A Narrative Review on Predictive Models for Bleeding Risk Associated With Anticoagulant Therapy in Venous Thromboembolism
Abstract
The prediction of bleeding risk is crucial for the standardized treatment of venous thromboembolism. This article introduces the definition and importance of predicting anticoagulant therapy–related bleeding in venous thromboembolism and reviews the latest research on existing anticoagulant therapy-related bleeding risk prediction models, especially HAS-BLED, RIETE, and VTE-BLEED models in recent years. It is found that existing clinical factor–based bleeding risk prediction models have certain predictive significance for bleeding events, but there is still a gap compared to ideal models, and dynamic evaluation is more important. This review also explores new models for predicting bleeding risk and provides a reference for clinical physicians to develop anticoagulant strategies.
1. Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PTE), is the third most common cardiovascular disease after coronary atherosclerotic heart disease (CAD) and ischemic stroke [1]. Anticoagulant therapy is the cornerstone of VTE treatment, and sufficient dosage and duration of anticoagulant therapy can significantly reduce the occurrence of thrombosis-related events. Generally speaking, the 1-year recurrence risk of VTE in patients with low recurrence risk is only 1%, and anticoagulation can be terminated after 3 months; patients with high risk of recurrence (if there are persistent risk factors or unknown causes) have a recurrence risk of about 10% at 1 year, 25%–30% at 5 years, and 30%–40% at 10 years, and require indefinite anticoagulation [2]. However, the cost of anticoagulation is an increase in bleeding events. Improper anticoagulation can lead to VTE recurrence and anticoagulation-related bleeding events, as well as long-term complications such as postthrombotic syndrome, which significantly reduce the quality of life of patients and result in heavy economic and medical burdens. In severe cases, it can cause disability and death [3]. Therefore, predicting the risk of bleeding is crucial for the standardized treatment of VTE.
2. The Definition and Predictive Importance of Anticoagulant Therapy–Related Bleeding
Accurate assessment of bleeding risk is particularly important in the treatment of VTE. The International Society on Thrombosis and Hemostasis (ISTH) defines the severity of bleeding as follows: (1) Major bleeding is characterized by bleeding that occurs in key organs, such as intracranial hemorrhage, spinal canal, intraocular, retroperitoneal, intra-articular, pericardial or intermuscular compartment syndrome, or bleeding leading to a decrease of hemoglobin by more than 20 g/L, or requiring infusion of ≥ 2 U red blood cells. (2) Not meeting the criteria for major bleeding but requiring hospitalization for medical intervention is clinically relevant nonmajor bleeding. (3) Other bleeding events are referred to as mild bleeding [4–6]. Bleeding events often occur within the first 3–6 months of initiating anticoagulation [7]. Meta-analysis shows that the total mortality rate of VTE within the first 3 months is 11.3% (including VTE events and major bleeding), with VTE events being the main cause; after 3 months, the overall mortality rate gradually decreased and remained stable, but the proportion of deaths from major bleeding caused by anticoagulation gradually increased [8], indicating that bleeding risk factors change with the course of the disease. Predicting bleeding risk is becoming increasingly important in VTE management [9, 10].
An ideal bleeding risk prediction model should have the following functions: (1) identifying reversible bleeding factors and removing them to reduce bleeding risk; (2) identifying high-risk bleeding populations that require more frequent monitoring and follow-up; and (3) estimating the risk of individual bleeding during anticoagulant therapy. In addition, the model should be simple, operable, and easy to implement in daily clinical practice. When VTE is diagnosed, although the results of the bleeding risk prediction model do not affect the initiation of anticoagulation, they provide certain reference significance for clinical physicians to determine duration of anticoagulation, the type, and dosage of anticoagulant drugs. At the same time, clinical physicians are reminded to remove reversible bleeding risk factors as much as possible to minimize the risk of bleeding. At present, there are 8 types of VTE bleeding risk prediction models in clinical practice, as well as several bleeding risk prediction models derived from atrial fibrillation. Each model includes 3–18 risk factors, including demographic biology, clinical information, and biomarkers [4], but there is no consensus yet. The American College of Chest Physicians (ACCP) established a model based on 18 bleeding factors to predict the risk of major bleeding in the VTE disease antithrombotic treatment guidelines and expert consensus [9] (Table 1). The 2019 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism identified advanced age (> 75 years old), previous hemorrhagic diseases or anemia, cancer progression, history of cerebrovascular accidents, chronic kidney or liver disease, combination of antiplatelet or nonsteroidal anti-inflammatory drugs, concomitant severe acute and chronic diseases, and fluctuations in anticoagulation intensity as eight main risk factors used to predict bleeding risk [10].
| Bleeding risk stratification | Risk of major bleeding (%) | ||
|---|---|---|---|
| Low risk (0 risk factors) | Moderate risk (1 risk factor) | High risk (≥ 2 risk factors) | |
| 0–3 months | 1.6 | 3.2 | 12.8 |
| After 3 months | 0.8 | 1.6 | ≥ 6.5 |
- Note: There are 18 risk factors: (1) age > 65 years, (2) age > 75 years old, (3) history of previous bleeding, (4) malignancy, (5) metastatic tumor, (6) renal failure, (7) liver failure, (8) thrombocytopenia, (9) history of previous stroke, (10) diabetes mellitus, (11) anemia, (12) antiplatelet therapy, (13) poor anticoagulation control, (14) comorbidities or limited mobility, (15) recent surgery, (16) frequent falls, (17) alcohol abuse, and (18) nonsteroidal anti-inflammatory drugs.
3. Current Status of Anticoagulant Therapy–Related Bleeding Risk Prediction Models
3.1. HAS-BLED Bleeding Risk Prediction Model
The HAS-BLED bleeding risk prediction model (hypertension, abnormal renal/liver function, stroke, bleeding history or preparation, laboratory international normalized ratio, elderly, and drugs/alcohol consistency) consists of eight well-defined variables (Table 2), which are widely used in the field of atrial fibrillation and are recommended by multiple international atrial fibrillation anticoagulation guidelines [11]. Multiple studies have evaluated the effectiveness of the HAS-BLED model in VTE. The incidence of bleeding in the medium risk group was 7.5% (95% CI: 5.1%∼11.0%), and the incidence of major bleeding was 2.0% (95% CI: 0.9%∼4.3%) [12]. The incidence of bleeding in the high-risk group was 12.2% (95% CI: 7.6%∼18.8%), and the incidence of major bleeding was 2.7% (95% CI: 0.9%∼7.2%). Compared to models such as the ACCP bleeding risk prediction model and the modified outpatient bleeding risk index (mOBRI), its predictive value is higher (AUC = 0.68, 95% CI: 0.59%∼0.78%). Kooiman et al. [13] followed up 537 patients with acute VTE who received anticoagulation therapy for 6 months and found that the incidence of major bleeding was 1.3% (95% CI: 0.1%∼2.5%) in the non–high-risk group (HAS-BLED < 3) and 9.6% (95% CI: 2.2%∼17.0%) in the high-risk group (HAS-BLED ≥ 3) (p < 0.001), and HR = 8.7 (95% CI: 2.7–28.4). Among them, renal dysfunction (HR = 10.8, 95% CI: 1.9–61.7) and a history of bleeding (HR = 10.4, 95% CI: 2.5–42.5) were the strongest risk factors. The largest VTE cohort study currently included 132,280 patients and found that when HAS-BLED ≥ 4, the 6-month cumulative bleeding incidence was 7.3% (95% CI: 6.6%–8.0%), and the incidence of major bleeding was 3.1% (95% CI: 2.7%∼3.6%), significantly higher than the bleeding incidence at HAS-BLED ≥ 3 (5.3%, 95% CI: 4.9%∼5.6%, p < 0.001). Therefore, it can be seen that the model with the increased threshold has better predictive performance for bleeding risk. For every 1 point increase in the HAS-BLED score, the incidence of bleeding increases by 20%–30%. In addition, the study also found that tumors are an independent risk factor for bleeding and can serve as a supplement to the content of bleeding history (B) [14].
| Abbreviation | Risk factors | Score |
|---|---|---|
| H | Hypertension: systolic blood pressure > 160 mmHg (1 mmHg = 0.133 kPa). | 1 |
| A |
|
1 or 2 |
| S | History of stroke | 1 |
| B | History of bleeding: a history of previous bleeding and/or triggers of bleeding | 1 |
| L | Unstable international normalized ratio (INR): high INR or not in the therapeutic range (< 60%) | 1 |
| E | The elderly > 65 years old | 1 |
| D | Drugs: antiplatelet drugs, nonsteroidal anti-inflammatory drugs, and alcoholism | 1 or 2 |
- Note: Hazard stratification: Low hazard: 0 min; medium danger: 1∼2 min; high danger: 3∼9 min.
3.2. RIETE Bleeding Risk Prediction Model
The RIETE bleeding risk prediction model is a bleeding risk prediction scoring system developed based on data from the International Multicenter Registry (RIETE). The model includes 6 variables (Table 3) and has been internally validated in a cohort of over 19,000 VTE patients. However, the follow-up time was only 3 months, and external validation showed unsatisfactory predictive ability [15]. Zhang et al. [16] conducted a prospective observational study comparing four bleeding risk prediction models, including the RIETE model (Kuijer model, Kearon model, and Nieuwenhuis model), with AUC (95% CI) of 0.56 (0.45∼0.71), 0.57 (0.44∼0.68), 0.75 (0.60∼0.89), and 0.59 (0.41∼0.74) for predicting major bleeding or clinically relevant nonmajor bleeding within 3 months of anticoagulation therapy. Therefore, it is believed that the value of the RIETE bleeding risk prediction model is limited for acute VTE patients in China. A real-world study involving 82,239 VTE patients from 2001 to 2019 found that the RIETE bleeding risk prediction model had positive predictive values of 3.9%, 1.3%, 2.3%, and 1.7% for major bleeding within 30, 90, 180, and 360 days, respectively, while its negative predictive values were 98.7% (AUC = 0.71, 95% CI: 0.70–0.73), 99.5% (AUC = 0.70, 95% CI: 0.68–0.72), 99.7% (AUC = 0.69, 95% CI: 0.67–0.73), and 99.6% (AUC = 0.72, 95% CI: 0.68–0.72), which helps to confirm whether VTE patients can safely receive anticoagulant therapy [17]. Skowrońska et al. [18] found in another prospective observational study that RIETE > 4 has a certain predictive value for clinically related bleeding events during hospitalization (sensitivity of 60% and specificity of 80%) and found that D-dimer levels > 5750 ng/mL are important factors related to bleeding events (OR = 2.3, 95% CI: 1.05–5.00, p = 0.035). Combining RIETE > 4 and D-dimer levels > 5750 ng/mL can improve the efficacy of predicting bleeding events.
| Risk factors | Score |
|---|---|
| Age > 75 years | 1.0 |
| Recent bleeding | 2.0 |
| Tumor active phase | 1.0 |
| Abnormal renal function: creatinine > 106.08 μmol/L | 1.5 |
| Anemia: hemoglobin < 130 g/L (male), hemoglobin < 120 g/L (female). | 1.5 |
| Pulmonary embolism | 1.0 |
- Note: Low danger: 0 min; medium danger: 1.0–4.0 min; high danger: > 4 min.
3.3. VTE-BLEED Bleeding Risk Prediction Model
The VTE-BLEED bleeding risk prediction model is developed based on the analysis of data from two randomized controlled studies, RE-COVER and RE-COVER II, consisting of six clinical variables (Table 4). The variable definition is clear, stable, and easy to apply, and the results are objective. The low high risk binary risk stratification avoids the fuzzy zone of “moderate risk” and can assist clinical physicians in making anticoagulation decisions. During internal validation, the VTE-BLEED bleeding risk prediction model showed good predictive performance for major bleeding events after 30 days, whether targeting direct oral anticoagulant (DOAC) or warfarin treatment. In the DOAC group, the incidence of major bleeding in high-risk patients was 6.5 times higher than that in low-risk patients (OR = 6.5, 95% CI: 2.0–21), and the same results were obtained in the warfarin group (OR = 6.5, 95% CI: 2.8–15). The Hokusai-VTE study externally validated the VTE-BLEED bleeding risk prediction model and found that the incidence of major bleeding in high-risk patients was significantly higher than that in low-risk patients (OR = 4.04, 95% CI: 2.51–6.48), and the warfarin group was more pronounced (OR = 5.04, 95% CI: 2.62–9.69) [19, 20]. The XALIA real-world study followed up VTE patients who received rivaroxaban or low molecular weight heparin-bridged warfarin anticoagulation for 12 months and found that for every one point increase in the VTE-BLEED score, the incidence of major bleeding increased by 1.4 times (95% CI: 1.2–1.7). Major bleeding was not related to age, gender, or anticoagulant drugs, but this characteristic was not found in tumor-related VTE patients. The consistency index of major bleeding events increased from 0.68 to 0.71 after 3 months, indicating that the model is more suitable for predicting the risk of major bleeding in long-term (> 6 months) anticoagulant therapy patients, especially those with unexplained VTE [21]. A larger scale COMMAND VTE registration study compared the 5-year cumulative incidence of major bleeding between the high-risk and low-risk groups of VTE-BLEED and found that the high-risk group had significantly higher rates of major bleeding and fatal bleeding than the low-risk group (13.2% vs. 5.4%, p < 0.001; 4.1% vs. 1.4%, p = 0.073), indicating that the model can effectively identify high-risk populations who undergo long-term (> 6 months) anticoagulation for major bleeding [22].
| Risk factors | Score |
|---|---|
| Active tumors | 2.0 |
| Men with uncontrolled blood pressure | 1.0 |
| Anemia | 1.5 |
| History of bleeding | 1.5 |
| Renal dysfunction (creatinine clearance 30∼60 mL/min) | 1.5 |
| Age ≥ 60 years | 1.5 |
- Note: Low risk: 0–1.5 points; high risk: ≥ 2 points.
It is worth mentioning that some studies have used the VTE-BLEED bleeding risk prediction model to evaluate the recurrence risk of VTE. PADIS-PE is a randomized, double-blind, placebo-controlled study using the VTE-BLEED bleeding risk prediction model to explore the recurrence risk of VTE after completing 6 months of anticoagulation and 24 months of anticoagulation for non–tumor-related VTE. It was found that after discontinuing anticoagulation, there was no statistically significant difference in the recurrence rate of thrombosis between the high-risk and low-risk VTE-BLEED groups (16.4% vs. 14.6%, HR = 1.16, 95% CI: 0.62∼2.19). This result has certain significance for determining the optimal duration of anticoagulation [23].
3.4. Comparison of Existing Anticoagulant Therapy–Related Bleeding Risk Prediction Models
At present, the three widely used bleeding risk prediction models in clinical practice (HAS-BLED, RIETE, and VTE-BLEED) have their own characteristics. Some scholars believe that HAS-BLED and RIETE bleeding risk prediction models can help identify individuals with high bleeding risk in the near future (< 3 months) during initial anticoagulation, while VTE-BLEED bleeding risk prediction models have advantages in evaluating bleeding risk in the extended stage (> 6 months) [24]. In addition, the selection of existing models should also consider their operability, such as RIETE and VTE-BLEED bleeding risk prediction models with simple content and clear variable definitions, which are easy to implement and promote in anticoagulant clinics. The ACCP bleeding risk prediction model contains a lot of content and is suitable for evaluation during hospitalization. The research on the HAS-BLED bleeding risk prediction model includes the tumor population, which seems to be more suitable for patients with tumor-related thrombosis. de Winter et al. [25] compared the predictive performance of various bleeding risk prediction models, including ACCP, HAS-BLED, RIETE, and VTE-BLEED, in the VTE population and found that existing bleeding risk prediction models cannot be relied on to make decisions to stop or extend anticoagulation after 3 months. Overall, although the bleeding risk prediction model based on clinical factors has certain predictive significance for bleeding events, there is still a certain gap compared to the ideal model [4]. It is worth noting that such bleeding risk prediction models have high negative predictive values, such as RIETE and HAS-BLED, which have a greater exclusion value and are more valuable in assisting in determining the safety of long-term anticoagulation therapy for VTE patients, especially those at high risk of thrombosis recurrence. In addition, it should be pointed out that the concept of dynamic evaluation is more important than evaluating the quality of a bleeding risk prediction model. The guidelines suggest that low bleeding risk patients should undergo an annual bleeding risk assessment, while high bleeding risk patients should undergo a dynamic evaluation of bleeding risk every 3–6 months [1].
4. A New Model for Predicting the Risk of Bleeding Related to Anticoagulant Therapy
4.1. PE-SARD Bleeding Risk Prediction Model
The PE-SARD (The Syncope, Anemia, Renal Dysfunction Bleeding Score) bleeding risk prediction model developed by Chopard et al. [26] was derived from a multicenter prospective registration study (BFC-FRANCE registration) of bleeding events during hospitalization (within 30 days). Three major risk factors for major bleeding were identified through a multivariate regression model, including anemia (OR = 3.89, 95% CI: 2.41–6.28), syncope (OR = 2.32, 95% CI: 1.28∼4.21), and renal insufficiency (OR = 1.74, 95% CI: 1.08∼2.81), and were assigned weights, respectively (Table 5). The incidence of bleeding gradually increases according to the assessed risk level, with only 0.97% (95% CI: 0.53%∼1.62%) in the low-risk group, 8.93% (95% CI: 6.15%∼12.44%) in the high-risk group, and a consistency index of 0.74 (95% CI: 0.73∼0.76). The PE-SARD bleeding risk prediction model is the first prediction model for bleeding risk in acute phase PTE (within 30 days). Compared with other existing models, it is simple and clear and has better predictive performance for early major bleeding risk. The next step is to determine its clinical application value for acute PTE patients through external validation.
| Risk factors | Score |
|---|---|
| Swoon | 1.5 |
| Anemia: hemoglobin < 120 g/L | 2.5 |
| Renal insufficiency: Creatinine clearance < 60 mL/min | 1.0 |
- Note: Low risk: 0 points; medium risk: 1.0–2.5 points; high risk: > 2.5 points.
4.2. ABC Bleeding Risk Prediction Model
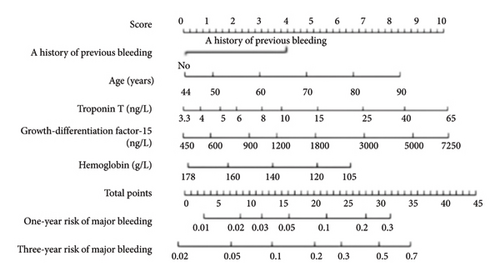
The N-terminal brain natriuretic peptide precursor (NT proBNP), highly sensitive cardiac troponin T (hs cTnT), inflammatory markers such as interleukin-6 and C-reactive protein, growth differentiation factor-15 (GDF-15), vitamin E, hemophilia factor, and gene polymorphism have all been confirmed to be associated with increased bleeding risk in patients with cardiovascular disease [24]. Hijazi et al. [27] found that GDF-15, hemoglobin concentrations were the most important biomarkers for predicting large bleeding. Combining age and past bleeding history, they developed an ABC bleeding risk prediction model for atrial fibrillation anticoagulation (Figure 1), with a higher consistency index than the HAS-BLED and ORBIT bleeding risk prediction models (0.68 (95% CI: 0.66–0.70) compared to 0.61 (95% CI: 0.59–0.63), p < 0.001). Compared to 0.65 (95% CI: 0.62∼0.67, p < 0.001), external validation also showed advantages [0.71 (95% CI: 0.68∼0.73), 0.62 (95% CI: 0.59∼0.64), p < 0.001, 0.68 (95% CI: 0.65∼0.70), p = 0.002]. In addition, the improved ABC bleeding risk prediction model (hematocrit, high sensitivity cardiac troponin I, cystatin C, or creatinine clearance) has better predictive performance than HAS-BLED and ORBIT bleeding risk prediction models. The ABC bleeding risk prediction model has been used to assist atrial fibrillation patients in making anticoagulation decisions. At present, there is no bleeding risk prediction model based on VTE specific biomarkers, but the ABC bleeding risk prediction model may also be a promising model for VTE patients [24].
5. Conclusion
The precise management of VTE patients not only requires active anticoagulation therapy to reduce the risk of thrombosis recurrence but also to avoid bleeding caused by anticoagulation. It is necessary to weigh the pros and cons between the two. Therefore, clinical decision-making requires accurate prediction of bleeding risk. Although multiple bleeding-related risk factors have been identified, there is currently no ideal bleeding risk prediction model. The overall predictive performance of existing models is poor, and some have not been validated in prospective studies. However, they can still provide a large amount of key information to assist clinical physicians in identifying and removing reversible bleeding risk factors as much as possible. At the same time, through dynamic evaluation, it can minimize anticoagulant therapy–related bleeding events. The future exploration direction should focus on bleeding risk prediction models based on biological markers.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors have made significant contributions to this study and have read and approved the final version of the manuscript.
Funding
There was no funding to our paper or any financial support.
Open Research
Data Availability Statement
The data used in this review are available in the cited literature.




