee.
2.2. Population and Sample
Patients aged 18 and above who were assessed for PE at the Kahramanmaras Sutcu Imam University Faculty of Medicine Chest Diseases and Thoracic Surgery department were retrospectively reviewed. Cases where the effusion nature was determined through thoracentesis were included. Thoracentesis was repeated when deemed necessary, and surgical procedures such as video-assisted thoracoscopy (VATS) were performed in cases where diagnosis remained elusive. Under general anesthesia, multiple pleural biopsies and fluid samples were obtained uniportally from the hemithorax with pleural fluid via double-lumen intubation. Complete blood count and biochemical parameters from venous blood were concurrently measured in all cases during thoracentesis.
Patients with missing data and those under the age of 18 were excluded from the study. Additionally, cases where thoracentesis was contraindicated or not feasible owing to patient reasons and those where venous blood was not concurrently collected during thoracentesis were not included. Demographic characteristics, complete blood count parameters, biochemical parameters, and cytology results were extracted from the hospital data system for analysis.
Complete blood count parameter values were calculated and recorded using the Sysmax XN 3000 complete blood count analyzer, including white blood cell count (WBC); neutrophil count (Neu); lymphocyte count (Lym); monocyte count (MO); eosinophil count (EO) and percentages; immature granulocyte (IG) count and ratio; Plt; PCT; MPV; PDW; hemoglobin (Hb); neutrophil-lymphocyte ratio (NLR); and PLR.
2.3. Study Groups
Following thoracentesis, pleural fluid was classified as either transudate or exudate based on Light’s criteria [8]. Pleural fluid samples from all cases underwent cytological, microbiological, and biochemical analyses. The exudate group was further categorized into malignant and benign pleural fluids, while the transudate group was assessed for fluid due to cardiac, hepatic, or renal failure. Causes of exudative effusions included MPE, parapneumonic PE (PPE), empyema, tuberculous pleurisy (TP), and others. Adenosine deaminase (ADA) levels were examined in cases with uncertain diagnoses.
2.4. Definition of Endpoints
Investigating the role of PLT parameters in exudate-transudate differentiation was our first endpoint in this study, and the effect of these parameters on mortality and morbidity was our second endpoint.
2.5. Statistical Analysis
The descriptive statistics of the data included mean, standard deviation, median, minimum, maximum, frequency, and ratio values. The distribution of variables was assessed using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Quantitative independent data were analyzed using the Mann–Whitney U test, while qualitative independent data were analyzed using the Chi-square test. The effect level and cut-off value were determined using the receiver operating characteristic (ROC) curve. The effect level was further examined through univariate and multivariate logistic regression analyses. The analyses were conducted using the SPSS 27.0 software.
3. Results
The study encompassed a cohort of 155 cases comprising 67 (43.2%) females and 88 (56.8%) males, with a mean age of 66.1 ± 16.6 years. Thoracentesis revealed 103 cases (66.5%) of exudative effusions and 52 cases of (33.5%) transudative effusions. Cytology analysis indicated that 111 cases (71.6%) were benign, whereas 44 cases (28.4%) were malignant. MPE accounted for the highest proportion (27.7%) among all causes, followed by cardiac failure (25.8%) and PPE (12.9%). Primary lung cancer (27.3%) predominated in cytology results (Table 1).
| Min-max | Median | Mean ± sd/n-% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 18.0–96.0 | 69.0 | 66.1 ± 16.6 | |||||||
| Sex | Female | 67 | 43.2 | |||||||
| Male | 88 | 56.8 | ||||||||
| Diagnosis | Benign | 111 | 71.6 | |||||||
| Malign | 44 | 28.4 | ||||||||
| Subdiagnosis | MPE | 44 | 28.4 | |||||||
| Cardiac failure | 40 | 25.8 | ||||||||
| PPE | 20 | 12.9 | ||||||||
| Empyema | 17 | 11.0 | ||||||||
| Inflammation | 14 | 9.0 | ||||||||
| TP | 7 | 4.5 | ||||||||
| Hepatic failure | 6 | 3.9 | ||||||||
| Renal failure | 5 | 3.3 | ||||||||
| Connective tissue disease | 1 | 0.6 | ||||||||
| Hemothorax | 1 | 0.6 | ||||||||
| n | % | |||||||||
| Malignancy type | Lung cancer | 12 | 27.3 | |||||||
| Breast cancer | 6 | 13.6 | ||||||||
| Gynecologic tumors | 5 | 11.4 | ||||||||
| GİS tumors | 5 | 11.4 | ||||||||
| Mesothelioma | 3 | 6.8 | ||||||||
| Thyroid cancer | 2 | 4.5 | ||||||||
| Others | 10 | 22.7 | ||||||||
- Abbreviations: GIS, gastro intestinal system; MPE, malignant pleural effusion; PPE, parapneumonic effusion; TP, tuberculous pleurisy.
No significant differences in age or gender distribution were observed between the exudate and transudate groups (p > 0.01–p > 0.01). However, parameters such as WBC (p < 0.001), Neu (p < 0.001), MO (p < 0.01) Plt (p < 0.01), PLR (p < 0.001), PCT (p < 0.001), and Hb (p < 0.001) levels were notably higher in the exudate group compared with the transudate group (Table 2). Conversely, Neu (p > 0.01) and MO (p > 0.01) percentages and, Lym (p > 0.01), EO (p > 0.01), and IG (p > 0.01) percentages and counts did not significantly differ between the two groups. MPV (p = 0.001), and PDW (p < 0.001) were significantly lower in the exudate group than in the transudate group.
| Transudate | Exudate | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± sd/n-% | Median | Mean ± sd/n-% | Median | |||||||
| Age | 69.9 ± 14.1 | 71.5 | 64.1 ± 17.5 | 66.0 | 0.055m | |||||
| Sex | Female | 22 | 42.3 | 45 | 43.7 | 0.870X2 | ||||
| Male | 30 | 57.7 | 58 | 56.3 | ||||||
| WBC | 9.9 | ± | 5.1 | 8.5 | 11.3 | ± | 5.4 | 10.0 | 0.033m | |
| % neutrophil | 72.4 | ± | 15.5 | 75.9 | 75.5 | ± | 11.8 | 76.2 | 0.397m | |
| Neutrophil | 7.4 | ± | 4.7 | 6.6 | 9.3 | ± | 7.4 | 7.4 | 0.040m | |
| % lymphocyte | 15.4 | ± | 8.1 | 14.9 | 14.2 | ± | 8.1 | 13.0 | 0.378m | |
| Lymphocyte | 1.34 | ± | 0.66 | 1.20 | 1.46 | ± | 0.82 | 1.36 | 0.424m | |
| PLT | 274.2 | ± | 140.3 | 240.5 | 365.8 | ± | 123.2 | 351.0 | ∼0.001m | |
| NLR | 7.0 | ± | 5.3 | 5.1 | 9.6 | ± | 10.5 | 6.0 | 0.245m | |
| PLR | 242.3 | ± | 146.4 | 227.1 | 367.2 | ± | 370.6 | 258.9 | 0.015m | |
| % monocyte | 7.6 | ± | 2.6 | 7.6 | 8.0 | ± | 3.0 | 8.1 | 0.499t | |
| Monocyte | 0.98 | ± | 1.83 | 0.69 | 0.86 | ± | 0.38 | 0.83 | 0.022m | |
| % eosinophil | 1.78 | ± | 1.69 | 1.30 | 1.90 | ± | 4.85 | 0.80 | 0.134m | |
| Eosinophil | 0.15 | ± | 0.16 | 0.11 | 0.20 | ± | 0.56 | 0.09 | 0.430m | |
| %IG | 0.58 | ± | 0.49 | 0.50 | 0.88 | ± | 1.20 | 0.50 | 0.535m | |
| IG | 0.07 | ± | 0.13 | 0.04 | 0.15 | ± | 0.31 | 0.05 | 0.085m | |
| PCT | 0.26 | ± | 0.11 | 0.25 | 0.35 | ± | 0.11 | 0.35 | ∼0.001m | |
| MPV | 10.4 | ± | 1.0 | 10.5 | 9.8 | ± | 1.2 | 9.8 | 0.001m | |
| PDW | 12.0 | ± | 2.2 | 11.7 | 10.8 | ± | 2.0 | 10.6 | ∼0.001t | |
| Hb | 10.9 | ± | 2.1 | 10.5 | 12.3 | ± | 2.3 | 12.1 | ∼0.001t | |
- Note: The bold values, i.e., p < 0.05, represent significance.
- Abbreviations: Hb, hemoglobin; IG, immature granulocyte; MPV, mean platelet volume; NLR, neutrophil-lymphocyte ratio; PCT, plateletcrit; PDW, platelet distribution width; PLR, platelet-lymphocyte ratio; PLT, platelet; Sd, standard deviation; WBC, white blood cell.
- X2Ki-Kare test.
- mMann–Whitney U test.
- tIndependent sample t-test.
In the univariate analysis, PLT (p < 0.001), PLR (p < 0.001), PCT (p < 0.001), MPV (p∼0.001), PDW (p∼0.001) and Hb (p∼0.001) exhibited significant discriminatory power in distinguishing between exudative and transudative effusions while WBC (p > 0.001), Neu (p > 0.001), and MO (p > 0.001) did not.
In the multivariate model, PCT (p < 0.001), PDW (p < 0.01), Hb (p < 0.01) and diagnosis (p < 0.01) independently demonstrated significant discriminatory abilities in distinguishing exudates from transudates (Table 3).
| Univariate model | Multivariate model | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | %95 CI | p | OR | %95 CI | p | |||
| WBC | 1.060 | 0.985–1.141 | 0.122 | |||||
| NEU | 1.069 | 0.989–1.156 | 0.094 | |||||
| PLT | 1.006 | 1.003–1.009 | ∼0.001 | |||||
| PLR | 1.003 | 1.000–1.005 | 0.019 | |||||
| MO | 0.916 | 0.682–1.231 | 0.561 | |||||
| PCT | > 100 | 81.60–> 100 | ∼0.001 | > 100 | 10.80–> 100 | ∼0.001 | ||
| MPV | 0.536 | 0.366–0.785 | 0.001 | |||||
| PDW | 0.726 | 0.604–0.874 | 0.001 | 0.712 | 0.560–0.905 | 0.005 | ||
| Hb | 1.347 | 1.137–1.597 | 0.001 | 1.356 | 1.107–1.661 | 0.003 | ||
| Diagnosis | 17.21 | 3.97–74.64 | ∼0.001 | > 100 | 4.926–> 100 | ∼0.001 | ||
- Note: The bold values, i.e., p < 0.05, represent significance.
- Abbreviations: CI, confidence interval; Hb, hemoglobin; MO, monocyte; MPV, mean platelet volume; NEU, neutrophil; PCT, plateletcrit; PDW, platelet distribution width; PLR, platelet-lymphocyte ratio; PLT, platelet; WBC, white blood cell.
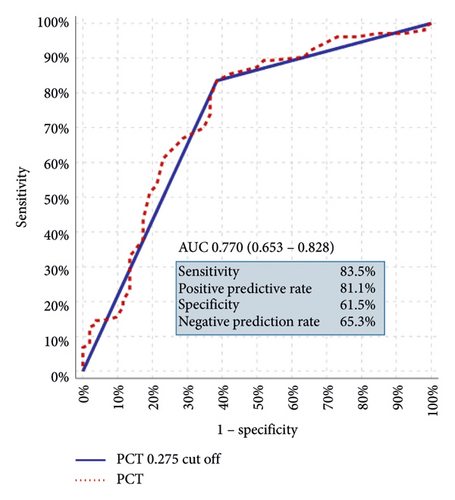
PCT values demonstrated significant effectiveness (area under the curve, 0.740 [0.653–0.828]) in distinguishing patients with exudates from those with transudates (Table 4).
| AUC | % 95 CI | p | |||
|---|---|---|---|---|---|
| PCT | 0.740 | 0.653–0.828 | ∼0.001 | ||
| PCT 0.275 cut-off | 0.725 | 0.636–0.815 | ∼0.001 | ||
| Transudate group | Exudate group | % | |||
| PCT | ≤ 0.275 | 32 | 17 | Sensitivity | 83.5 |
| > 0.275 | 20 | 86 | PPR | 81.1 | |
| Specificity | 61.5 | ||||
| NPR | 65.3 | ||||
- Note: The bold values, i.e., p < 0.05, represent significance.
- Abbreviations: AUC, area under curve; CI, confidence interval; NPR, negative prediction ratio; PCT, plateletcrit; PPR, positive prediction ratio; ROC, receiver operating characteristic.
A cut-off value of 0.275 for PCT yielded significant effectiveness (area under the curve, 0.725 [0.636–0.815]), with a sensitivity of 83.5%, a positive predictive value of 81.1%, a specificity of 61.5%, and a negative predictive value of 65.3% (Figure 1).
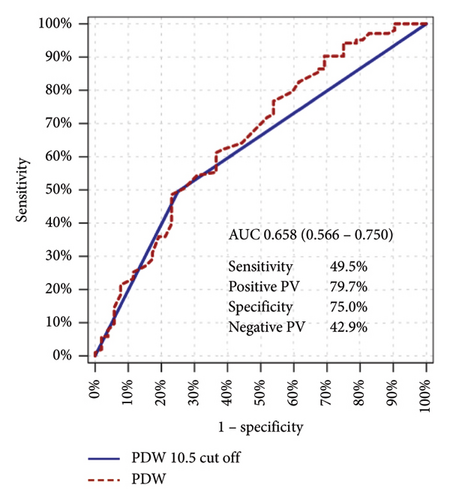
For a PDW cut-off value of 10.5 the sensitivity was 49.5%, the positive predictive value was 79.7%, the specificity was 75.0%, and the negative predictive value was 42.9% (Figure 2).
4. Discussion
In various series, the etiology of PE remains elusive in up to 20% of cases [12]. In our study, consistent with recommendations in the literature, thoracentesis was performed in all cases, and transudate/exudate differentiation was based on Light’s criteria. Exudative PE predominated in our cases aligning with findings in the literature [8].
The literature presents a wide array of information on the etiologies of exudative PE with infections and malignancies often cited as the leading causes [22–25]. In our series, MPE ranked highest with 43 cases (27.7%) consistent with some findings in the literature. Notably, primary lung cancer emerged as the most common etiological cause of MPE in our cases akin to trends documented in the literature [6, 26].
Congestive heart failure (CHF) accounts for approximately 55% of transudative effusions. Similarly, fluids secondary to failures represented the most frequent cause of transudative PE in our study aligning with trends in the literature [7]. There are studies in the literature showing that PLT-related parameters, such as the PLT-lymphocyte ratio (PLR) and the NLR, are potential diagnostic and prognostic biomarkers in many inflammatory diseases, autoimmune diseases and malignant tumors [27, 28].
In our series, WBC, Neu, Plt, PLR, PCT and Hb values were significantly higher in the exudate group compared with the transudate group, mirroring findings in the literature [29–31]. The elevated WBC and Neu counts in the exudate group can likely be attributed to infective PE such as PPE and empyema. However, contrary to reports in the literature, Neu, Lym, NLR, MO, EO percentages, and %IG and IG values did not exhibit significant differences between the exudate and transudate groups. Consequently it was inferred that NLR values might not be effective in distinguishing transudate from exudate [32].
In our study, Plt, PLR, PCT, MPV, PDW, and Hb levels obtained from venous blood samples taken simultaneously with thoracentesis from all our cases exhibited significant effectiveness in distinguishing between exudate and transudate patients. This has led to the need to investigate the effect of other hematologic parameters in differentiating exudate from transudate. Plt and MPV/PLT were used to predict mortality in transudative pleural fluid in a study by Garcia-Pachon et al. [15].
Interestingly previous studies have highlighted the diagnostic potential of Plt values in differentiating TP and MPE [6, 29]. Additionally, Plt levels have been reported to be significant in pancreatic adenocarcinomas and are associated with the postoperative prognosis of patients with non-small-cell lung cancer [6, 29, 30]. Similarly, PCT is significantly associated with mortality in PE due to heart failure [31]. Increasing evidence suggests that inflammatory factors play an important role in the emergence and recurrence of tumors [33].
However, few studies have emphasized PLT-related parameters. It was proven in recent years that PLTs, an important factor, are indispensable in tumors. PLTs also play a vital role in many other physiological and pathophysiological processes, such as inflammation, angiogenesis and cancer progression. Studies point to a potential relationship between PLT function and the spread of cancer through the bloodstream [34, 35].
PLTs also play an important role in inflammatory reactions by releasing factors that activate neutrophils, lymphocytes and macrophages [36]. Recent studies have shown that PLTs play a role in inflammation and infection prevention [37]. Many invading microbial pathogens can alter PLT count or function by directly or indirectly targeting host PLTs [38].
When these studies in the literature are taken into consideration, it can be concluded that PLT series can show differences in inflammatory diseases, infectious pathologies, and malignancies. In our study, PCT levels were significantly higher in exudative PE than in transudative fluids. Considering the studies in the literature demonstrating a relationship between PLTs and PLT series with inflammation, infection, and malignancies, it should not be a surprise that platalet and series were found to be high in exudative PE because the etiologies of exudative PE are malignancies, infectious diseases, and inflammatory pathologies [39].
Studies in the literature mention that PLTs increase in malignancies, inflammation and infections. However, in our literature search, we could not find any other study mentioning the role of PLTs in the differentiation of transudates from exudates in PE. In our study, PLT, PLR, PCT, MPV, PDV, Hb levels obtained from venous blood samples obtained simultaneously with thoracentesis from all our patients showed significant (p < 0.05) diagnostic efficiency in the differentiation of exudate and transudate group patients. Studies have shown that inflammation causes tissue edema by disrupting endothelial function; it creates a tendency to coagulation through released pro-hemostatic proteins and as a result, PLT parameters increase. It is known that inflammation is the main cause of exudative pleural fluids. This study supports our results [40]. PLTs have also been found to play a proinflammatory role in tuberculosis [41].
In a study by Ai L et al., the diagnostic efficacy of PLT values in the differentiation of TP and MPE was mentioned, while in another study by Wang L et al. PLT levels were found to be significant in pancreatic adenocarcinomas. Hur JY et al. reported that PLTs were associated with the postoperative prognosis of patients with non-small-cell lung cancer. Budak et al. mentioned that PCT was significantly associated with mortality in PE due to heart failure [14, 21, 39, 42].
We observed the significance and efficacy of the PCT value in differentiating patients in the exudate and transudate groups with a cut-off value of 0.275. We have not found any other study in the literature in which PCT values have shown such significance in differentiating transudate and exudate groups with a cut-off value. This aspect of our study stands out as unique and adds new insights to the field.
The differential diagnosis of PE poses challenges and often necessitates numerous laboratory investigations, including invasive procedures. Despite ongoing efforts, a standardized pleural marker has yet to be established. There is a pressing need for easily accessible procedures that are less invasive for the differential diagnosis of PE. Thoracentesis remains a common diagnostic approach for PE with a transudate/exudate differentiation made based on Light criteria. However, in cases where thoracentesis is contraindicated or unfeasible for the patient, alternative procedures are actively sought [13].
PLT parameters offer a promising avenue for investigation, as they can be easily obtained using routine hematology analyzers during peripheral blood sampling. This method is less invasive and can be conducted in many centers where complete blood count analysis is available without requiring hospitalization. Moreover, compared with other prognostic factors, PLT parameters are more cost-effective and, thus, more widely accessible [14, 39].
The limitations of our study are that it was conducted in a single center and was retrospective. We did not include cases without data or instances where thoracentesis was contraindicated or impossible to perform. Additionally, the low number of cases in our study may be attributed to various external factors, including the aftermath of an earthquake that occurred in our hospital’s vicinity in 2023.
5. Conclusions
PLTs and their series are known to increase in malignancies, infections and inflammation. In our study, the sensitivity and specificity of PLT parameters in differentiating patients in transudate and exudate groups were found to be high. We found that PLT parameters have a role in differentiating transudates from exudates, but we did not find any effect on mortality or morbidity. Working with these parameters is noninvasive, does not require hospitalization and has low cost. Therefore, this method can be applied at every center. It may represent hope in the search for new parameters in the diagnosis of PE. We think that further studies with larger series will enable us to make significant contributions to the literature in this field.
Ethics Statement
Ethical permission was obtained from the Sutcu Imam University Medical Research Ethics Committee for this study with date 18.07.2023 and number 02, and Helsinki Declaration rules were followed to conduct this study. Since our study was retrospective, informed consent could not be obtained from the patients. This information was given to the ethics committee and ethics committee approval was obtained.
Consent
Please see the Ethics Statement.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
B.A. designed this investigation and wrote the manuscript. F.K. contributed to study design. B.A. and F.K. collected the data. B.A. conducted statistical analysis and revision of the manuscript. F.K. designed this investigation and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




