Carpal Tunnel Syndrome Diagnostics Revisited: A Comprehensive Review
Abstract
Carpal tunnel syndrome (CTS) remains the most common peripheral entrapment neuropathy worldwide. There are several challenges associated with CTS diagnostic process. The most widely accepted gold standard is clinical assessment. Its subjective nature and the experience required pose an insurmountable challenge when aiming to achieve satisfactory accuracy and consistency. Nerve conduction and imaging studies are not widely available to general practitioners and have to be performed by specialists, which prolongs the diagnostic process. Meanwhile, the syndrome may progress, leading to the exacerbation of symptoms. Furthermore, the therapeutic decisions have to be well-founded, as invasive surgeries are often considered as the treatment of choice in more severe CTS cases. A resurging alternative method, which shows a lot of promise, is quantitative sensory testing. For the purposes of this review, databases available in Medical University of Warsaw resources were screened and evaluated by two independent reviewers. 29 studies were identified for further assessment. There is a need to reconsider the commonplace trends and establish proper diagnostic guidelines. The available diagnostic methods should be viewed as complementary rather than standalone. Choosing the right combination can provide a more accurate and comprehensive evaluations of CTS, helping to confirm the diagnosis, assess severity, and guide treatment options.
1. Introduction
Academic textbooks define entrapment neuropathies as damage to peripheral nerves, resulting from chronic pressure exerted on the nerve in a narrow and nonstretching anatomical canal. The causes of injury may be diverse and difficult to detect. Kochanowski mentions, among others, the occupation, practiced sport discipline, developmental disorders, and diseases conducive to nerve damage. Damage can occur both as a result of repetitive movement or vibration, as well as due to osteoarticular injuries [1].
Carpal tunnel syndrome (CTS) remains the most common peripheral entrapment neuropathy worldwide. According to Aboonq, it is responsible for as much as 90% of all entrapment neuropathy cases [2]. In the United States, it is estimated to be the most expensive musculoskeletal disease of the upper extremity, costing approximately $2 billion annually [3]. Even though CTS is thought to be the most widely studied nerve entrapment syndrome, there is no worldwide consensus on specific diagnostic protocols [4]. This is exemplified by the variability of reported prevalence and incidence of CTS.
It is believed that the pathophysiology of CTS includes elements of compression and pulling. They can lead to disorders of nervous microcirculation, demyelination, and disruption of the structure of the surrounding connective tissue. Additionally, the role of changes in fluid pressure (increasing up to 10 times) within the canal occurring during carpal movements cannot be understated [5, 6]. Repeated occurrence of pressurized conditions can result in nerve injury, demyelination, and microcirculatory dysfunction. Damage to the neurovascular equilibrium leads to the destruction of the blood-nerve barrier, protein imbalance, increase in local inflammation and edema. Mechanical irritation of connective tissue structures, including synovial membranes, changes their structure. The tendon apparatus can also become inflamed and scarring can lead to further nerve damage [7].
It is important to note that, such as the name suggests, this neuropathy is a syndrome, rather than a disease in and of itself and therefore is defined by a set of symptoms. Depending on the presentation and underlying conditions, the choice of diagnostic methods (both systemic and aimed specifically at CTS) may vary. Furthermore, a significant proportion of CTS cases are secondary to underlying systemic conditions (as outlined in Table 1). In such situations surgery should be initially avoided, as the nerve compression may resolve with proper therapy. Operating before treating the cause may be ineffective or unnecessary [8]. If, after appropriate treatment of the underlying disease, CTS symptoms remain, then surgical decompression may be considered.
| Category | Underlying condition example |
|---|---|
| Endocrine/metabolic/toxic | Diabetes |
| Alcoholism | |
| Vitamin deficiency or toxicity | |
| Hypothyroidism | |
| Kidney failure | |
| Acromegaly | |
| Rheumatologic | Rheumatoid arthritis |
| Psoriatic arthritis | |
| Scleroderma | |
| Systemic lupus erythematosus (SLE) | |
| Fluid retention/physiologic | Pregnancy |
| Oral contraceptive use | |
| Menopause | |
| Structural/anatomical | Obesity |
| Wrist trauma (e.g., fractures, dislocations) | |
| Ganglion cysts or tumors | |
| Storage diseases | Amyloidosis (especially familial or dialysis-related) |
| Hemochromatosis | |
| Infectious | Lyme disease |
| Iatrogenic/medication-related | Long-term corticosteroid use |
| Occupational | Repetitive strain injury, working with vibrating tools |
The most widely accepted diagnostic gold standard is clinical assessment and taking history [4]. Its subjective nature and the experience required pose an insurmountable challenge when aiming to achieve satisfactory accuracy and consistency, which in turn is required to make informed decisions about further therapeutic options.
Standard confirmatory tests currently include classical electrophysiological nerve conduction studies (NCS). They provide crucial information about the severity of nerve compression and its site, as well as help in the differential diagnosis of other possible symptom causes and monitoring of the syndrome after therapy. However, as it has been pointed out by Gervasio et al. “clinical examination and electrophysiological tests are not able to differentiate idiopathic forms from secondary forms of CTS, and discrepancies are possible between clinical examination and electrophysiological tests (false negatives).” Complementary information about the nature of the syndrome (primary or secondary), as well as anatomical conditions, could be provided by imaging studies, such as ultrasonography (USG) and magnetic resonance imaging (MRI) [9].
A resurging alternative method, which shows a lot of promise, is quantitative sensory testing (QST), in which thermal and nociceptive stimuli are applied. Most authors research large myelinated nerves in their studies, however the involvement of small fibers in CTS symptom presentation seems almost equally as important. The results of the available studies, however, seem inconsistent, with no clear conclusions regarding sensitivity, extraterritorial hyperalgesia and the aspect of possible central sensitization [10].
There are several challenges associated with CTS diagnostic process, the availability and costs (both direct and indirect). Specialized tools (such as MRI and NCS) are not widely available to general practitioners and require equipment that has to be consistently operated and maintained. Shortages of trained personnel to perform or interpret the tests, affect both access and quality. These issues may prolong the diagnostic process by several weeks or even months and can be exacerbated in low-resource settings. Indirect costs, such as transportation, time off work, or the cost of a delayed diagnosis are not always factored into economic evaluations but are significant in practice. Furthermore, the therapeutic decisions have to be well-founded, as invasive surgeries are often considered as the treatment of choice in more severe CTS cases.
In recent months, numerous excellent studies and meta-analyses have been published regarding several problems of CTS diagnostics. However, they have either focused on one narrow issue, or tried to summarize all aspects of CTS (including anatomy, diagnostics, treatment options and outcomes). We strongly believe, that the results of the former, in isolation, do not provide sufficient context, while in the latter case, it is impossible to provide an exhaustive summary on the problem of diagnostics alone. The primary objective of this study is to summarize the performance of each diagnostic method in terms of sensitivity, specificity, and its correlation with clinical severity across various patient groups. Rather than determining which method is superior over the others, the focus is on exploring how these tests can complement one another to enhance diagnostic consistency.
2. Materials and Methods
This review was performed according to PRISMA guidelines (Figure 1). Several databases available in Medical University of Warsaw resources, including PubMed, SCOPUS, The Cochrane Library, Embase, and Polska Biblioteka Lekarska, were screened through December 2022. As this article aimed to be a comprehensive review and summary, rather than a focused meta-analysis, in our work we included (1) original studies, reviews and meta-analyses on the issues of QST, provocation tests, neurophysiology and imaging studies in CTS, (2) assessing the sensitivity and/or specificity of the aforementioned methods, (3) which were written in English, (4) and the participants had to be > 18 years old. Reference lists of all primary studies and review articles were then screened for additional relevant articles.
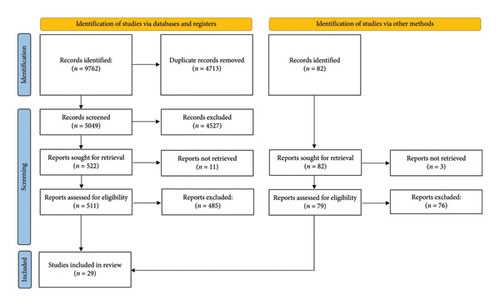
Studies were excluded if they: (1) did not address the diagnostic methods under investigation, (2) did not report diagnostic performance indicators such as sensitivity or specificity, (3) were not written in English, (4) included participants under the age of 18, (5) were of poor methodological quality or provided insufficient data relevant to the aims of the review.
Additionally, duplicates and original studies exhaustively covered in the reviews were removed, full articles, if possible, were obtained and assessed. The screening and evaluation was performed by two independent reviewers, any differences or disagreements were resolved, the data was then summarized.
3. Results and Discussion
Twenty nine studies were identified for further assessment. They are summarized in Table 2.
| Study type | Tests included | |
|---|---|---|
| Sobeeh et al. [10] | Meta-analysis | QST |
| Tai et al. [11] | Meta-analysis | USG |
| Cartwright et al. [12] | Review | USG |
| Fowler et al. [13] | Review | NCS, USG |
| Ghasemi-Rad et al. [14] | Review | Tinel’s test, Phalen’s test, hand elevation test, tourniquet test, NCS, QST (Weinstein enhanced sensory test, two-point discrimination test, tuning fork, vibrometer) |
| Ibrahim et al. [7] | Review | Tinel’s test, Phalen’s test, NCS, USG, MRI |
| MacDermid and Wessel [15] | Review | Tinel’s test, Phalen’s test, carpal compression, QST (Weinstein enhanced sensory test, tuning fork, vibrometer) |
| Osiak et al. [16] | Review | Tinel’s test, Phalen’s test, NCS, USG |
| Padua et al. [4] | Review | Tinel’s test, Phalen’s test, USG |
| Werner and Andary [17] | Review | NCS |
| Kokubo and Kim [18] | Review abstract | NCS |
| Aggarwal et al. [19] | Original research | USG |
| Azami et al. [20] | Original research | USG |
| Boland and Kiernan [21] | Original research | Phalen’s test, modified carpal compression test |
| Drakopoulos et al. [22] | Original research | NCS, USG |
| El Miedany et al. [23] | Original research | USG |
| Emril et al. [24] | Original research | USG |
| Goswami et al. [25] | Original research | USG |
| Kasundra et al. [26] | Original research | Tinel’s test, Phalen’s test, USG, MRI |
| Kotevoglu and Gülbahce-Saglam [27] | Original research | USG |
| Kutlar et al. [28] | Original research | USG |
| Nakamichi and Tachibana [29] | Original research | USG |
| Ng et al. [30] | Original research | USG |
| Ng et al. [31] | Original research | MRI |
| Ng et al. [32] | Original research | MRI |
| Park et al. [33] | Original research | MRI |
| Ratasvouri et al. [34] | Original research | USG |
| Ting et al. [35] | Original research | USG |
| Rosario and De Jesus [36] | Book | NCS |
| Jablecki et al. [8] | Report | NCS |
- Abbreviations: MRI = magnetic resonance imaging, NCS = nerve conduction studies, QST = quantitative sensory testing, USG = ultrasonography.
3.1. Provocation Tests
Correct CTS diagnosis requires a thorough evaluation, including a physical examination and patient history. Provocation tests, also known as provocative maneuvers or clinical tests, are used during a physical examination to assess for the presence of symptoms or signs that may indicate CTS. These tests are designed to provoke or reproduce the symptoms of CTS, such as numbness, tingling, or pain in the hand and fingers, by manipulating the wrist and hand in specific ways. The most commonly used and cited provocation tests for CTS include:
Phalen’s Test: The patient flexes the wrists and holds them in a maximally flexed position for about 60 s. If numbness, tingling, or pain develops in the median nerve distribution (thumb, index, middle, and half of the ring finger), it may be indicative of CTS.
Tinel’s Sign: The healthcare provider taps or lightly strikes over the median nerve at the wrist. If this elicits tingling, numbness, or a shock-like sensation in the median nerve distribution, it may suggest CTS.
These methods are simple and low-cost; however, they are associated with significant limitations.
According to Kasundra, Phalen test had greater sensitivity in mild-moderate, moderate, and severe groups, while Tinel’s had greater sensitivity in mild group [26]. Conversely, Ghasemi-Rad reported that no trends were identified between testing positive for various provocative tests and the severity of CTS [14]. The reported sensitivity and specificity, as demonstrated in Table 3, is highly variable and dependent on method of examination and interpretation, and thus these tests, along with other provocative maneuvers (such as the aforementioned hand elevation test, tourniquet test, and carpal compression test), especially in isolation, have low positive predictive value [7].
| Study | Test | Sensitivity | Specificity |
|---|---|---|---|
| Ghasemi-Rad et al. [14] | Tinel’s test | 23%–60% | 64%–87% |
| Phalen’s test | 57%–91% | 33%–86% | |
| Hand elevation test | 75.5% | 98.5% | |
| Tourniquet test | 21%–59% | 36%–87% | |
| Ibrahim et al. [7] | Tinel’s test | 48%–73% | 30%–94% |
| Phalen’s test | 67%–83% | 40%–98% | |
| MacDermid and Wessel [15] | Phalen’s test | 68% | 73% |
| Tinel’s test | 50% | 77% | |
| Carpal compression | 64% | 83% | |
| Osiak et al. [16] | Tinel’s test | 28%–73% | 44%–95% |
| Phalen’s test | 46%–80% | 52%–91% | |
| Padua et al. [4] | Tinel’s test | 38%–100% | 55%–100% |
| Phalen’s test | 42%–85% | 54%–98% | |
| Boland and Kiernan [21] | Phalen’s test | 64% | 75% |
| Modified carpal compression test | 14% | 96% | |
| Kasundra et al. [26] | Tinel’s test | 78.5% | 91% |
| Phalen’s test | 84.9% | 74%–83% | |
3.2. NCS
NCS can help in the diagnosis of CTS by providing objective information about the function of the physiological health of the median nerve across the carpal tunnel on a quantitative basis [14].
NCS are specifically very sensitive in examining median nerve dysfunction caused by damage to the nerve and can define the degree of demyelination and axonal loss that has occurred [4]. One of the undeniable advantages of NCS is the ability to help identify dysesthesia conditions that may mimic or accompany CTS, such as cervical radiculopathy, ulnar nerve disease, polyneuropathy, brachial plexopathy, thoracic outlet syndrome, central nervous system (CNS) disorders, or other median nerve entrapment syndromes [14]. Moreover, preoperative NCS can provide an objective baseline for comparison in cases where surgical intervention is planned. This baseline can be useful in postoperative follow-up to assess treatment outcomes and identify any persistence or recurrence of symptoms [15].
Although the specificity of said method remains consistently above 80%, the sensitivity has been reported to be as low as 56% (Table 4). Even though many treating physicians and patients appreciate the additional information NCS provide in terms of confirming the diagnosis and ruling out other potential causes, as well as providing prognostic information, it has its limitations. Apart from unsatisfactory sensitivity, the disadvantages include potential discomfort for patients, and increased costs [37].
| Study | Sensitivity | Specificity |
|---|---|---|
| Fowler et al. [13] | 80.2% | 92.8% |
| Ghasemi-Rad et al. [14] | 56%–85% | > 94% |
| Ibrahim et al. [7] | 80%–92% | 80%–99% |
| Osiak et al. [16] | > 85% | 95% |
| Werner and Andary [17] | 85%–90% | 82%–85% |
| Kokubo and Kim [18] | 57%–94% | 51%–97% |
| Drakopoulos et al. [22] | 97% | 89% |
| Rosario and De Jesus [36] | 75%–95% | |
| Jablecki et al. [8] | 56%–85% | 94%–99% |
NCS base on the relative comparison of nerves. Several attempts have been made at establishing methodological criteria; however, due to insufficient literature, these findings should be confirmed by larger, multicenter collaborative efforts [38]. It has been stated that “establishing consensus on a reference standard for the diagnosis for CTS is the most important research goal in this area” [38].
If a patient does not exhibit symptoms that are consistent with CTS involving the typical distribution of the median nerve (i.e., the first three digits), performing NCS may not provide valuable information in confirming or ruling out CTS [36]. Therefore, the diagnostic accuracy depends on the clinical correlation between the patient’s symptoms and the nerve conduction findings. Similarly, normal NCS may not definitively rule out CTS. As pointed out by Sonoo et al., “it is possible to have all the symptoms of CTS with minimal or even no electrophysiological evidence of a MNW” (MNW = median neuropathy at the wrist) [39]. In case of mild CTS, results may be normal in up to one-third of the patients [40].
There are several reasons why NCS may have limitations in diagnosing CTS. Firstly, the short length of the involved nerve segment in the wrist-finger region may result in overall normal conduction velocity. Secondly, there is inter-individual variability in nerve conduction velocity, which can limit cross-nerve comparisons. Additionally, bilateral CTS may be as common as 60% of CTS cases [41], which can obscure side-to-side comparisons [4].
What’s more, the cutoff values used for defining CTS may need to be adjusted to account for differences in normal values and specific population characteristics to avoid false positives or false negatives. For example, in diabetic patients, the NCS may show slightly prolonged latencies compared to the general population even in the absence of CTS. If the typical cutoff value of 0.5 ms, is applied to diabetic patients, it may result in a higher number of false positives, as cases with relative latencies of 0.5–1.0 ms may actually be normal for this specific population. The chance of false positives is also higher for workers with hand-intensive jobs, who have vague hand symptoms. Finally, during standard NCS, small fiber axons are not examined, while pain is commonly the most distressing symptom, prompting the patients to seek medical help [39].
3.3. Imaging: USG
USG can provide real-time imaging of the soft tissues, including nerves, tendons, ligaments, and other structures in the wrist, and therefore be used as a complementary diagnostic test to assess the wrist for structural abnormalities. USG is cost-effective, widely available, noninvasive, and well tolerated by patients [14]. Therefore, it may be considered as a feasible alternative for diagnosing CTS in clinical settings as the first-line confirmatory test [13], especially where access to electrodiagnostic facilities may be limited [24].
As reported by Gervasio et al. [9] and expanded upon in Table 5, the sensitivity and specificity of USG are similar to NCS. Diagnostic accuracy of CTS based on class I studies using neuromuscular ultrasound is comparable to electrodiagnostic studies (Level A), and class II evidence consistently demonstrates the ability of USG to detect underlying structural abnormalities. Thus, “neuromuscular USG should be used to screen for structural abnormalities in the setting of suspected CTS (Level B) [12].” Lesions, which display symptoms similar to CTS, such as tenosynovitis, mass lesions, and anatomic defects, can be excluded from differential diagnosis [14].
| Study | Sensitivity | Specificity |
|---|---|---|
| Tai et al. [11] | 87.3% | 83.3% |
| Cartwright et al. [12] | 65%–97% | 72.7%–98% |
| Fowler et al. [13] | 77.6% | 86.8% |
| Ibrahim et al. [7] | 64.7% | — |
| Osiak et al. [16] | 77.6% | 86.8% |
| Padua et al. [4] | 77.6% | 86.8% |
| Aggarwal et al. [19] | 95% | 100% |
| Azami et al. [20] | 99.2% | 88.3% |
| Drakopoulos et al. [22] | 94% | 55% |
| El Miedany et al. [23] | Mild 98% | 100% |
| Moderate 98% | 97% | |
| Severe 97% | 99% | |
| Emril et al. [24] | 88.5% | 65% |
| Goswami et al. [25] | 91.7% | 87.1% |
| Kasundra et al. [26] | Depending on site and technique 76%–84% | 84%–97% |
| Kotevoglu and Gülbahce-Saglam [27] | 89% | 100% |
| Kutlar et al. [28] | Doppler 90% | 94% |
| Hypervascularization 93.4% | 90% | |
| Nakamichi and Tachibana [29] | > 95% | 43%–57% |
| Ng et al. [30] | CSA proximal to the tunnel 75% | 87.5% |
| CSA distal to the tunnel 63.6% | 100% | |
| Ratasvouri et al. [34] | 87% | 91% |
| Ting et al. [35] | Normal vs. abnormal 75% | 97% |
| Severe vs. nonsevere 80.6% | 63.2% | |
- Abbreviation: CSA = cross-sectional area.
USG can be used to quantify the degree of swelling of the median nerve in the carpal tunnel by calculating the nerve cross-sectional area (CSA). It has been suggested that this method may even be used to confirm clinical CTS diagnosis in pregnant women [42]. A normal median nerve typically has a transverse area of less than 10 mm2. If the nerve’s transverse area exceeds this threshold, it may indicate swelling or compression of the nerve, which can be associated with varying degrees of nerve suffering. A transverse area between 10 and 12.9 mm2 may indicate mild suffering, an area between 13 and 14.9 mm2 may indicate moderate suffering, while if the transverse area exceeds 15 mm2, it may indicate severe suffering of the nerve. USG has been reported to be more sensitive in detecting moderate to severe cases of CTS compared to mild cases [26]. The risk of false positives may be reduced in cases of mild suffering by measuring the difference between the maximum transverse area of the nerve in the carpal tunnel and the area of the nerve evaluated in the forearm at the level of the proximal third of the pronator quadratus muscle. For a difference above 2 mm2, Gervasio et al. reported a sensitivity and specificity close to 100% [9].
According to Bang et al., the CSA of the median nerve proximally to carpal tunnel inlet and at the carpal tunnel inlet level, increases with the severity of CTS with statistically significant differences, while only the examination at the inlet was able to differentiate normal reference values from mild CTS [43]. This is in line with Park et al.’s findings, who concluded that measuring the nerve at the carpal tunnel inlet shows the highest rates of sensitivity [33]. Azami et al. also reported that the difference in CSA of the median nerve in mild, moderate, and severe CTS was statistically significant [20]; Ahmed et al. showed a strong correlation between CSA on USG and severity of CTS on NCS [44], while Kim et al. pointed out that USG provides a reliable correlation with the grade of electrodiagnostic abnormalities and clinical severity [45]. In another study, high-resolution USG, with a wrist-forearm ratio of 1.7, showed 96.1% accuracy in detecting CTS and correlated positively with median nerve conduction latency [46]. Ng et al. recommended median nerve CSA of > 14.1 mm2 for mild CTS and > 19.5 mm2 for severe CTS, whereas Ting et al. found optimal CSA cutoff points to discriminate between normal versus abnormal (10.5 mm2), normal/mild versus moderate/severe (13.4 mm2), and normal/mild/moderate versus severe groups (16.4 mm2).
Furthermore, Kutlar et al. reported, that Doppler USG is highly sensitive and specific (Table 5) and the results correlate strongly with CTS severity [28]. Furthermore, the utilization of Doppler USG may serve as a valuable approach in the diagnosis and evaluation of the severity of CTS, and can be considered as a dependable supplementary technique in the examination of CTS [47].
To summarize, there was a positive correlation and proportional increase between the USG measurements and the severity of CTS observed in NCS [21]. Additionally, ultrasound parameters could be used to predict both the initial treatment response and the likelihood of relapse [25]. Moreover, the presence of typical clinical symptoms, along with at least one sonographic measurement of the median nerve that displays pathological increase, can be a highly accurate predictor [27].
USG examination, however, is not without its limitations. Even though CSA measurements seem promising, there is currently no agreement on a universal cut-off value of CSA that can be used to diagnose CTS, nor is there a consensus on the location of maximum swelling of the median nerve, whether it is at the tunnel inlet, tunnel outlet, or within the tunnel itself [19]. Kasundra et al. noted that different ranges have been reported for “normal” USG parameters, with the CSA ranging from 9 mm2 to 15 mm2 [26]. While the measurement location at the level of the pisiform is the most common, the use of other anatomic landmarks (i.e., distal radioulnar joint, radiocarpal joint, wrist crease, intratunnel space, hook of the hamate, and distal flexor retinaculum edge) has also been reported. Furthermore, different studies have used varying reference standards for validation, with some relying on electrodiagnostic studies, while others depended on a combination of clinical examination and NCS [48].
Additionally, when dealing with advanced cases of CTS in elderly patients, it is possible for the nerve to exhibit less swelling than anticipated, given the extent of the damage. This occurs due to a decrease in the number of axons and a reduction in edema, which is replaced by fibrotic tissue. Consequently, the neural bundles tend to display greater echogenicity, and the external epineurium becomes thickened and hyperechoic [9]. Oliveira et al., in their study, concluded that USG was unable to differentiate between the various groups based on their indicative signs and symptoms of CTS [49].
3.4. Imaging: MRI
MRI is a valuable tool both for evaluating primary nerve pathologies and assessing space-occupying lesions that may compress nerves. MRI can provide detailed information about morphological damage, inflammation, and the extent of nerve compression [14]. By visualizing the nerves directly, MRI can reveal any structural abnormalities, such as nerve thickening, enlargement, or discontinuity, which can aid in diagnosis and treatment planning. MRI excels in accurately showing the location of the lesion and determining the severity of nerve compression. This information is crucial for guiding surgical interventions. Additionally, MRI can be valuable in cases of rare pathological causes of conditions like CTS—it can accurately identify ganglia, hemangiomas, or bony deformities that can lead to nerve compression and mimic CTS symptoms, as well as localize these rare causes, enabling proper diagnosis and subsequent treatment [7, 15].
As outlined in Table 6 above, MRI seems to be a highly sensitive and specific method; however, the data available is limited. Early studies have shown that there is agreement between fractional anisotropy and the apparent diffusion coefficient values derived from MRI and the results of NCS [4]. MRI may provide information about CTS severity—it has been reported to be highly accurate at diagnosing CTS and moderately accurate at determining CTS severity. Park et al. states that measuring the nerve at the carpal tunnel inlet shows the highest rates of sensitivity and CSA of the median nerve was 15.1 mm2 or larger [33]. Ng et al. suggested CSA cutoff values at > 15 mm2 either proximal to or distal to the tunnel and > 19 mm2 proximal to the tunnel as a diagnostic criterion for CTS in general and in severe cases, respectively, (sensitivity, specificity, and accuracy of prediction of severe CTS using for CSA > 19 mm2 were 75.0%, 65.9%, and 69.6%, respectively) [31]. In their later work, median nerve CSA > 14.1 mm2 to diagnose mild CTS and > 19.5 mm2 for severe CTS has been recommended [32].
MRI can help identify associated pathologies that may not be evident on NCS or USG (58.97% of MRIs done as reported by Kasundra et al. [26]), which becomes especially crucial when surgical interventions, such as endoscopic procedures, are being considered. Furthermore, MRI plays a role in patients who have previously undergone carpal tunnel release surgery and subsequently develop recurrent symptoms. In such cases, MRI can be employed to assess the postoperative anatomy and identify any potential factors contributing to the recurrence of symptoms, such as residual or recurrent compressive lesions or scar tissue formation.
This method is, however, costly, time-consuming, and not readily available. Additionally, the variability in diagnostic criteria leads to variations in the profiles of patients included, impacting the accuracy of the tests [14].
Novel solutions are slowly being explored. Zhou et al. tested a deep neural network in CTS identification. The model achieved 0.63 accuracy of intersection over union and 0.17 s segmentation efficiency, even though the image training pool was relatively small (333 images) [50]. Funahashi et al. concluded that useful information about nerve morphology can be obtained from 3-D MRI [51]. As of now, these techniques remain relevant mostly as scientific research tools; however, they may become useful as complementary options once their cost-effectiveness and availability improve.
3.5. QST
QST can help in quantifying sensory abnormalities and provide additional insights into the severity and nature of nerve dysfunction in CTS. QST allows for the assessment of both large and small nerve fiber function, enabling a comprehensive evaluation of sensory abnormalities in CTS. Even though QST methods have been explored for several years, the data is still scarce (Table 7). Based on the promising preliminary results, however, this area is worth exploring to pinpoint the most effective approach among the plurality of different techniques.
An excellent meta-analysis, albeit focusing mostly on the pain detection aspect, has been recently published by Sobeeh et al. In the paragraphs related to thermal stimuli, they have reported a significant loss of warm sensation measured at the index finger and the middle finger with considerable and moderate heterogeneity, respectively. There has also been a significant loss of cold detection sensation, with moderate heterogeneity and no significant publication bias [10].
Numerous other studies showed promising results. Clarke et al. demonstrated that, after post hoc analysis, cold detection thresholds (CDT) of the severe group were significantly lower compared to the healthy subjects and the trend of CDT impairment was correlated with CTS severity. Warm perception impairment was notably more severe in the group with severe CTS when compared to the control group. Furthermore, the severe CTS group exhibited significantly greater impairment in warm perception compared to both the mild and moderate CTS groups [52]. Goadsby and Burke found clear evidence of abnormal small-fiber function, with higher thresholds for both hot and cold sensation [53]. In another study, deficits were revealed in all detection thresholds in the combined patient groups compared with healthy controls [54]. The patient groups exhibited diminished thermal and mechanical detection function within the primary pain area and dermatome when compared to healthy reference data, as reported by Tampin et al. [55]. A study by Lang et al. revealed a significant increase in thresholds for warmth, cold, and heat pain on the index finger compared to the control subjects [56].
Furthermore, as reported by Tamburin et al. overall severity of CTS sensory symptoms, as measured with the BCTQ Symptoms Severity Scale, was significantly correlated with CDT. Single CTS symptom analysis also showed a significant correlation with CDT, not with warm detection thresholds (WDT) however. This would indicate Ad- and C-fiber involvement in electrodiagnostic-negative CTS patients [57].
It is worth noting that, within the patient groups, the WDT can be higher in the affected arm compared to the unaffected arm. Similarly, within the control group, the WDT may be higher on the dominant side compared to the nondominant side. Additionally, heat hyperalgesia was more prominent in patients with unilateral CTS [58]. Age has also been shown to influence thermal detection thresholds (the magnitude is small, however) [52].
Another major component of CTS patient care is postoperative management. Kennedy et al. quantitatively tested the patients at 6 months postsurgery. They observed a moderate to large effect size in the change of QST parameters, specifically for thermal detection, thermal pain, and mechanical detection thresholds. However, there was no statistically significant change in the measures of mechanical pain. These findings indicate that the sensory phenotype derived from thermal QST is sensitive to clinically significant changes in sensory perception [59].
Even though QST aims at objectivizing the results, several researchers argue, that considerable subjective components are inextricable [7]. The patient groups involved in the studies are small and heterogenous. The data remains scarce and the available articles have variable methodology. This is especially important, as QST effectiveness may vary depending on the protocol, as exemplified by the analysis by Sobeeh et al. which revealed no difference of warm sensation at the thenar eminence or carpal area [10].
Apart from thermal stimuli, another aspect of QST is mechanical, pain, and vibration sensation. However, according to the meta-analysis of Sobeeh et al. either no significant differences between groups were shown in most of the pain modalities (i.e., cold, heat, mechanical, and pressure thresholds), or the heterogeneity and publication bias was significant [10]. This is in line with Schmid’s findings. The study revealed that patients exhibited higher thermal detection thresholds (p < 0.0001), suggesting dysfunction in C and Aδ nerve fibers, however, there were no significant differences in mechanical and thermal pain thresholds between the patient and control groups (p > 0.13). A skin biopsy was then performed and revealed a substantial reduction in intraepidermal nerve fiber density in patient group (p < 0.0001), confirming a significant impairment of small nerve fibers [60].
3.6. Summary
The questions around a true gold standard in diagnosing CTS are a fundamental challenge in evaluating the accuracy of different diagnostic measures. Clinical symptoms and signs, NCS, and surgical outcomes are three commonly used criteria, but none of them are perfect and can have false negatives and false positives.
To accurately evaluate the sensitivity and specificity of a diagnostic modality, it is important to compare it against a reference standard that is independent of that modality. For example, when evaluating the diagnostic yield of NCS for CTS, the reference standard should be based on clinical criteria, as suggested by Jablecki et al. [61]. However, the reference standard itself may have limitations and can also have false negatives or false positives, further complicating the evaluation process [39]. Additionally, there has been a lack of comprehensive head-to-head studies comparing some of approaches, which limits our ability to fully evaluate their comparative effectiveness [4].
This inherent challenge underscores the complexity of diagnosing this condition and highlights the need for a comprehensive approach that takes into consideration multiple factors, including clinical symptoms, signs, and results from various diagnostic modalities, to arrive at an accurate diagnosis.
According to Sonoo et al. in the absence of a definitive gold standard for CTS, it is advisable for clinicians to employ all reasonable diagnostic measures, including assessing symptoms and signs, as well as conducting NCS, to enhance diagnostic accuracy. By utilizing multiple diagnostic tools, clinicians can gather comprehensive information and make well-informed decisions regarding the diagnosis and management of CTS [39].
Provocation tests can serve as a first-line evaluation tool, quickly identifying potential CTS. However, they are not definitive, so they should be followed by more objective methods like NCS and USG to confirm the diagnosis.
There is a growing body of research papers claiming the effectiveness of carpal tunnel surgery without prior diagnostic investigation [62]. According to Fowler, based on their clinical experience, many surgeons operate without arranging NCS because of patient satisfaction. However, the placebo effect of the intervention is often not taken into account. Therefore, there exists a divergence of opinion between surgeons, who do not consider NCS as an essential component of their routine management for patients, and neurophysiologists, who believe that these tests provide clinical value and should be conducted in all patients with suspected CTS before surgery. Fowler then concludes that the key factor contributing to this discrepancy is the oversimplified perception of NCS for CTS as solely a binary confirmatory test, aiming to determine the presence or absence of the disease. From his experience, however, out of 21,000 patients who tested negative for CTS using standard diagnostic tests, 7% exhibited neurophysiological evidence of an alternative neurological condition that could account for their symptoms. Conversely, among patients with confirmed evidence of CTS, 5% also demonstrated indications of an additional neurological disorder.
These findings highlight the importance of identifying coexisting conditions, as they can impact the treatment outcomes for CTS or necessitate separate treatment approaches based on their individual merits [62]. Depending on the suspected condition, the use of different diagnostic techniques may be warranted. NCS can help assess the physiological damage to the median nerve, while USG can provide structural insights into nerve swelling and compression.
While MRI is excellent for visualizing nerve pathologies and associated conditions that may not be evident on NCS or USG [63], it is more costly and time-consuming. It may be recommended in situations where the patient is unresponsive to therapy and/or for research purposes. However, it does not offer sufficient additional valuable information to replace routine evaluation. It’s worth noting that some patients prefer MRI over NCS for diagnostic purposes [14]. Combining MRI with NCS or USG may be especially helpful in complex or recurrent CTS cases, where additional structural information is needed to guide treatment decisions.
In cases where the sensory symptoms of CTS are difficult to quantify using traditional methods, QST can be used to assess sensory abnormalities in more detail. This is particularly valuable for tracking changes in sensory function post-treatment or surgery and understanding the pathophysiology of CTS, especially when electrodiagnostic tests show normal results despite clear clinical symptoms.
Overall, these diagnostic methods should be viewed as complementary rather than standalone. Using a combination of clinical tests, NCS, USG, MRI, and QST, can provide a more accurate and comprehensive evaluation of CTS, helping to confirm the diagnosis, assess severity, and guide treatment options (Table 8). The suitable tools available should be primarily used to provide information required of them, rather than arbitrarily classify CTS as “confirmed” or “ruled out”.
| Technique | Advantages | Shortcomings | Application |
|---|---|---|---|
| Provocation tests | • Low cost and time required | • Subjectivity and variability (dependent on method of examination and interpretation) | • Preliminary diagnosis |
| • Availability | • Highly variable sensitivity and specificity | • Screening | |
| • Easy to learn | • Limited value in assessing severity | ||
| NCS | • High specificity | • Questionable sensitivity | • Assessment of median nerve dysfunction specifically |
| • Objectivity | • Questionable correlation between symptom severity and NCS results | • Differential diagnosis, ruling out other potential causes | |
| • Providing quantitative data | • Discomfort for the patient | • Baseline for physiological comparison | |
| • Ability to identify accompanying/mimicking conditions | • Not readily available | ||
| • Baseline for comparison before and after surgery | • Comparative study (between sides), interindividual variability in nerve conduction velocity | ||
| • No small fiber assessment | |||
| USG | • Cost-effectiveness | • No agreement on a universal CSA cut-off value | • Confirmation of anatomical changes of the median nerve (e.g., swelling) |
| • Availability | • No consensus on the site of measurement | • Screening for structural abnormalities | |
| • Noninvasiveness | • Varying reference standards for validation | • Differential diagnosis | |
| • Well-tolerated | • Qualification for surgery and baseline for anatomical comparison | ||
| • Real-time | |||
| • Accurate anatomical assessment | |||
| • Possible correlation with CTS severity (both clinical and electrophysiological) | |||
| • Sensitivity and specificity at least comparable with NCS | |||
| MRI | • Evaluating primary nerve pathologies | • High cost | • Rare pathological causes |
| • Assessment of morphological damage, inflammation, and the extent of nerve compression | • Limited availability | • Additional information before surgery, especially in case of reoperation | |
| • Accurately showing the location of the lesion | • Time-consuming | • Scientific research tools | |
| • High sensitivity and specificity | • Variability in diagnostic criteria | ||
| QST | • Low cost | • Scarce research | • Potential early detection |
| • Assessment of small nerve fiber function | • No normative data | • Potential baseline for comparison | |
| • Possible correlation between the severity of sensory symptoms and QST findings | • No clear universal protocol | • Scientific-additional information about CTS pathophysiology | |
| • Subjective components | |||
- Note: In bold: suggested primary application.
- Abbreviations: CSA = cross-sectional area, CTS = carpal tunnel syndrome, MRI = magnetic resonance imaging, NCS = nerve conduction studies, QST = quantitative sensory testing, USG = ultrasonography.
4. Conclusions
- 1.
The available diagnostic methods should be viewed as complementary rather than standalone. Choosing the right combination can provide a more accurate and comprehensive evaluations of CTS, helping to confirm the diagnosis, assess severity, and guide treatment options.
- 2.
USG seems to provide most of the data needed for qualification for surgical procedures, while its low cost, good tolerability by patients, and increasing availability warrant its ascendance as the primary diagnostic option to be considered after screening. It may be considered as a feasible first-line confirmatory test, especially where access to electrodiagnostic facilities may be limited.
- 3.
Even though some researchers go as far as to consider NCS as outdated and therefore redundant, this seems to be a major simplification. Despite some of its advantages being well covered by other techniques, its effectiveness in assessing the physiological health of the median nerve is unparalleled. This is especially valuable in some aspects of differential diagnosis and postoperative care. It seems, however, that routine NCS for CTS “confirmation” may become unnecessary.
- 4.
QST seems to be a promising technique, uniquely providing additional data about small fiber damage specifically. Even though preliminary results seem promising (especially in the case of thermal thresholds), until further investigations (including multicenter large-scale studies) warrant the establishment of universal normative data, diagnostic protocols and criteria, this technique remains a complementary option. Its time- and cost-effectiveness, however, justify the necessity of urgent further research.
- 5.
MRI is limited by its expensiveness and long duration, therefore it should only be used whenever the cost is justified and in scientific settings.
- 6.
CSA appears to be the most useful measurement in both USG and MRI, however further multicenter clinical investigations are required to establish diagnostic protocols and criteria, especially in specific patient groups.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.




