Sleep-Breath–Related Characteristics in Males With Chronic Rhinosinusitis
Abstract
Background: Patients with chronic rhinosinusitis (CRS) often exhibit sleep impairments that are closely associated with obstructive sleep apnea (OSA). However, objective evaluations of sleep quality in inpatients with CRS are lacking. This study explored the sleep-breath–related characteristics of CRS patients without nasal polyps (CRSsNP).
Methods: In this cross-sectional single-center study, we recruited 147 adult male inpatients diagnosed with CRSsNP between March 2019 and April 2020. OSA was diagnosed using standard PSG. The patients were classified into two groups based on the apnea–hypopnea index: CRSsNP with OSA and CRSsNP without OSA groups. Demographic features and sleep parameters were evaluated and compared between the groups.
Results: Inpatients with CRSsNP were at high risk of OSA (61.2%). Interestingly, the oxygen desaturation index (ODI), sleep efficiency (SE), and wake duration showed no differences between the CRSsNP with OSA and CRSsNP without OSA patients. The CRSsNP with OSA group exhibited a higher prevalence of obesity, slept for less time, was more easily aroused, and exhibited higher SE and larger CT90 scores during sleep (p < 0.05) compared to the CRSsNP without OSA group. Linear regression analysis revealed that the average SpO2 value (β = 1.182; p = 0.036) and ODI (β = 0.818; p < 0.001) remained significantly associated with OSA in CRS patients even after adjusting for age and body mass index.
Conclusions: Male inpatients with CRSsNP exhibited a high prevalence of OSA. CRSsNP patients evidenced severe hypoxia and exhibited more awakenings during sleep.
1. Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disorder of the nasal cavity and paranasal sinuses that causes nasal obstruction [1]; the prevalence is 8% in the Chinese population [2]. CRS can be generally classified into two phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP) [3]. CRS significantly compromises the quality of life (QOL) and increases healthcare utilization and sleep disturbance [4, 5]. Sleep problems, such as snoring, daytime sleepiness, difficulty falling asleep or maintaining sleep, and early morning awakening [6, 7], occur in approximately 50%–90% of patients with CRS and further contribute to depression, impaired cognition, and memory loss [7–10].
The typical characteristics of obstructive sleep apnea (OSA), such as upper airway collapse, fragmented sleep, and breathing cessation during sleep, have been reported in 49.7% of men and 23.4% of women [11, 12]. One study found that up to 64.7% of patients with CRS had OSA and 38% suffered from daytime sleepiness; the OSA prevalence was higher than that in the general population [8]. One interesting study that employed home sleep testing and used nasal symptom questionnaires found that new and worsening CRS was an independent risk factor for OSA even after adjusting for age, sex, and body mass index (BMI) [13]. A large-scale 10-year follow-up epidemiological study reported a causal relationship between the development of CRS and self-reported sleep problems, such as snoring, insomnia, and difficulty maintaining sleep [14]. A case–control study by Alt et al. [15] using the Pittsburg Sleep Quality Index (PSQI), Epworth Sleepiness Scale (EpSS), and a WatchPAT device reported increased awakenings and night snoring, and a lower average oxygen saturation (SpO2), in patients with CRS than without CRS. Patients with OSA who complained of sinonasal symptoms have been assessed using the 22-item Sino-Nasal Outcome Test (SNOT-22) [16]. Furthermore, it was reported that endoscopic surgery improved daytime sleepiness and OSA in patients with CRS [17, 18]. However, the sample sizes of these studies were small, and any involvement of OSA in the clinical manifestations of CRS remains to be elucidated.
One prospective study [19] showed that patients with CRSsNP were at higher risk of OSA than those with CRSwNP. Using the SNOT-22 and a visual analog scale, another study [20] concluded that patients with CRSsNP had significantly poorer sleep disturbance scores than those with CRSwNP. To date, most studies used questionnaires to evaluate the sleep quality of patients with CRS; few employed standard polysomnography (PSG) to evaluate the sleep impairment and sleep characteristics of patients with CRSsNP, especially those with OSA. In terms of the typical characteristics of OSA, the prevalence is notably higher in men at 49.7% compared to just 23.4% in women. And previous research has also identified a predominant sex distribution in males with CRS [12, 21]. Therefore, male participants were recruited in the present study. In the present study, we used standard PSG to explore the prevalence of OSA in male CRSsNP patients and determine the objective association between CRSsNP and OSA as male patients slept.
2. Methods
2.1. Subjects
This cross-sectional study evaluated the sleep characteristics of CRSsNP in Chinese men. A total of 188 male inpatients diagnosed with CRSsNP using nasal endoscopy and computed tomography according to the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 [3], and who required endoscopic sinus surgery, were initially enrolled at the Otorhinolaryngology Department of Shanghai Sixth People’s Hospital between March 2019 and April 2020. Inpatients complaining of nasal congestion, nasal discharge, facial pain, changes in sense of smell, and difficulty sleeping were examined and then underwent standard overnight PSG in the sleep laboratory; PSG was supervised by a technician, and the results were scored by two skilled physicians. As per the study design, patients with nasal polyps were not included in the analysis. The inclusion criteria were diagnosis of CRSsNP via nasal endoscopy and computed tomography; subjective sleep problems; and a strongly expressed desire for surgical treatment. In other words, all subjects were of the strong view that surgical intervention would effectively solve their CRS-related issues. Ultimately, 147 inpatients were enrolled in the analysis. The results will be disseminated to all enrolled patients via a variety of appropriate channels (discharge summaries or phone calls). The study was approved by the Ethics Committee of Shanghai Sixth People’s Hospital. Informed consent was obtained from all participants.
2.2. Assessment of Sleep Variables and Definition of Poor Sleep Quality
We monitored the sleep of all participants using standard overnight PSG (Alice 4 or 5; Respironics Inc., Pittsburgh, PA, USA) following the American Academic Sleep Medicine (AASM) guidelines [22]. The total sleep time (TST), number of wakes, wake duration, lowest and average SpO2, and arousal times were recorded during sleep. In line with the 2007 AASM manual, hypopnea was defined as a ≥ 3% decrease in oxygen desaturation coupled with a ≥ 50% airflow reduction from that at baseline, and apnea was defined as respiratory airflow cessation for at least 10 s [23]. The apnea–hypopnea index (AHI) was defined as the sum of all hypopneas and apneas per hour of sleep. The AHI was used for OSA classification: AHIs < 5, 5–14.9, 15–30, and ≥ 30 per hour indicated no OSA and mild, moderate, and severe OSA, respectively. The microarousal index (MAI) was defined as the abrupt arousal frequency per hour during sleep. The oxygen desaturation index (ODI) was the number of desaturation episodes during which the mean SpO2 decreased by 4% per hour during the sleep recording time. The sleep efficiency (SE) was the ratio of the TST to time in bed (TIB). The cumulative sleep time at SpO2 < 90% (CT90) was also recorded. The BMI was the weight in kilograms divided by the height squared in meters. The following parameters were used as indicators of poor sleep quality: TST < 240 min, minimum SpO2 < 90%, average SpO2 < 95%, ODI > 10 events/h, CT90 ≥ 5%, SE (%) < 85%, and MAI classified according to the interquartile range (IQR); a value > 6.25 indicated poor sleep quality [24, 25]. Additionally, a BMI ≥ 30 kg/m2 was considered to be a risk factor for OSA.
2.3. Statistical Analysis
All statistical analyses were conducted using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). We compared the polysomnographic variables between patients with CRSsNP with and without OSA. Continuous variables are presented as means ± standard deviations (SDs) or medians (IQR). Differences between the two groups were evaluated using the Kolmogorov–Smirnov test. Categorical variables are presented as numbers with percentages and were compared using the Pearson chi-squared test or Fisher’s exact test. Linear regression was used to explore the linear relationships between single dependent variables and other independent variables, with data on one or more explanatory variables used to calculate the statistical likelihood of a diagnosis of OSA. The TST, SE, minimum and average SpO2 values, number of wakes, and CT90, MAI, and ODI scores were subjected to linear regression analyses to determine the linear changes thereof according to CRS and comorbid OSA status. Independent variables were selected depending on the results of CRSsNP and OSA analyses and included the TST, SE, minimum and average SpO2 values, number of wakes, and CT90, MAI, and ODI scores. The dependent variable was the AHI (comorbid vs. not comorbid with OSA). The regression coefficients (β) and standard errors (SEs) were used to describe the linear changes. The sample size was calculated by G∗Power 3.1.9.7 statistical software. p values < 0.005 were considered significant, and p values between 0.05 and 0.005 were interpreted as trends.
3. Results
3.1. Baseline Characteristics
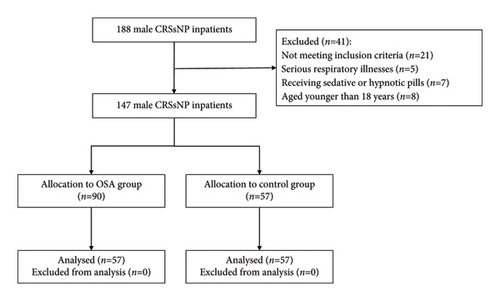
A total of 147 male patients with CRSsNP, with a mean age of 48 years (SD, 14 years), were recruited after the application of the inclusion and exclusion criteria (Figure 1). The prevalence of OSA was 61.2% (non-OSA, 38.8%; mild OSA, 17.0%; moderate OSA, 17.7%; severe OSA, 19.7%). Patient demographics and sleep parameters are presented in Table 1. The mean age of all CRS subjects was 47.75 years (SD: 13.86 years), the mean BMI was 26.22 kg/m2 (SD: 3.99 kg/m2), the mean TST was 296.17 min (SD: 79.45 min), the median MAI was 6.27 (range: 2.80–22.73), the median minimum SpO2 was 83.50 (range: 75.00–90.00), the median average SpO2 was 96.01 (range: 94.54–96.83), the median ODI was 8.10 (range: 2.10–31.60), the median AHI was 11.00 (range: 2.29–31.00), the median wake duration was 89.00 min (range: 50.50–135.50 min), the median SE was 0.85 (range: 0.74–0.95), and the median CT90 was 0.57 (range: 0.00–4.07). The BMI was significantly higher in the CRSsNP with OSA group than in the CRSsNP without OSA group (p < 0.001). The TST was 279.53 min in adults with CRSsNP and OSA and 321.86 min in adults with CRSsNP without OSA (p = 0.002). Patients with OSA had an MAI of 7.30, whereas those without OSA had an MAI of 3.69 (p = 0.001). The number of wakes was 15.00 in the CRSsNP without OSA group, whereas that in patients with OSA was 10.00 (p = 0.010). The SE was 0.81 in the CRSsNP without OSA group and 0.88 in OSA patients (p = 0.002). As expected, a significantly higher CT90 score (2.25) was seen in the CRSsNP with OSA group, whereas the CT90 score of those without OSA was 0.00% (p < 0.001). The mean AHI of patients with CRSsNP without OSA was 2.13, whereas that of patients with OSA was 24.2 (p < 0.001). There were no significant differences in age, the minimum SpO2, the average SpO2, wake duration, or ODI between the groups with and without OSA (p = 0.119, 0.719, 0.325, and 0.653, respectively).
| Characteristic | All | CRS without OSA | CRS with OSA | p value |
|---|---|---|---|---|
| N (%) | 147 | 57 (38.8) | 90 (61.2) | |
| Demographics | ||||
| Age (years) | 47.75 ± 13.861 | 45.42 ± 15.09 | 49.22 ± 12.89 | 0.119 |
| BMI (kg/m2) | 26.22 ± 3.99 | 24.04 ± 3.38 | 27.69 ± 3.70 | < 0.001 |
| Sleep parameters | ||||
| TST (min) | 296.17 ± 79.45 | 321.86 ± 77.03 | 279.53 ± 76.93 | 0.002 |
| MAI | 6.27 (2.80–22.73) | 3.69 (2.38–8.20) | 7.30 (2.56–25.60) | 0.001 |
| Minimum SpO2 | 83.50 (75.00–90.00) | 83.00 (74.00–90.00) | 84.00 (76.00–90.00) | 0.719 |
| Average SpO2 | 96.01 (94.54–96.83) | 96.22 (94.39–97.14) | 96.00 (94.57–96.76) | 0.325 |
| ODI | 8.10 (2.10–31.60) | 6.10 (2.40–31.9) | 10.48 (2.00–31.76) | 0.653 |
| AHI | 11.00 (2.29–31.00) | 2.13 (1.28–2.66) | 24.20 (12.62–39.00) | < 0.001 |
| Wake duration | 89.00 (50.50–135.50) | 93.75 (60.88–136.50) | 83.00 (44.00–134.25) | 0.115 |
| Number of wakes | 13.00 (5.00–18.50) | 15.00 (10.25–19.00) | 10.00 (4.00–18.00) | 0.010 |
| SE | 0.85 (0.74–0.95) | 0.81 (0.72–0.87) | 0.88 (0.75–0.97) | 0.002 |
| CT90 | 0.57 (0.00–4.07) | 0.00 (0.00–0.07) | 2.25 (0.30–9.36) | < 0.001 |
- Note: Normally distributed data are presented as mean ± SD; skewed data are presented as the median (IQR). Differences in baseline characteristics between the two groups were examined using the independent samples t-test or Mann–Whitney U test, as appropriate. Bold values are statistically significant (p<0.05).
- Abbreviations: AHI, apnea–hypopnea index; BMI, body mass index; CRS, chronic rhinosinusitis; CT90, cumulative time at SpO2 < 90%; IQR, interquartile range; MAI, microarousal index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SD, standard deviation; SE, sleep efficiency; SpO2, oxygen saturation; TST, total sleep time.
3.2. Sleep Parameters
The results obtained when comparing the sleep-related parameters of the CRSsNP with and without OSA groups are listed in Table 2. The numbers and percentages of patients with poor sleep quality and disturbed respiration are listed. The proportion of patients with TST > 240 min was higher in the CRSsNP without OSA group than in the CRSsNP with OSA group (p = 0.044). The number and proportion of patients with CT90 ≥ 5% were significantly higher in the CRSsNP with OSA group than in the non-OSA group (p < 0.001). Also, the number of patients with SE < 85% was higher in the CRS without than with OSA group (p = 0.005). When the MAIs of the two groups were compared, the CRSsNP with OSA group did not exhibit significantly higher scores except for patients in the third quartile (6.27 < MAI ≤ 22.73, p = 0.005). In addition, there was no significant difference in the TST, minimum SpO2, average SpO2, or ODI between the two groups.
| Parameter | CRS without OSA N (%) |
CRS with OSA N (%) |
Statistic | p value |
|---|---|---|---|---|
| BMI ≥ 30 kg/m2 | 3 (5.8) | 20 (26.0) | 8.649 | 0.003 |
| TST > 120 min | 57 (100) | 86 (97.7) | 1.314 | 0.520 |
| TST > 240 min | 50 (87.7) | 65 (73.9) | 4.047 | 0.044 |
| TST > 300 min | 34 (59.6) | 25 (28.4) | 13.989 | < 0.001 |
| TST > 360 min | 17 (29.8) | 16 (18.2) | 2.667 | 0.102 |
| Minimum SpO2 < 90% | 38 (69.1) | 65 (73.0) | 0.259 | 0.610 |
| Average SpO2 < 95% | 9 (27.3) | 17 (33.3) | 0.344 | 0.557 |
| ODI > 10 events/h | 24 (42.1) | 46 (51.1) | 1.135 | 0.287 |
| CT90% ≥ 5% | 2 (3.6) | 32 (36.4) | 20.403 | < 0.001 |
| SE (%) < 85% | 36 (63.2) | 35 (39.3) | 7.900 | 0.005 |
| ODI IQR | ||||
| ODI ≤ 2.10∗ | 13 (22.8) | 24 (26.7) | 0.430 | 0.672 |
| 2.10 < ODI ≤ 8.10∗ | 19 (33.3) | 18 (20.0) | 1.535 | 0.126 |
| 8.10 < ODI ≤ 31.60∗ | 11 (19.3) | 26 (28.9) | 0.897 | 0.384 |
| ODI > 31.60∗ | 14 (24.6) | 22 (24.4) | 0.162 | 0.885 |
| MAI IQR | ||||
| MAI ≤ 2.80∗ | 19 (33.9) | 16 (19.0) | 1.192 | 0.243 |
| 2.80 < MAI ≤ 6.27∗ | 19 (33.9) | 16 (19.0) | 0.017 | 0.987 |
| 6.27 < MAI ≤ 22.73∗ | 9 (16.1) | 26 (31.0) | 2.755 | 0.005 |
| MAI > 22.73∗ | 9 (16.1) | 26 (31.0) | 1.510 | 0.138 |
- Note: Data are presented as numbers and percentages. Differences between the two groups were examined using Pearson’s chi-square test or Fisher’s exact test. Bold values are statistically significant (p<0.05).
- Abbreviations: BMI, body mass index; CRS, chronic rhinosinusitis; CT90, cumulative time at SpO2 < 90%; MAI, microarousal index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SE, sleep efficiency; SpO2, oxygen saturation; TST, total sleep time.
- ∗p value and statistics from the Mann–Whitney U test.
3.3. Linear Regression Analysis
The objective sleep and breathing parameters recorded via standard PSG were subjected to linear regression analysis. The correlations between patients with CRSsNP with and without OSA (Table 3), and between patients with CRSsNP without OSA and with moderate-to-severe OSA (Table 4), were assessed. As shown in Table 3, linear regression analysis showed that the average SpO2 (β = 1.182; p = 0.036) and ODI (β = 0.818; p < 0.001) remained significantly associated with OSA in CRS patients even after adjusting for age and BMI. When differences in PSG parameters were compared between individuals without OSA and those with moderate-to-severe OSA (Table 4), we found that moderate-to-severe OSA was associated with a lower minimum SpO2 (β = −0.339; p = 0.027) and a reduced ODI (β = 0.802; p < 0.001) after adjusting for age and BMI.
| Covariates | Unstandardized coefficients | t | p value | 95% CI for β | ||
|---|---|---|---|---|---|---|
| β | SE | Lower bound | Upper bound | |||
| TST | 0.006 | 0.012 | 0.509 | 0.612 | −0.017 | 0.029 |
| SE | 3.821 | 6.237 | 0.613 | 0.542 | −8.621 | 16.264 |
| Minimum SpO2 | −0.211 | 0.113 | −1.863 | 0.067 | −0.436 | 0.015 |
| Average SpO2 | 1.182 | 0.553 | 2.136 | 0.036 | 0.078 | 2.285 |
| Number of wakes | −0.037 | 0.11 | −0.335 | 0.739 | −0.256 | 0.183 |
| CT90 | −0.112 | 0.106 | −1.058 | 0.294 | −0.324 | 0.099 |
| MAI | 0.09 | 0.122 | 0.738 | 0.463 | −0.154 | 0.334 |
| ODI | 0.818 | 0.079 | 10.413 | < 0.001 | 0.662 | 0.975 |
- Abbreviations: BMI, body mass index; CRS, chronic rhinosinusitis; CT90, cumulative time at SpO2 < 90%; MAI, microarousal index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SE, sleep efficiency; SpO2, oxygen saturation; TST, total sleep time.
| Covariates | Unstandardized coefficients | t | p value | 95% CI for β | ||
|---|---|---|---|---|---|---|
| β | SE | Lower bound | Upper bound | |||
| TST | 0.008 | 0.013 | 0.604 | 0.548 | −0.018 | 0.034 |
| SE | 2.128 | 6.859 | 0.310 | 0.758 | −11.636 | 15.892 |
| Number of wakes | −0.061 | 0.128 | −0.473 | 0.638 | −0.317 | 0.196 |
| Minimum SpO2 | −0.339 | 0.149 | −2.279 | 0.027 | −0.637 | −0.040 |
| Average SpO2 | 1.151 | 0.669 | 1.720 | 0.091 | −0.192 | 2.493 |
| CT90 | −0.095 | 0.115 | −0.829 | 0.411 | −0.325 | 0.135 |
| MAI | 0.071 | 0.137 | 0.518 | 0.607 | −0.204 | 0.346 |
| ODI | 0.802 | 0.090 | 8.905 | < 0.001 | 0.621 | 0.982 |
- Note: Bold values are statistically significant (p<0.05).
- Abbreviations: BMI, body mass index; CRS, chronic rhinosinusitis; CT90, cumulative time at SpO2 < 90%; MAI, microarousal index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; SE, sleep efficiency; SpO2, oxygen saturation; TST, total sleep time.
3.4. Sample Size Calculation
All subjects were enrolled in this study between March 2019 and April 2020. Thus, we used the post hoc test for evaluation; we employed G∗Power 3.1.9.7 software to calculate the power when the Type 1 error rate and effect size were set to 0.05 and 0.5, respectively; our sample size (147 subjects) afforded an 81.7% power.
4. Discussion
This cross-sectional study sought to identify the sleep characteristics of male inpatients diagnosed with CRSsNP. We found that male inpatients with CRSsNP exhibited a high prevalence of OSA (61.2%). All subjects were middle-aged (mean age, 47.75 years) and nonobese (mean BMI, 26.22 kg/m2). Doctors who regularly encounter CRS should enhance their awareness of sleep-related disorders, even in middle-aged nonobese people. Interestingly, ODI, SE, and the number of awakenings showed no differences between CRSsNP with OSA and CRSsNP without OSA patients. To a certain extent, the level of hypoxia and the extent of sleep disorder caused by CRS are in line with the level of hypoxia induced by OSA.
Many previous studies have assessed the relationship between OSA and CRS. One observational study of 916 African American patients with CRS reported that the OSA risk was significantly higher than that for white patients. Furthermore, patients with CRSsNP were at higher risk of OSA, with an odds ratio of 1.63 compared to patients with CRSwNP [19]. One retrospective cohort study found that OSA patients were at higher risk of subsequent CRS compared to patients without OSA [26]. A 6-year follow-up study showed that CRS should be targeted when seeking to prevent the development of OSA [27]. One prospective multisite cohort study with 405 participants concluded that OSA was the primary independent risk factor for CRS evaluated using the Rhinosinusitis Disability Index (RSDI) survey, the SNOT-22, and the PSQI [28]. CRS worsened sleep quality, and endoscopic sinus surgery was proposed as the treatment of choice for symptomatic patients [29]. Sleep quality then improved, as evaluated using the ESS, PSQI, AHI, and SNOT-22 [4, 30]. Subjective questionnaires are important in terms of patient assessment [31]. Few studies have concentrated on the sleep quality of CRSsNP patients with comorbid OSA using standard PSG, nasal endoscopy, and computed tomography.
The high prevalence of OSA in male inpatients with CRSsNP indicates that other risk factors may contribute to the development of OSA in patients with CRS. The chronic (and increasing) nasal congestion in patients with CRS may create negative pressure in the pharynx and thus trigger pharyngeal collapse, associated with oral breathing, snoring, and OSA [32]. Sleep impairment in CRS patients may be attributable to nasal congestion, inflammatory conditions, and/or upper airway resistance [26, 33]. The obstructive symptoms of CRS are predictors of whether surgical or medical therapies will be successful [34]. The relationship between CRS and OSA may be explained by a combination of two putative mechanisms: increased nasal airway resistance and inflammation of the upper airway [35]. Previous studies on OSA patients revealed variable extents of nasal inflammation, uvular mucosal congestion, and airway hyperreactivity, indicating that upper airway inflammation plays a role in the pathogenesis of OSA [36]. Sinusitis and chronic postnasal drainage may trigger secondary inflammatory changes in the upper airway, including the soft palate, and thus contribute to OSA development in patients with CRS [35]. Such development may also be explained by nasal obstruction and inflammatory cytokine infiltration into the nasal mucosa. A correlation between increased TGF-β and IL-4 levels (both are sleep-reducing cytokines) and poor PSQI sleep scores in patients with CRS has been reported previously [37], suggesting an important role for inflammation in CRS-related sleep impairment. One study on 601 subjects enrolled in the World Trade Center Health Program found a strong association between CRS and OSA and speculated that the higher risk of OSA in subjects with CRS may be attributable to increased upper airway inflammation and/or elevated nasal/upper airway resistance [13]. In addition, nasal congestion in patients with CRS can cause sleep arousal, awakening, and an increased frequency of apnea given the limited ventilation during sleep, thereby contributing to OSA [38]. Surgeries that treat nasal obstruction, such as sinus surgery and rhinoplasty, improve OSA symptoms and increase the continuous positive airway pressure tolerance [39]. Previous research revealed that OSA led to a significantly higher rate of comorbidities among patients with CRSsNP than those with CRSwNP (12.2% vs. 8.5%) [19]. The interplay between inflammation and increased airway resistance is complex. The mechanisms underlying these differences remain unclear and require further investigation.
Our study showed that the hypoxia events revealed by the ODI scores and SpO2 values differed (after adjustment for age and BMI) between CRSsNP patients with and without OSA. Previous studies have shown that patients with CRS did not exhibit obvious declines in SpO2 compared to non-CRS patients; however, some dyspnea was apparent in CRS patients, the cause of which was unclear [40]. CRSsNP is associated with OSA; however, apart from the SpO2 and ODI, there was no significant difference in any PSG parameter, indicating that a causal relationship is absent. Given the high rates of comorbidity associated with CRS and OSA, it is important to assess sleep-related breathing events in such patients. OSA is characterized by upper airway collapse that triggers intermittent hypoxia, subsequently inducing inflammatory pathways and endothelial dysfunction. Intermittent hypoxia is one of the main manifestations of OSA, which may explain why comorbid OSA exaggerates hypoxia in the presence of CRS. Inflammatory cytokines, including IL-1, IL-6, IL-8, and TNF-α, are expressed by OSA patients, and many related mediators have also been identified in studies on CRS pathophysiology. Therefore, progression of the two conditions may be deeply linked [41, 42]. Nasal congestion can partially or totally block the upper airway tract and may thus contribute to an insufficient oxygen supply. Cho et al. [43] observed overexpression of hypoxia-inducible factor 1, a regulator of inflammation, in the sinus mucosa of patients with CRS, suggesting that chronic nasal inflammation is one cause of hypoxia in patients with CRSsNP. Nasal and/or paranasal obstruction or inflammation may influence both subjective and objective sleep parameters [42]. It remains unclear whether hypoxia caused by inflammation or obstruction predisposes CRS patients to OSA, or whether a random correlation confuses the results. Taken together, our results suggest that when CRS is comorbid with or without OSA, the inflammation triggered by both diseases may contribute to the hypoxia events observed during sleep.
Our findings suggest that patients diagnosed with CRS should be evaluated in terms of primary sleep disorders. If a patient with CRS is diagnosed with OSA, treatment of both the CRS and OSA should be considered to improve sleep disruption. Our results are robust for the following key reason: we used standard PSG, not subjective questionnaires, to assess sleep quality in patients with CRSsNP. To our knowledge, such kind of work has not been reported before or published before.
This study had some limitations. First, there was bias associated with the selection of only CRSsNP inpatients, where we excluded nonsurgical cases and those lacking sleep issues. However, we compared CRSsNP patients with and without OSA. Second, possible confounding factors, such as smoking, alcohol consumption, anxiety, and depression, were not considered; only age, BMI, and sex were evaluated. Third, the cross-sectional nature of the study did not allow us to discuss any possible causal link between CRSsNP and OSA. Also, the impact of CRSsNP severity on sleep was not explored. Fourth, patients with fungal ball sinusitis or other identifiable etiologies were not well represented. Finally, this was a single-center study and the fact that all patients were ethnically Chinese limits the generalizability of our findings. A larger multicenter study is required for validation.
5. Conclusions
This study explored the sleep characteristics of male inpatients diagnosed with CRSsNP. Our findings indicate that male inpatients with CRSsNP exhibit a high prevalence of OSA and are prone to more hypoxic events, suggesting the importance of screening patients with CRSsNP in terms of sleep disturbances.
Disclosure
A preprint of this manuscript was previously made available online [44]. It has since been substantively revised and should not be viewed as the final version. For accurate and complete research findings, refer to the published version of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Prof. Xinyi Li and Yunhai Feng had full access to all of the data in the study. Study design: Xinyi Li, Yunhai Feng, and Haibo Ye; data collection: Wenjun Xue, Qianqian Zhang, Xiaolin Wu, Huaming Zhu, Xinyi Li, and Yunhai Feng, Haibo Ye; statistical analysis: Wenjun Xue and Qianqian Zhang; manuscript draft: Wenjun Xue, Qianqian Zhang, and Xinyi Li; manuscript revised: Wenjun Xue, Xinyi Li, Yunhai Feng, and Haibo Ye.
Funding
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (STI2030-Major Projects 2021ZD0201900), National Natural Science Foundation of China (82000967), Natural Science Foundation of Shanghai (22ZR1447200), Shanghai Sixth People’s Hospital (ynts202103), and Medical Research Projects of Xuhui District (SHXH202003 and SHXH202102).
Acknowledgments
The authors would like to thank all the subjects for their participation and acknowledge the skillful work of the entire medical staff.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




