The Impact of Silymarin on Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
Numerous studies have investigated into the antioxidant and anti-inflammatory properties of silymarin, as well as its ability to reduce reactive oxygen species and inflammation biomarkers. The effect of silymarin on inflammation and oxidative stress was investigated using the keywords “milk thistle” OR “Silybum marianum” OR “Silybum” OR “silymarin” OR “Silibinin” AND “MDA” OR “Malondialdehyde” OR “TAC” OR “total antioxidant capacity” OR “IL-10” OR “Interleukin-10” OR “IL-6” OR “Interleukin-6” OR “TNF-α” OR “tumor necrosis factor alpha” on the Web of Science, Scopus, Embase, PubMed, and Cochrane Library, up until March 2023. Data were combined using a random-effects model, and the weighted/standardized mean differences (WMDs/SMDs) with a 95% confidence interval (CI) were used as the overall effect size. Our meta-analysis indicated that silymarin supplementation resulted in a significant decrease in levels of C-reactive protein (CRP) (WMD: −3.39 mg/L, 95% CI: −5.99, −0.79, p = 0.01) and malondialdehyde (MDA) (SMD: −1.69, 95% CI: −2.62, −0.76, p < 0.001). Furthermore, silymarin significantly increases IL-10 (WMD: 2.03 pg/mL; 95% CI: 1.04, 3.01, p < 0.001), superoxide dismutase (SOD) (SMD: 3.39, 95% CI: 1.42, 5.37, p = 0.001), and glutathione peroxidase (GPx) (SMD: 1.94; 95% CI, 0.89 to 2.99; p < 0.001) level. However, silymarin supplementation did not have significant effects on TAC (SMD: 2.91; 95% CI: −0.30, 6.11, p = 0.076) and IL-6 (WMD: −0.70 pg/mL; 95% CI: −1.42, 0.02, p < 0.056) level. Silymarin supplementation may significantly improve oxidative stress and inflammation in adults by decreasing CRP and MDA and increasing IL-10, SOD, and GPx. However, additional studies with longer study periods are required to ascertain the long-term effects of silymarin on oxidative stress and chronic inflammation.
1. Introduction
In the pathogenesis of chronic diseases, namely diabetes, cardiovascular diseases (CVDs), obesity, and metabolic syndrome, inflammation plays a significant role [1]. Various studies have demonstrated that elevated levels of inflammatory biomarkers, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), have a substantial impact on the occurrence of ischemic events [2]. On the other hand, a positive correlation has been observed between elevated levels of inflammatory biomarkers and endothelial dysfunction. IL-6, a proinflammatory cytokine, is secreted by various cells including leukocytes [3, 4]. This cytokine serves as an intermediary for initiating inflammatory processes in blood vessels. Additionally, some studies have reported that adiponectin possesses anti-inflammatory and antiatherogenic properties [5]. Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the capacity of antioxidant defense mechanisms to counteract them. Extensive research has demonstrated that oxidative stress plays a pivotal role in the onset of over 100 diverse illnesses and disorders within the human body [6]. Superoxide anion is a biologically important ROS, with a recognized capacity to generate other toxic species (such as hydrogen peroxide, peroxynitrite, and peroxynitrite decomposition products) leading to oxidative stress [7]. Superoxide dismutase (SOD) is widely recognized as the primary physiological scavenger responsible for neutralizing superoxide radicals. This remarkable enzyme efficiently transforms superoxide into hydrogen peroxide. Subsequently, the hydrogen peroxide undergoes further conversion to water (H2O) through the collaborative efforts of glutathione peroxidase (GPx) and catalase [8]. Several existing studies have demonstrated the protective effects of prescribing SOD exogenous gene against a range of abnormalities, including cancer and CVD. Antioxidant enzymes can enhance the interaction of nuclear factor erythroid 2-related factor 2 (Nrf2) with antioxidant response element (ARE) and reduce the expression of nuclear factor kappa B (NF-Κb) [9]. These positive effects have drawn increasing attention to the prescription of antioxidants such as SOD, which serve as strong antioxidants and anti-inflammatory drugs for human diseases. The use of herbal compounds and nutrients in the prevention and management of oxidative stress and the diseases caused by it has been the focus of various researchers in recent years [10]. Many studies indicate that medicinal plants like silymarin and antioxidant supplements can reduce inflammation and oxidative stress [11]. Silymarin, also known as “Milk Thistle,” is a plant that has been recognized since ancient times for its effectiveness in treating diseases such as diabetes and CVDs, among others [12]. Silymarin has been reported to have anti-inflammatory and antioxidant activities, as well as protective effects against endothelial dysfunction, in both in vitro and in vivo studies [13]. Nieuwenhuizen et al. [14] have concluded that silymarin possesses protective effects against lung injury. They found that silymarin is capable of reducing the production of nitric oxide, infiltration of inflammatory cells, myeloperoxidase activity, and the levels of proinflammatory mediators including catalase, superoxide, dismutase, and glutathione (GSH). Furthermore, in 2015, Ebrahimpour-Koujan et al. [15] conducted a study to investigate the impact of silymarin on diabetic patients. The findings of the study demonstrated that silymarin supplementation for duration of 45 days improved certain antioxidant indicators such as SOD and GPx and reduced the levels of high-sensitivity C-reactive protein (hs-CRP) in diabetic patients. Although the association between silymarin consumption and inflammatory biomarkers and oxidative stress has been well established, the results of studies are contradictory. Given the detrimental impact of inflammation and oxidative stress on health, as well as their role in various inflammatory conditions like cancer and rheumatoid arthritis, it is crucial to comprehend how effective doses of silymarin can influence inflammatory factors and oxidative stress levels. Moreover, due to the limited research on the link between silymarin and inflammation plus oxidative stress, the primary objective of this study is to assess the impact of silymarin on inflammatory markers (such as TNF-α, IL-6, IL-10, and CRP) and oxidative stress indicators (including SOD, GPx, malondialdehyde (MDA), and TAC) through a comprehensive analysis.
2. Method
This study employed a methodical review and meta-analysis approach, following the established guidelines outlined in the PRISMA statement criteria (Supporting Table 1). The review protocol for this study has been appropriately recorded in the PROSPERO database of Systematic Reviews, under the registration number: CRD42022353487.
2.1. Search Strategy
The study’s search strategy was carried out on the Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria. To identify relevant studies, multiple international databases such as the Embase, Web of Science, PubMed, Scopus, and Cochrane Library were searched without any time limitations up until March 2024. The search terms used included both non-MESH and MESH terms related to the topic. Key phrases such as “milk thistle” [non-MESH], “Silybum marianum” [non-MESH], “Silybum” [non-MESH], “silymarin” [MESH], “Silibinin” [MESH], “MDA” [MESH], “malondialdehyde” [MESH], “TAC” [non-MESH], “total antioxidant capacity” [MESH], “IL-10” [MESH], “interleukin-10” [MESH], “IL-6” [MESH], “interleukin-6” [MESH], “TNF-α” [MESH], and “tumor necrosis factor alpha” [MESH] were searched. Furthermore, we conducted a comprehensive examination of the citation lists of eligible articles to guarantee that no pertinent studies were excluded from our review. Also gray literature was explored to fill any possible gap of unpublished studies.
2.2. Eligibility Criteria
In order to perform this meta-analysis, a careful selection process was undertaken to identify the studies for analysis. This involved the application of specific criteria for inclusion and exclusion. Initially, two researchers named A.K. and M.V. carried out an initial assessment of the papers to determine their potential eligibility for inclusion in the study. The titles and abstracts of all identified studies were carefully assessed based on the predetermined selection criteria. Subsequently, full-text versions of publications that appeared to meet the eligibility requirements were retrieved and evaluated. Ultimately, only randomized controlled trials (RCTs) that examined the effects of silymarin supplementation on inflammation and stress oxidative markers were chosen for analysis. The RCTs could have been structured with either parallel or cross-over designs. In instances where disagreements arose during the process of selecting studies, a third researcher (S.A.) was involved to facilitate open discussions and achieve a consensus. The qualified research studies for this examination were required to fulfill all of the subsequent inclusion requirements: (1) human experimental research studies ; (2) research studies with parallel or crossover structure; (3) research studies carried out on individuals (≥ 18 years); (4) silymarin consumption as an interference (without mixed treatment); (5) research studies contemplating a suitable control set as a comparator; and (6) reporting the changes in markers of inflammation such as CRP, IL-6, IL-10, TAC, MDA, GPx, and SOD during the intervention or at the beginning and end of it.
2.3. Exclusion Criteria
Exclusion criteria from this review include (1) animal studies; (2) in vivo studies; (3) studies conducted on individuals under 18 years old; (4) noninterventional studies, including observational studies, review articles, short communications, and letters to the editor; and (5) studies without a suitable control group.
2.4. Data Extraction
During our analysis, we collected the following information from each study: the name of the first author, the year of publication, the trial location (country), the type of study, the dose of silymarin supplement, and the duration of the intervention. In case we needed clarification or additional data, we contacted the respective authors via email. To minimize any potential errors, two authors (M.V. and A.K.) independently gathered the data. In instances where disagreements arose, we relied on consensus-based discussions to reach resolutions. Our primary outcomes for the selection of articles include inflammation (CRP, IL-6, and IL-10) and secondary outcomes (MDA, TAC, GPx, and SOD) include oxidative stress.
2.5. Risk of Bias Assessment
The risk of bias assessment was done independently by two reviewers (M.V. and A.K.). Any disagreement was resolved by consensus. To assess the quality of the included article, the updated Cochrane risk-of-bias for randomized trials method was used [16]. The following domains were evaluated: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other source of bias. The terms are defined as follows: (1) Good, if all domains are assessed as having “low risk”; (2) Fair, if one or two domains are assessed as having “unclear risk”; and (3) Poor, if one or more domains are assessed as having “high risk” or if more than three domains are assessed as having “unclear risk”.
2.6. Statistical Analysis
The statistical analyses for this study were conducted using Stata software Version 14 (Stata Crop, College Station Texas, USA). DerSimonian and Laird random-effects models were employed to evaluate the overall effect sizes [17] and were expressed as the weighted/standardized mean differences (WMDs/SMDs) with a 95% confidence interval (CI). To calculate the standard deviations (SDs) of the mean differences, the following equation was used: SD change = square root [(SD baseline)2 + (SD final)2 − (2 × 0.98 × SD baseline × SD final)] in cases where they were not reported. For trials that reported the standard error of the mean (SEM), SD was calculated using the formula: SD = SE × square root (n), where n represents the number of subjects in each group. Statistical heterogeneity between studies was assessed by Cochrane’s Q test and I2 index (significance point at I2 > 50%). The random-effects model was chosen over a fixed-effects model based on the assumption that there is likely to be clinical and methodological heterogeneity among trials. The results of random- and fixed-effects models are the same in analyses with heterogeneity, and if heterogeneity is present, the random-effects model will generally be considered more conservative. To determine potential sources of heterogeneity, a subgroup analysis was performed using pre-established variables. The analysis took into account various factors such as CRP, IL-6, IL-10, TAC, and MDA including dosage (< 420 vs. ≥ 420 mg/day), treatment duration (< 2 vs. > 12 weeks), age (< 45 vs. > 45 years), quality of studies (good, fair, and poor), and health status (diabetes, thalassemia, and others). A sensitivity analysis was carried out by systematically excluding one study at a time to evaluate its impact on the overall effect size. Funnel plots and Egger’s test were used to assess publication bias for 153 the outcome with ≥ 10 studies [18]. We include Supporting Table 2 from describing Funnel plots and Egger’s test in the Supporting section of the manuscript.
3. Results
3.1. Study Selection
In our primary search, we identified a total of 957 studies; 256 duplicates were identified and removed using EndNote 9. After screening based on title and abstract, 42 studies were retained for further assessment. In the next step, 26 studies were excluded based on the full-text review. Finally, 16 papers were included in this meta-analysis [19–34] (Figure 1).
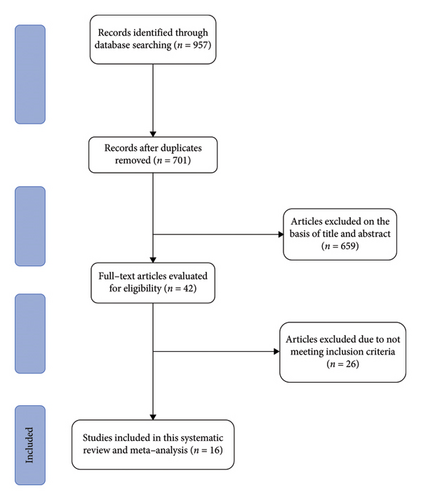
3.2. Characteristics of the Included Trials
The summary of the general characteristics of included studies is shown in Table 1. These trials were conducted in Iran, Italy, Iraq, Thailand, Australia, and Pakistan [19, 23, 25, 26, 28, 38]. Included studies were published between 1997 and 2022. A total of 1306 individuals (522 subjects in the silymarin group and 514 in the control group) with a mean age of 22–63 years are included in the current meta-analysis. Most included studies were conducted on both genders, while one study was conducted on males. The dosage of silymarin ranged from 140 to 600 mg per day, and intervention duration varied between two and 48 weeks. All included trials are carried out in different target population including diabetes, painful knee osteoarthritis hemodialysis, β-thalassemia, pulmonary tuberculosis, multiple sclerosis, hepatitis C, and healthy subjects [20, 23, 34, 35, 39, 40].
| Author (ref) | Year/location | Subjects | Participants | Gender (male/female) | Mean age | Design | Supplement | Comparator | Dose (mg/day) | Duration (week) | Main results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | ||||||||||
| Velussi et al. [25] | 1997/Italy | Insulin-treated diabetic patients | 30 | 30 | Both | 63 | 62 | RPC | Silymarin | Not treated | 600 | 48 | Sig. decrease in MDA |
| Hussain et al. [24] | 2009/Iraq | Painful knee OA patients | 40 | 35 | Both | — | — | RDBPC | Silymarin + piroxicam | Piroxicam | 300 | 8 | Sig. decrease in IL-8 |
| Roozbeh et al. [33] | 2011/Iran | Hemodialysis patients | 20 | 20 | Both | 50 | 44 | RPC | Silymarin | Not treated | 420 | 3 | Sig. increase in GPx |
| Fallahzadeh et al. [30] | 2012/Iran | Type 2 diabetic patients with overt nephropathy | 30 | 30 | Both | 55.9 | 57.6 | RDBPC | Silymarin | Placebo | 420 | 12 | Sig. decrease in MDA |
| Balouchi et al. [27] | 2014/Iran | Homozygous β-thalassemia major patients | 13 | 9 | Both | 22.62 | 23.77 | RDBPC | Silymarin | Placebo | 420 | 24 | Sig. decrease in IL-10 |
| Ebrahimpour-Koujan et al. [15] | 2014/Iran | T2DM patients | 20 | 20 | Both | 43.5 | 46.1 | RDBPC | Silymarin | Placebo | 420 | 6.42 | Sig. increase in SOD, GPx, and TAC, sig. decrease in hs-CRP and MDA |
| Luangchosiri et al. [31] | 2015/Thailand | Patients with pulmonary tuberculosis | 27 | 28 | Both | 56 | 51.5 | RDBPC | Silymarin | Placebo | 420 | 4 | Sig. decrease in SOD |
| Darvishi-Khezri et al. [35] | 2017/Iran | Patients with β-thalassemia major | 37 | 37 | Both | 20.4 | 20.4 | Randomized, triple-blind, crossover trial | Silymarin | Placebo | 420 | 12 | Sig. decrease in MDA, sig. increase in TAC |
| Vidimce et al. [36] | 2021/Australia | Healthy subjects | 17 | 17 | Male | 31.8 | 31.8 | Randomized, single-blind, placebo-controlled crossover trial | Silymarin | Placebo | 420 | 2 | NS |
| Ghiasian et al. [22] | 2021/Iran | Patients with relapsing–remitting multiple sclerosis | 24 | 24 | Both | 34.9 | 33.7 | RDBPC | Silymarin | Placebo | 140 | 24 | Sig. decrease in MDA, sig. increase in TAC |
| Ahmed et al. [37] | 2022/Pakistan | Hepatitis C patients | 15 | 15 | Both | Randomized miniature clinical trial | Silymarin | Not treated | 400 | 8 | Sig. decrease in MDA, sig. increase in SOD | ||
| Elgarf et al. [28] | 2015/Egypt | Parallel, R, PC, SB | 20 | 20 | Both | 51.5 | 51.5 | Randomized, and placebo-controlled, single-blinded, pilot study | Silymarin | Placebo | 420 | 12.85 | Sig. decrease in MDA, hs-CRP, increase in SOD |
| Mirzaei et al. [41] | 2021/Iran | Parallel, R, PC, DB | 45 | 45 | Both | 52.02 | 52.02 | Randomized, double-blinded, placebo-controlled clinical trial | Silymarin | Placebo | 420 | 2 | Sig. decrease in MDA, increase in TAC |
| Mirzaei et al. [29] | 2022/Iran | Parallel, R, PC, DB | 35 | 35 | Female | 36.49 | 36.49 | Phase II, double-blind, randomized, placebo-controlled trial | Silymarin | Placebo | 280 | 12 | Sig. decrease in IL-6 |
| Darvishi-Khezri et al. [19] | 2021/Iran | Crossover, R, PC, TB | 69 | 69 | Both | 20.4 27.7 | 20.4 27.7 | Phase II, double-blind, randomized, placebo-controlled trial | Silymarin | Placebo | 420 | 12 | Sig. decrease = significantly decrease |
| Rajendra et al. [32] | 2022/India | Fatty liver index (FLI) between 31 | 28 | 28 | Both | 40.2 | 40.2 | Randomized, triple-blind, crossover trial | Silymarin | Placebo | 320 | 12 | Sig. decrease MDA Sig. increase SOD |
3.3. Quality Assessment
Table 2 presents the details of the quality of each trial. The risk of bias for included studies was evaluated by the Cochrane collaboration’s assessment tool. As represented in Table 2, 16 studies were assessed and 10 studies were considered as low risk, 3 of them were in category of moderate risk, and 3 of them had high risk of bias.
| Author (year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other source of bias | Overall quality |
|---|---|---|---|---|---|---|---|---|
| Velussi et al. [25] (1997) | L | L | L | L | L | L | L | G |
| Hussain et al. [24] (2009) | L | L | L | L | L | L | L | G |
| Roozbeh et al. [33] (2011) | L | L | H | H | L | L | L | P |
| Fallahzadeh et al. [30] (2012) | L | L | L | L | L | L | U | F |
| Balouchi et al. [27] (2014) | L | L | L | L | L | L | L | G |
| Ebrahimpour-Koujan et al. [15] (2015) | L | L | L | L | L | L | L | G |
| Luangchosiri et al. [31] (2015) | L | L | H | H | L | L | L | P |
| Darvishi-Khezri et al. [35] (2017) | L | L | L | L | L | L | L | G |
| Vidimce et al. [36] (2021) | L | L | L | L | L | L | U | F |
| Ghiasian et al. [22] (2021) | L | L | U | H | L | L | L | P |
| Ahmed et al. [37] (2022) | L | L | H | H | L | L | L | P |
| Elgarf et al. [28] (2015) | L | L | L | L | L | L | L | G |
| Mirzaei et al. [41] (2021) | L | L | L | L | L | L | L | G |
| Mirzaei et al. [29] (2022) | L | L | L | L | L | L | L | G |
| Darvishi-Khezri et al. [19] (2021) | L | L | U | U | L | L | L | F |
| Rajendra et al. [32] (2022) | L | L | L | L | L | L | L | G |
- Abbreviations: F = fair, G = good, H = high, L = low, and P = poor.
3.4. Effect of Silymarin Supplementation on CRP
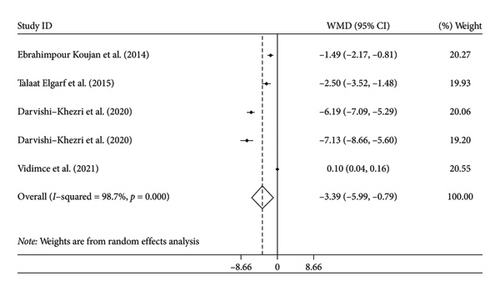
Four studies with five arms reported changes in CRP as an outcome measure. We combined the findings using the random-effects model and showed significant reduction in CRP following silymarin intervention (WMD: −3.39 mg/L, 95% CI: −5.99, −0.79, p = 0.01) (Figure 2(a)). There was significant heterogeneity among the trials (I2 = 98.7%, p < 0.01). Subgroup analysis showed that health status was potential source of heterogeneity. Moreover, subgroup analysis revealed that in studies with duration longer than 12 weeks, studies with good quality, and those conducted on patients with thalassemia, silymarin could further reduce CRP levels.
3.5. Effect of Silymarin Supplementation on IL-10
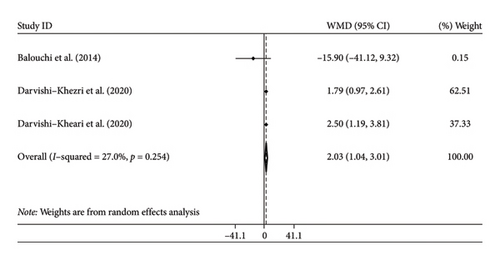
Two studies with three arms reported changes in IL-10 as an outcome measure. We combined the findings using the random-effects model and showed significant increase in IL-10 following silymarin intervention (WMD: 2.03 pg/mL; 95% CI: 1.04, 3.01, p < 0.001) (Figure 2(b)). There was insignificant heterogeneity among the trials (I2 = 27%, p = 0.254). We could not perform subgroup analysis by age for IL-10 levels due to the limited studies.
3.6. Effect of Silymarin Supplementation on IL-6
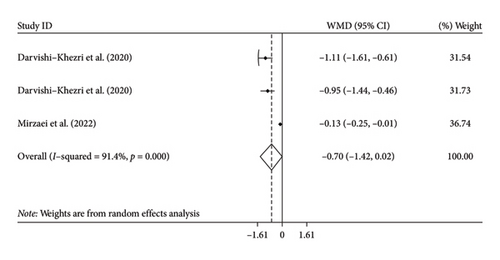
Two studies with three arms examined the effects of silymarin supplementation on IL-6 levels. However, the results showed that silymarin supplementation did not significantly decrease IL-6 levels compared to the control group (WMD: −0.70 pg/mL; 95% CI: −1.42, 0.02, p = 0.056) (Figure 2(c)). Additionally, there was a significant amount of variation among the studies, indicating heterogeneity (I2 = 91.4%, p < 0.001). We could not perform subgroup analysis by age for IL-6 levels due to the limited studies.
3.7. Effect of Silymarin Supplementation on MDA
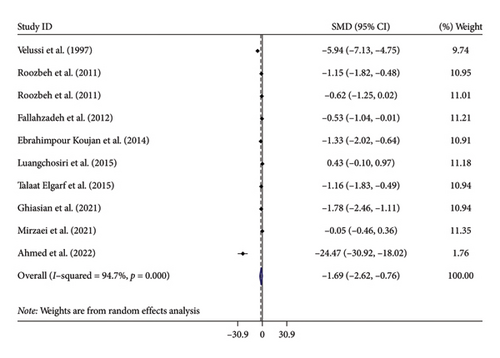
Nine studies with 10 arms examined the effects of silymarin supplementation on MDA levels. The combined findings from the random-effects model demonstrated a significant decrease in MDA following the administration of silymarin (SMD: −1.69, 95% CI: −2.62, −0.76, p < 0.001). However, there was substantial heterogeneity observed among the studies (I2 = 94.7%, p < 0.001) (Figure 3(a)). Subgroup analysis showed that age and health statuses were potential sources of heterogeneity. Moreover, subgroup analysis indicated that silymarin could further decrease MDA levels in studies that met the following criteria: a duration surpassing 12 weeks, studies with good quality, a dosage less than 420 mg/day, and those involving patients with diabetes. No publication bias was detected with Egger test or by inspection of the funnel plot (p = 0.643) (Supporting Figure 1).

3.8. Effect of Silymarin Supplementation on SOD
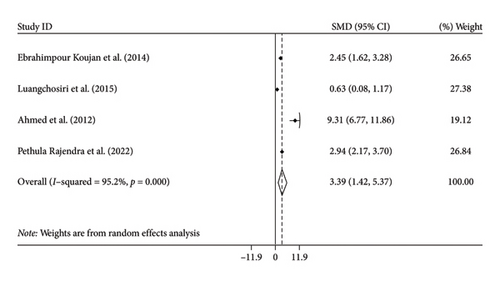
Pooling effect sizes from four trials, we reached a significant effect of silymarin intake on SOD (SMD: 3.39, 95% CI: 1.42, 5.37, p = 0.001) with significant heterogeneity among the trials (I2 = 95.2%, p < 0.001) (Figure 3(b)). We could not perform subgroup analysis by age for SOD levels due to the limited studies.
3.9. Effect of Silymarin Supplementation on TAC
Four different research studies examined the effects of silymarin supplementation on TAC levels. However, the results showed that silymarin supplementation did not significantly increase TAC levels compared to the control group (SMD: 2.91; 95% CI: −0.30, 6.11, p = 0.076) (Figure 3(c)). Additionally, there was a significant amount of variation among the studies, indicating heterogeneity (I2 = 95.2%, p < 0.001). Furthermore, the subgroup analysis suggested that in studies with duration of less than 12 weeks and studies with good quality, silymarin could potentially enhance TAC levels.
3.10. Effect of Silymarin Supplementation on GPx
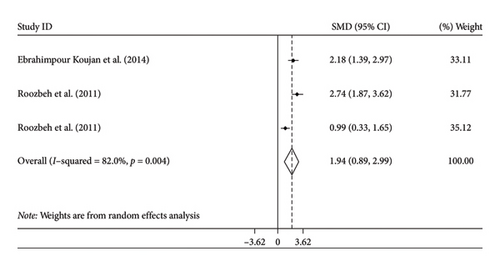
The combined estimation obtained from the random-effects model demonstrated a significant impact on GPx levels following the supplementation of silymarin, in comparison to the control group (SMD: 1.94; 95% CI, 0.89 to 2.99; p < 0.001). However, there was noticeable heterogeneity in the results (I2 = 82%, p = 0.004) (Figure 3(d)). We could not perform subgroup analysis by age for SOD levels due to the limited studies.
3.11. Sensitivity Analysis
Each study was a step-wisely removed from the overall analysis, to determine the effect of individual studies on the combined effect size. No single study had a significant effect on the combined effect size of CRP, MDA, SOD, TAC, and GPx. However, the effect of silymarin on IL-10 and IL-6 was significantly changed after removing studies by Darvishi-Khezri et al. [19] (WMD = −2.21; 95% CI: −17.95, 3.52) and Mirzaei et al. [29] (WMD = −1.02; 95% CI: −1.37, −0.68) (Supporting Figure 2).
4. Discussion
The present systematic review and meta-analysis compiled findings from 16 eligible studies examining the impact of silymarin on and inflammation and stress oxidative stress. Our results showed that silymarin could reduce the inflammation and oxidative stress induces such as CRP and MDA and increase IL-10, SOD, and GPx. Various antioxidant supplements have been introduced as a reasonable strategy to inhibit the deleterious effects of different oxidants [33]. Silymarin, a polyphenolic flavonolignan that is extracted from ripe seeds of Silybum marianum or milk thistle, has shown anti-inflammatory and antioxidant effects in previous animal studies [42–44]. The present study reviewed 11 clinical trials to assess the effects of silymarin on inflammatory and antioxidative features in humans. We found a significant decrease in CRP and MDA as well as a remarkable increase in IL-10, SOD, and GPx after silymarin supplementation. However, we failed to find any significant effect of silymarin intake on TAC and IL-6 levels. None of the mentioned variables were significantly affected by silymarin supplementation after performing sensitivity analysis.
We found a significant reduction in CRP after silymarin supplementation which was in line with previous clinical trials [15, 19]. Moreover, studies with more than 60 participants and in thalassemia patients showed more decreasing effect. However, no significant effect was found about the effect of silymarin supplementation in IL-6 which was in accord with previous studies [19]. According to previous studies, silymarin has a potential inhibitory effect in proinflammatory cytokine production resulting from suppressing different inflammatory pathways [45, 46]. Enzymatic peroxidation by lipoxygenase and cyclooxygenase as a key pathway in inflammation could be suppressed by silymarin and therefore 5-lipoxygenase products like leukotrienes could be decreased [47, 48]. A previous study by Kang et al. showed that silymarin could prevent the activation of NF-ƙB/Rel transcription factor resulting from suppressing IƙBα phosphorylation in mouse macrophages and RAW 264.7 cells [49]. Another study in human histiocytic lymphoma U-937 cells found the suppressive effect of silymarin in the activation of MEK and JNK signaling pathways stimulated by TNF-α [45]. Since NF-ƙB transcription factor and MEK and JNK signaling pathways could lead to various inflammatory mediator syntheses, silymarin could affect CRP through these mechanisms [15, 49]. Regarding IL-6, there were limited number of studies which impeded performing subgroup analysis. On the other hand, sensitivity analysis revealed that removing the study by Darvishi-Khezri et al. changed the results of IL-6. Therefore, more studies are required to reach final accurate results about IL-6 [19].
IL-10 is an immunomodulatory and anti-inflammatory cytokine and inhibitory factor for human cytokine synthesis [50]. Based on our results, IL-10 significantly increased after supplementation with silymarin which was in agreement with one previous clinical trial [19]. On the contrary, a significant reduction in IL-10 levels was found in the Balouchi et al. study which could be due to the small sample size and intervening effect of desferrioxamine combination therapy [27]. Silymarin could probably apply its anti-inflammatory effect by inhibiting NF-ƙB proinflammatory cascade which could upregulate IL-10 expression [51, 52]. Significant reduction in MDA levels was found after silymarin supplementation in the present study which was the most in studies with < 420 mg/d silymarin supplementation. This decreasing effect by silymarin could be due to its inhibitory effect on lipid peroxidation [53] and could result in increased amounts of intracellular GSH and hepatoprotective effects [22, 54]. Our findings were in accord with the results of 6 previous clinical trials that showed silymarin supplementation decreases MDA levels compared to placebo in various study populations [15, 22, 25, 30, 35, 37].
The present pooled study found significant SOD improvement after silymarin supplementation which had the same results as 2 previous trials [15, 37] and was in contrast with the results of a study by Luangchosiri et al. [55]. The last mentioned study had been conducted in tuberculosis patients who had imbalanced SOD status induced by various medications and silymarin could not significantly affect SOD compared to placebo [55]. Silymarin could stimulate DNA-dependent RNA polymerase I and consequently induce gene expression and activity of SOD [56]. TAC levels could represent the overall protective antioxidant capacity of the body [57]. Contrary to some studies that have shown significant increases in TAC levels due to silymarin supplementation [15, 22, 35], the present pooled analysis failed to show any significant effects. Ebrahimpour-Koujan et al. found significant post-intervention effect on TAC which could be possibly explained by using the formulation of silymarin with high bioavailability and a proper measuring method for total antioxidant activity [15].
GPx was significantly increased due to silymarin supplementation in the present study similar to previous trials [15, 33]. GSH pool is saved due to the cellular protection of silymarin and lower demand for GSH which results in high GSH and serum oxidant supply [58]. Furthermore, silymarin could upregulate gamma-glutamylcysteine synthetase (gamma-GCS) gene expression by stimulating DNA-dependent RNA polymerase I which leads to increase in intracellular GSH and increment in protein synthesis and GSH supply [56, 58]. GSH could provide the sulphydryl groups which are needed for DNA expression and increase defensive ability against ROS by enhancing antioxidant enzyme synthesis [56].
Among the reviewed studies, Gazak, Walterova, and Kren [59] did not find any significant effect on antioxidative and inflammatory factors after supplementation with silymarin. That could be related to the limited study population including male healthy subjects and short study duration [36]. Observing supplementation effects in the patient population depends on the underlying pathophysiology of diseases which often contain inflammation and oxidative stress [60]. Therefore, silymarin could better show its anti-inflammatory and antioxidant competencies in the patient population. Also, previous evidence showed that antioxidant activity in men’s bodies especially about GPx is less efficient than in women which could be another possible explanation for mentioned findings [61].
Due to its free radical scavenging properties [58], silymarin has been known as an antioxidant component that could increase the levels and activity of antioxidant enzymes [62] and inhibit lipid peroxidation [53]. It could directly affect scavenging ROS and decreasing free radicals which are related to lipid and protein peroxidation [58, 63, 64]. As silymarin could activate vitagenes which are responsible for producing protective molecules like sirtuins, thioredoxin, and heat shock proteins in stressful conditions, it could lead cells to optimal redox status [65]. Silymarin could demonstrate different antioxidant and anti-inflammatory effects through its inhibitory effect on leukotriene synthesis, prostaglandin formation, and NF-ƙB activation [66, 67], as it suppresses IL-1α and prostaglandin E 2 (PGE 2) in mouse peritoneal macrophages [49]. Also, it could stabilize mast cells, impede neutrophil migration, and inhibit TNF-α production [68–71]. The small molecular size and high lipophilicity of silymarin make it an effective component in intracellular reactions [72]. On the other hand, silymarin may interfere with the expression of apoptosis-related proteins and cell cycle regulators resulting in immunomodulatory effects [73, 74]. Also, the production of nitric oxide synthase could be stimulated by silymarin and increasing nitric oxide amounts may suppress inflammation [75, 76].
Due to the low bioavailability of silymarin which showed 20%–50% absorption through the gastrointestinal tract, using the dose of 420 mg/day could be enough for showing its antioxidant properties [77]. Different flavonoids including silymarin have various and unique anti-inflammatory activities unlike nonsteroidal anti-inflammatory drugs (NSAIDs) [24]. It could be used due to its accessibility, safety, and no major side effects [72, 73, 78, 79]. Except for some gastrointestinal disorders and headache, no major side effects have been reported [30, 80]. In a study, which had been conducted on tuberculosis patients treated with standard antituberculosis drugs, no serious adverse effects were reported except for some minor side effects such as nausea, vomiting, diarrhea, and lightheadedness which were similar in the placebo group [55].
4.1. Implications for Practice
Current meta-analysis showed that silymarin supplementation could exert anti-inflammatory and antioxidant properties which could lead to prevent various chronic diseases and improve health status of individuals. On the other hand, different variables like age, body mass index, health problems, and environmental situation could affect the improving effect of silymarin supplementation. Thus, more studies with greater study populations which evaluate various health conditions in longer period are required to make comprehensive decisions.
4.2. Implications for Research
Studies with larger and homogeneous study populations are recommended to accomplish more accurate conclusions. Moreover, various health conditions should be studied and various confounding factors such as lifestyle factors, absorption and excretion amounts, and food and drug interactions should be considered to generalize recommendations. Furthermore, the cost-benefit effects and the appropriate dosage of silymarin supplementation for applying anti-inflammatory and antioxidant effects should be determined by cost-benefit and dose escalation studies.
4.3. Strengths and Limitations
Present study was the first meta-analysis which evaluated the changes of inflammatory and antioxidant factors due to silymarin supplementation and most entered studies were categorized as good quality. Also, the validity and accuracy of results were verified via sensitivity and subgroup analysis. However, there were some limitations. There was high heterogeneity among reviewed studies which could be due to various supplementation doses, study populations, and study durations. Therefore, subgroup analysis was conducted to find the source of heterogeneity. Moreover, the efficacy of any antioxidant substances including silymarin depends on the oxidant/antioxidant status of subjects and their exposure to environmental oxidants which were not considered in the present study. Therefore, any recommendations should be given cautiously.
5. Conclusion
Silymarin demonstrated significant anti-inflammatory effects, evidenced by reduced levels of CRP and MDA and increased IL-10 levels, as well as notable antioxidant effects through the elevation of SOD and GPx levels. However, no significant impact was observed on TAC and IL-6 levels. Notably, the beneficial effects of silymarin on MDA SOD, GPx, and CRP are observed during intervention periods of 12 weeks or longer. However, additional studies with longer study periods are required to ascertain the long-term effects of silymarin on oxidative stress and chronic inflammation.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Shaghayegh Adeli, Arash Karimi, and Vahid Asghari Azar: conceptualization, methodology, writing–original draft, writing–review and editing, investigation, and formal analysis. Mahdi Vajdi, Vahid Asghari Azar, Arezoo Moini Jazani, and Ramin Nasimidoost Azgomi: conceptualization, writing–original draft, writing–review and editing, and formal analysis. Ajay Kumar: conceptualization, formal analysis, resources, writing–original draft, writing–review and editing, and supervision. Naveen Kumar Vishvakarma: conceptualization, methodology, validation, formal analysis, resources, and writing.
Mahdi Vajdi, Shaghayegh Adeli, Arash Karimi, Vahid Asghariazar, Arezoo Moini Jazani, Ramin Nasimidoost Azgomi.
Mahdi Vajdi and Shaghayegh Adeli have contributed equally to this work and share first.
Funding
The authors would like to thank the Ardabil University of Medical Sciences for its support.
Acknowledgments
The authors would like to thank the Ardabil University of Medical Sciences for its support.
Supporting Information
Supporting Table 1: PRISMA checklist. Supporting Figure 1: Sensitivity analysis of silymarin on CRP (a), IL-10 (b), MDA (c), SOD (d), TAC (e), and GPX (f). Supporting Figure 2: Funnel plot (with pseudo 95% CIs) of the WMD versus the se (WMD) for studies evaluating the association between silymarin supplementation and CRP (a), IL-10 (b), MDA (c), SOD (d), TAC (e), and GPX (f) values.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on a reasonable request.




