Paving the Way for Commercial Hydrogen Generation From Natural and Artificial Seawater Based on Photocathode of Manganese(II) Oxide–Manganese(IV) Oxide/Poly–1H Pyrrole Nanocomposite Seeded on Additional Poly–1H Pyrrole Film
Abstract
A MnO–MnO2Poly–1H pyrrole (P1HP) nanocomposite featuring a distinctive semi-pentagonal structure has been synthesized by integrating manganese oxides (MnO and MnO2) into P1HP through an oxidation process. The resulting nanostructure includes large pentagonal particles ranging from 500 to 600 nm in size, along with smaller dotted particles about 20 nm in diameter. This nanocomposite demonstrates excellent optical absorption across a broad range of spectra, with a bandgap of 1.8 eV. The MnO–MnO2–P1HP composite is deposited onto a P1HP substrate, forming a MnO–MnO2–P1HP/P1HP photocathode designed for hydrogen (H2) production using either natural Red Sea water or an artificial seawater solution free of heavy metals as the electrolyte. H2 production is evaluated in a three-electrode cell, where the current density under light (Jph) compared to dark conditions (Jo) serves as the primary indicator of the photocathode’s sensitivity. In natural seawater, Jph reaches −2.55 mA/cm2, while Jo measures −1.6 mA/cm2. In artificial seawater, these values decrease slightly to −2.3 mA/cm2 and −1.4 mA/cm2, respectively. The photocathode’s reproducibility is confirmed through chopped light experiments, which show consistent fluctuations in J values. Its high sensitivity is also demonstrated by its response to varying light energies, ranging from 3.6 to 1.7 eV, with peak Jph values at 3.6 eV (−2.50 mA/cm2) and a decrease to 2.25 mA/cm2 at 2.3 eV. Due to its crystalline structure and cost-efficiency, this MnO–MnO2–P1HP/P1HP photocathode offers a promising solution for the conversion of Red Sea water into H2 gas.
1. Introduction
Renewable and sustainable energy has emerged as a leading candidate in the quest for clean fuel sources [1, 2]. As concerns over climate change and the depletion of fossil fuel reserves grow, hydrogen (H2) presents a promising alternative capable of addressing these critical issues. However, producing H2 gas as a clean and renewable fuel necessitates the development of stable and efficient photocatalysts for future applications [3, 4]. H2 can serve as a viable substitute for fossil fuels, but its production through water electrolysis with 1.23 V due to the slow kinetics of oxygen evolution reactions. In 1972, Fujishima and Honda pioneered the use of ultraviolet light in the water-splitting photocatalytic method. Following this breakthrough, researchers began generating H2 gas using semiconductor photocatalysts. This method of producing H2 gas via direct water splitting offers a potential solution to the energy crisis [5, 6].
The unique characteristics of H2 energy position it as a crucial component in the global shift toward a low-carbon economy. Nevertheless, despite its vast potential, numerous challenges and limitations must be addressed to make H2 energy a widespread reality. The photoreactions used to generate H2 gas are typically performed using metal sulfides, oxides, or polymer materials, which exhibit excellent optical properties and benefits, such as mass production capabilities and low costs. To enhance H2 production, these materials need to possess a large surface area that provides numerous active sites, making nanomaterials, nanowires, and nanosheets ideal for the H2 generation process [7]. Several studies have focused on inorganic materials, such as ZnO, CuO, double oxide, and carbon derivatives, demonstrating improvements in the efficiency of these materials [8–10]. However, the efficiency of these materials has generally remained below 1%. Researchers aim to develop some polymers to achieve an optimal bandgap of 1.2–1.5 eV by enhancing photon capture within the molecular structure. This advancement is exemplified for responding to a wide range of light wavelengths [11, 12].
Photocatalytic H2 production offers significant potential for addressing global energy challenges. Current research in this area is focused on enhancing the efficiency and stability of photocatalysts by combining diverse materials and capitalizing on their unique properties. Scientists aim to develop advanced photocatalysts capable of efficiently utilizing solar energy to drive water splitting and H2 generation; creating composite materials and optimizing their structural and electronic characteristics are critical steps toward improving H2 production efficiency [1, 2]. The future of H2 energy relies on overcoming existing challenges and developing scalable, cost-effective technologies to minimize carbon emissions associated with conventional energy sources. With continued innovation and interdisciplinary collaboration, H2 energy is poised to play a vital role in the transition to a sustainable, low-carbon energy future [7, 13].
Electroactive conductive polymers have the ability to be oxidized or reduced by altering the electronic structure of their polymer backbone. This process is coupled with a charge compensation event where counterions move in or out of a layer, forming structures like ion-enriched sponges, ion gates in membranes, or hydrogels. For instance, poly–1H pyrrole (P1HP) becomes positively charged when oxidized and is neutral and hydrophobic when reduced. The exploration of electroactive conductive polymers for H2 production and other applications continues to evolve. The ability of these polymers to undergo oxidation and reduction, combined with their ion exchange capabilities, presents significant opportunities for developing efficient and versatile systems [14, 15]. The challenge remains to enhance the Jph values and overall performance of these materials to make them viable for large-scale H2 production and other advanced applications.
Research in this area seeks to optimize the structural and electronic properties of polymer composites to improve their efficiency. By combining different materials and leveraging their unique characteristics, scientists aim to create more effective photocatalysts for H2 generation. This involves a detailed understanding of the interactions between synthetic procedure parameters and the resulting material properties. Several literature have explored the use of polymer compounds for H2 gas generation. One area of research has focused on the PANI composite with TiO2, evaluating its photocatalytic performance in water solutions. However, the resulting Jph value showed limited effectiveness. Additionally, previous literature on polymer composites has often employed acidic mediums, such as H2SO4 or HCl, as electrolytes, but these also yielded limited Jph values. Examples of these materials include polyaniline/MoS2, poly(3-aminobenzoic acid) frameworks, and Ni/polyaniline [16–19].
In this study, a MnO–MnO2–P1HP nanocomposite with a unique pentagonal morphology has been synthesized and thoroughly characterized. XPS is employed to identify the peaks corresponding to the different oxidation states of the manganese oxides, while X-ray diffraction (XRD) confirms the crystalline structure of the material. Furthermore, FTIR analysis revealed changes in bond elongation within the P1HP network after the incorporation of manganese oxides. Theoretical modeling and SEM were also utilized to emphasize and verify the distinct pentagonal shape of the nanocomposite.
This MnO–MnO2–P1HP/P1HP photocathode was specifically designed for H2 gas production, using Red Sea water as the electrolyte or a synthetic equivalent that is free from heavy metals. The photocathode’s performance was assessed in a three-electrode cell, where the Jph compared to the Jo was used to measure the sensitivity of the photocathode. The results showed that the photocathode exhibited high sensitivity across a range of light energies, from 3.6 to 1.7 eV. Due to its crystalline structure and cost-effectiveness, the MnO–MnO2–P1HP/P1HP photocathode holds considerable promise for efficiently converting Red Sea water into H2 gas.
2. Materials and Methods
2.1. Materials
Potassium permanganate (KMnO4 99.9%, PIOCHEM co, Egypt), HCl (36%, Merck, Germany), ethanol (C2H5OH, 99.9%, Merck, Germany), Pyrrole (Across Co., 99.9%, USA), and (NH4)2S2O8 (99.9%, PIOCHEM co, Egypt).
2.2. The Fabrication of MnO–MnO2–P1HP/P1HP Photocathode Using the Polymerization Reaction
The fabrication of the MnO–MnO2-poly(1H-pyrrole)/poly(1H-pyrrole) photocathode is a two-step process (Figure 1a,b). The first stage involves creating a poly(1H-pyrrole) seeding layer on a glass substrate. To prepare this layer, a highly soluble solution of 0.06 M pyrrole is dissolved in 0.6 M HCl and stirred thoroughly. To deposit this layer, 0.15 M (NH4)2S2O8 is suddenly added while stirring, initiating the polymerization reaction and forming the P1HP seeding layer.
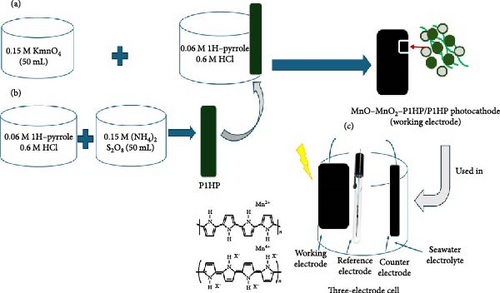
In the second step, the photocathode fabrication is finalized by carrying out a polymerization reaction using the same pyrrole mixture, with 0.15 M KMnO4 serving as the oxidant. This reaction facilitates the polymerization and integration of MnO–MnO2 into the P1HP network, forming the MnO–MnO2–P1HP nanocomposite. The resulting composite is then deposited onto the initial P1HP layer, with the reaction continuing for 24 h. Following fabrication, the layer undergoes treatment through immersion in distilled water, followed by drying at 60°C, completing the production of the MnO–MnO2–P1HP/P1HP photocathode.
2.3. The H2 Generation Photoelectrochemically Using the MnO–MnO2–P1HP/P1HP Photocathode
The H2 generation reaction is conducted using either Red Sea water or equivalent artificial seawater prepared in the lab with a specific chemical composition. The H2 gas estimation is carried out through photoelectrochemical methods, where the fabricated MnO–MnO2–P1HP/P1HP photocathode functions as the working electrode for a three-electrode cell setup. This cell uses the natural or equivalent artificial seawater as the electrolyte. All tests are conducted using the CHI 608E device, which measures the reaction through linear sweep voltammetry or time-current relationship estimation under white light illumination (Figure 1c).
3. Results and Discussion
3.1. The MnO–MnO2–P1HP Nanocomposite Physio-Chemical Analyses
The chemical composition of the synthesized MnO–MnO2–P1HP nanocomposite includes functional groups, crystalline structure, crystalline size, and elemental composition, along with their oxidation states, as demonstrated in Figure 2. The FTIR analysis (Figure 2a), where the expected bands corresponding to the MnO–MnO2–P1HP composite or the pristine P1HP material are identified, and their positions are noted.
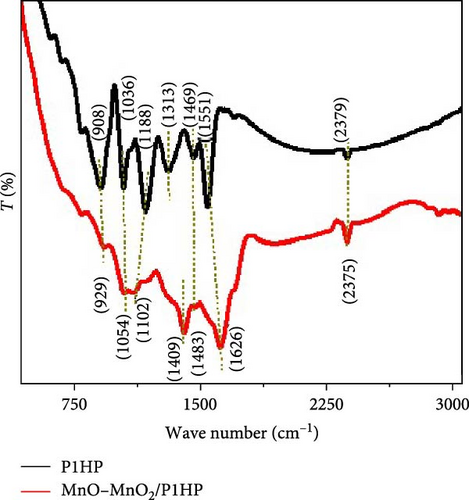
For the pristine P1HP polymer, the ring structure is characterized by bands at 908, 1036, 1188, 1313, 1469, and 1551 cm−1. In contrast, the MnO–MnO2–P1HP nanocomposite exhibits similar bands but at slightly shifted positions: 929, 1054, 1102, 1409, 1483, and 1626 cm−1. The observed minor red-shifts in most of these bands or the variations in their intensities can be attributed to the incorporation of the MnO–MnO2 groups [20].
To provide further clarity on the distribution of these bands and their association with specific functional groups, Table 1 is included in the study. This table outlines the precise positions of the bands and links them to the respective functional groups, thereby offering a detailed understanding of the structural modifications induced by the addition of the MnO–MnO2 components to the P1HP polymer.
| Group and its value (cm−1) | Function group | |
|---|---|---|
| MnO–MnO2–P1HP | P1HP | |
| 929 | 908 | C─H out of plan |
| 1054, 1102 | 1036, 1188 | C─H group |
| 1409 | 1313 | C─N [21] |
| 1483 | 1469 | C─C |
| 1626 | 1551 | C═C |
The FTIR analysis is crucial for confirming the successful synthesis of the nanocomposite and understanding the interaction between the P1HP polymer and the MnO–MnO2 groups. The shifts in the bands are indicative of changes in the chemical environment and bonding interactions within the nanocomposite. These spectral changes provide insights into how the MnO–MnO2 incorporation affects the overall structure and properties of the P1HP polymer.
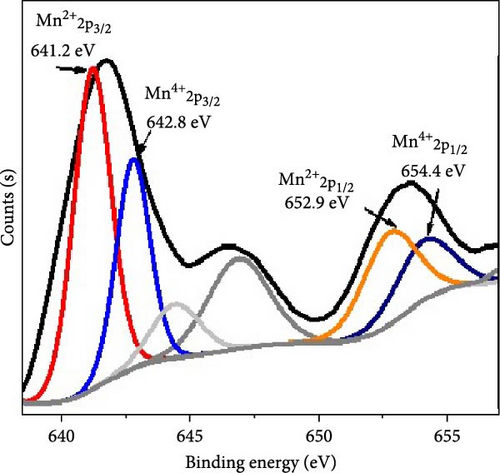
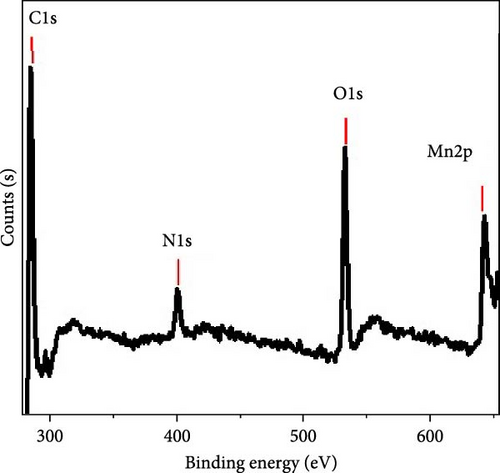
The chemical structure of the synthesized nanocomposite has been evaluated using XPS analysis, as illustrated in Figure 2b,c. Figure 2c presents the survey scan, while Figure 1b focuses on the Mn 2p region. In the Mn 2p spectrum, doublet peaks indicate the presence of two oxidation states of manganese within the composite. These peaks correspond to the 2p3/2 and 2p1/2 orbitals. For Mn(II), the peaks are located at 641.2 and 652.9 eV, whereas for Mn(IV), the peaks appear at 642.8 and 654.4 eV [22]. Additionally, the excitation of oxygen at 532 eV confirms the presence of MnO and MnO2 in the material.
The XPS analysis also detects other elements in the composite, such as carbon and nitrogen. The carbon peak at 285.1 eV is associated with C─C and C═C bonds, indicating the structural presence of carbon within the P1HP polymer. The nitrogen peak at 400 eV corresponds to C─N and C = N bonds, further detailing the chemical structure of the P1HP polymer.
The presence of Mn(II) and Mn(IV) oxidation states and their respective binding energies provide crucial insights into the oxidation states of manganese in the composite. The binding energies at 641.2 and 652.9 eV for Mn(II) and 642.8 and 654.4 eV for Mn(IV) are characteristic of these oxidation states and confirm the coexistence of MnO and MnO2 within the nanocomposite. This dual oxidation state of manganese plays a significant role in defining the chemical and physical properties of the material.
Furthermore, the excitation of the oxygen element at 532 eV in the XPS spectrum confirms the formation of oxides, specifically MnO and MnO2, which are integral to the nanocomposite’s structure. The XPS analysis not only identifies the oxidation states but also provides a detailed for the elemental composition and chemical environment within the material.
The detection of carbon and nitrogen elements, with binding energies at 285.1 eV and 400 eV, respectively, highlights the contribution of the P1HP polymer to the overall structure of the nanocomposite. The carbon signal indicates the presence of C─C and C═C bonds, typical of organic polymers, while the nitrogen signal suggests the presence of C─N and C═N bonds, which are characteristic of the P1HP polymer.
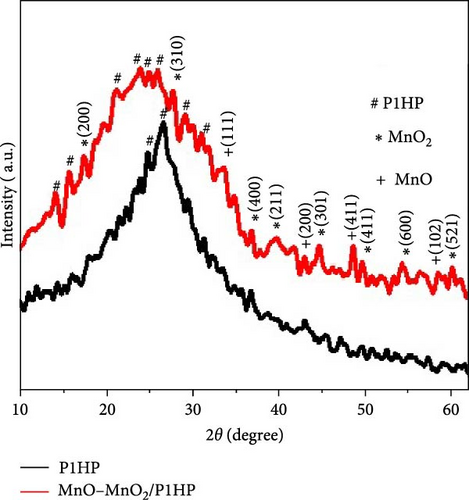
The chemical structure of the synthesized MnO–MnO2–P1HP nanocomposite is highlighted through XRD analysis, as illustrated in Figure 2d. This analysis reveals four peaks corresponding to MnO and eight peaks associated with MnO2, completing the chemical structure of the composite. Specifically, the MnO peaks are observed at 33.9°, 43.1°, 48.7°, and 58.5°, corresponding to the (111), (200), (411), and (102) crystal growth directions, respectively [23]. For MnO2, the peaks appear at 17.5°, 27.8°, 37.0°, 39.7°, 44.8°, 49.9°, 54.4°, and 60.2°, corresponding to the (200), (310), (400), (211), (301), (411), (600), and (521) crystal growth directions, respectively, (JCPDS 65-3333) [24].
In comparison, the pristine P1HP polymer exhibits peaks at 24.9° and 26.9°, indicating its crystalline nature. Within the MnO–MnO2–P1HP nanocomposite, P1HP displays six peaks ranging from 15.8° to 31.2°. These peaks collectively suggest that the MnO–MnO2–P1HP nanocomposite has a highly crystalline structure, which contributes to its excellent optical properties.
The crystalline size (D) of the nanocomposite is evaluated using the full width at half maximum (FWHM) method, yielding a size of approximately 75 nm. This crystalline structure is effective for photon capture when the composite is exposed to light illumination.
H2 gas evolution is driven by photochemical reactions, making the optical properties of the synthesized MnO–MnO2–P1HP nanocomposite particularly promising compared to the pristine P1HP. As depicted in Figure 3a, the optical behavior is assessed through the absorption characteristics of these materials up to 1000 nm. The nanocomposite demonstrates absorption up to 580 nm, whereas the P1HP polymer absorbs up to 370 nm. This disparity in absorption range and intensity underscores the influence of the MnO–MnO2 inorganic components in the nanocomposite, highlighting the synergistic effect of combining these materials with the P1HP polymer to achieve enhanced and broader absorption related to the formation of coordination bonds between the inorganic components and the P1HP polymer network [25, 26].
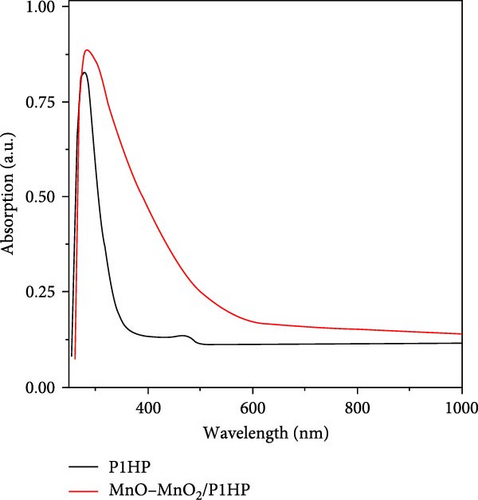
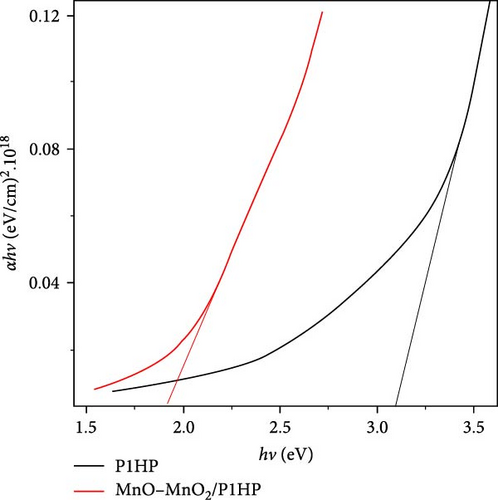
This improved optical behavior is also reflected in the bandgap measurements shown in Figure 3b. The nanocomposite has a bandgap of 1.8 eV, significantly lower than the 3.15 eV bandgap of the pristine P1HP polymer. This lower bandgap indicates that the composite is highly efficient in absorbing visible and ultraviolet light, which together constitute over 50% of sunlight.
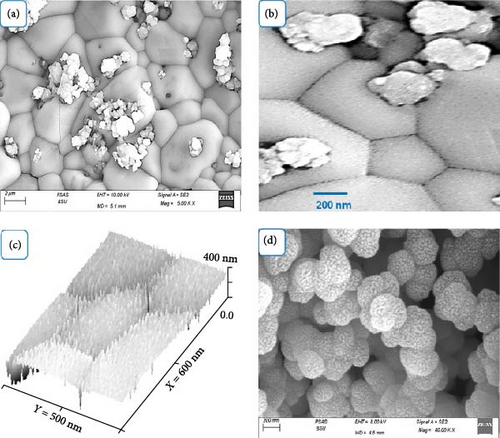
To understand the relationship between the morphological behavior and the optical properties as well as H2 gas generation, we examine the topography and morphology of the MnO–MnO2–P1HP nanocomposite demonstrated in Figure 4a that reveals a surface with significant roughness and densely packed particles, interspersed with small porous structures. This morphology is promising for photon trapping and capture. Figure 4b offers a closer look at these features, showing pentagon-shaped particles with very small spot particles on their surfaces. These tiny particles serve as excellent receptors for illuminated photons. The larger pentagonal particles measure approximately 500–600 nm, while the small spotted particles are about 20 nm in size. These small particles enhance photon trapping and facilitate the transfer of energy to receptor particles, generating hot electrons that activate nearby solutions [27]. Further details of the pentagonal particles are theoretically modeled in Figure 4c, which shows a clear pentagonal structure with dimensions around 500 nm x 600 nm. This structure is covered with small 20 nm particles, reinforcing their role in photon reception and energy transfer. Additionally, the P1HP component of the nanocomposite exhibits a semi-spherical morphology with high porosity due to the cross-linking of the polymer and the internal network formed within these particles. These semi-spherical particles are approximately 300 nm in size. Their morphology not only enhances light reception but also facilitates interaction with other particles in the composite [28], contributing to the overall efficiency of the nanocomposite in applications like H2 gas generation.
3.2. The MnO–MnO2–P1HP/P1HP Photocathode Applications for the H2 Gas Generation From the Natural Red Sea Water or an Equivalent Water
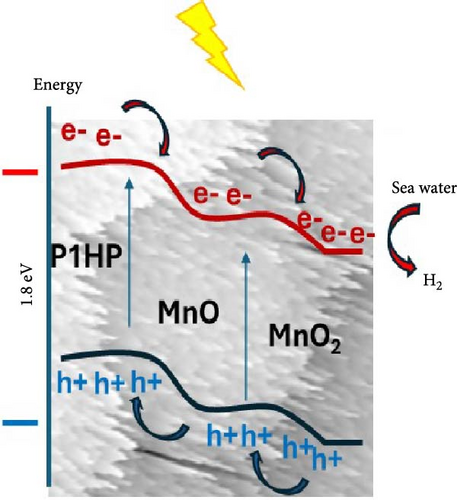
The estimation of green H2 generation is conducted using a specially fabricated MnO–MnO2–P1HP/P1HP photocathode. This electrode, characterized by its unique pentagonal shape and extremely small crystalline size, along with a limited bandgap of 1.8 eV, demonstrates exceptional properties that make it highly efficient in photocatalysis. These attributes enable the effective conversion of incident photons into hot electrons, which then interact with seawater to produce H2 gas. The use of seawater, a natural water source, in this process underscores its cost-effectiveness and sustainability with a promising eco-friendly behavior. Moreover, the influence of heavy metals on this reaction is examined by comparing the results with those obtained using an equivalent artificial metal-free water.
The impact of light illumination on the MnO–MnO2–P1HP/P1HP photocathode is assessed by evaluating the photocurrent density, which is directly related to the generation of hot electrons that are excited from the valence band by the energy transferred from photons [29] and then they traverse the small bandgap of 1.8 eV, leading to the formation of highly energetic electrons capable of efficiently splitting seawater to generate H2 gas. The photocathode exhibits a Jph of −2.55 mA/cm2, indicating its potential as a highly promising electrode for water splitting and H2 production. Under varying light conditions, the photocurrent density is measured at 0.8 V, while the Jo is estimated at 1.6 mA/cm2, demonstrating the electrode’s significant sensitivity to light and its effectiveness in utilizing seawater for H2 production (Figure 5a).
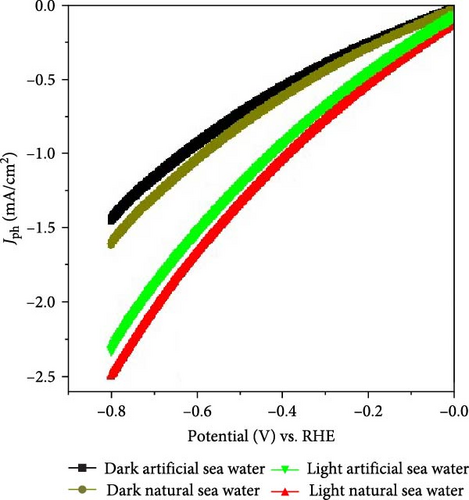
When artificial seawater is used, the values of Jph and Jo are observed to be −2.3 mA/cm2 and −1.4 mA/cm2, respectively, which are comparatively lower than those obtained with natural seawater.
The similar Jo values observed under both water conditions indicate that the presence of heavy metals in natural seawater has a negligible impact on their contribution or deposition during the water-splitting reaction. However, these metals play a significant role in facilitating the splitting reaction by enhancing mobility during the process.
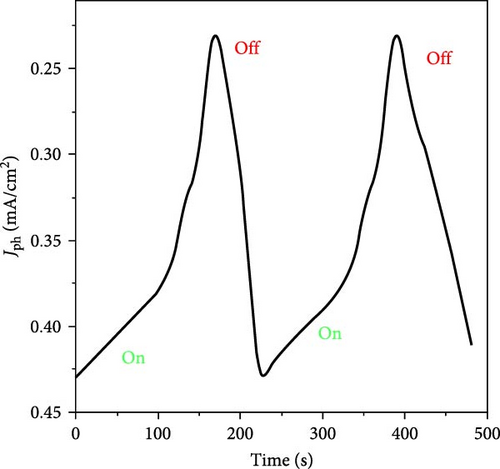
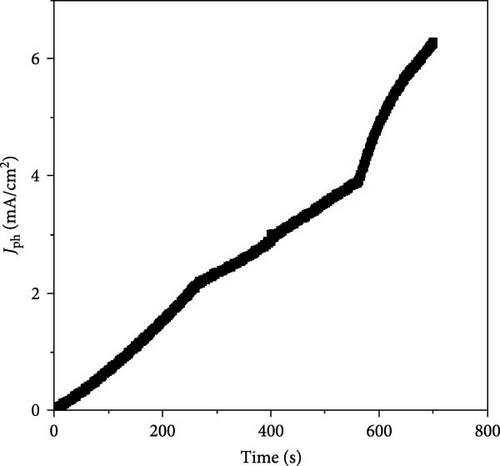
The significant variations in current density under light and dark conditions are further analyzed through chopped light illumination, revealing the material’s behavior and sensitivity to photon activation. The introduction of photons to the photocathode activates the materials, leading to the generation of hot electrons, which in turn create a strong electric field around the materials [30, 31], resulting in the generation of Jph values. These sequential changes highlight the material’s excellent response to photon energy (Figure 5b).
The stability of the MnO–MnO2–P1HP/P1HP photocathode is evaluated through multiple cycles of operation, which demonstrate the reproducibility and durability of the material. The use of P1HP as a covering material for both MnO and MnO2 provides enhanced stability to the entire photocathode, allowing it to effectively utilize both UV and visible light for water splitting. This stability and efficiency make the MnO–MnO2–P1HP/P1HP photocathode a promising candidate for sustainable H2 production from seawater, with potential applications in renewable energy systems.
The sensitivity of the fabricated MnO–MnO2–P1HP/P1HP photocathode is evaluated by examining its behavior under light illumination (Figure 6a). The responsivity of the photocathode varies, with changes in the Jph ranging from −2.5 to −2.22 mA/cm2 as the wavelength shifts from 340 to 730 nm, respectively. Each specific Jph value corresponds to the energy transfer from photons to the electrons within the MnO–MnO2–P1HP/P1HP structure. These materials, being semiconductor photocatalysts, are designed to efficiently absorb photons and generate hot electrons, which in turn create an electric field around the photocathode [32].
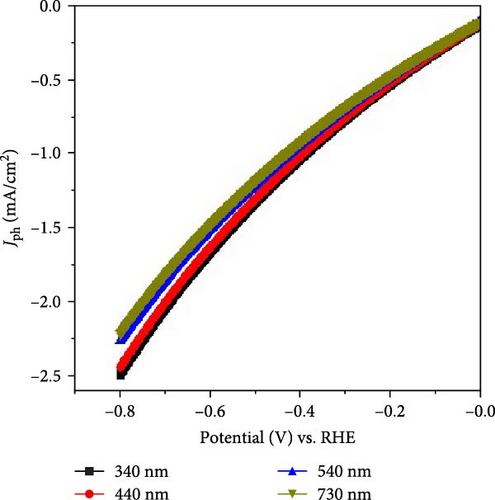
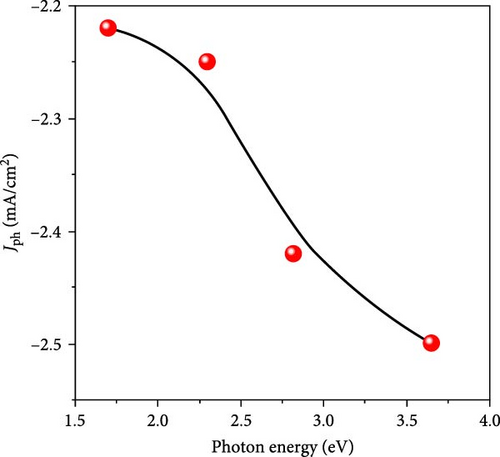
The energy of the photons is calculated using the equation E = hv where E, h, and v are with respect to the energy, Planck’s constant, and the frequency. Since frequency is the reciprocal of wavelength, the photon energies vary from 3.6 to 1.7 eV as the wavelength changes from 340 to 730 nm. Figure 6b illustrates the decrease in current density as photon energy decreases, with the highest absorption occurring at wavelengths of 340 nm and 440 nm, corresponding to maximum Jph values of −2.50 and −2.45 mA/cm2, respectively. This high absorption is attributed to the efficient electron transfer from the bonding (bi) to the antibonding (bi ∗) states within these specific optical regions [33]. This behavior aligns well with the data presented in Figure 3a, which also indicates strong absorption in these optical regions. At a wavelength of 540 nm, there is still some absorption, but the values are lower, resulting in a decrease in Jph to −2.25 mA/cm2, as shown in Figure 6b. As the wavelength increases further, the Jph values continue to decline, corresponding to the bond vibrations without additional electron transfer, leading to a final Jph of −2.22 mA/cm2.
The observed reduction in Jph with increasing wavelength and decreasing photon energy highlights the sensitivity of the MnO–MnO2–P1HP/P1HP photocathode to different light conditions. The photocathode exhibits optimal performance at shorter wavelengths, where photon energies are higher, leading to more effective electron excitation and transfer. As the wavelength increases and photon energy decreases, the photocathode’s efficiency diminishes, as reflected in the lower Jph values. This detailed analysis of the photocathode’s behavior under varying light conditions reflects its potential for efficient H2 generation in specific optical regions. The correlation between the optical absorption properties and the photocurrent response provides valuable insights into the material’s performance and guides the optimization of photocathode designs for enhanced solar-driven water splitting.
The electron transition mechanism within the MnO–MnO2–P1HP photocathode is driven by the variation in energy levels among these materials. This variation facilitates the sequential transfer of generated hot electrons, starting from P1HP to MnO and then to MnO2, while the holes move in the opposite direction [34]. The accumulation of hot electrons on the active surface is crucial, as it initiates the reaction with the neighboring Red Sea water, which contains heavy metals that significantly enhance the H2 generation process. This reaction is influenced by the photon energy, with the density of hot electrons increasing under high-frequency light and decreasing at longer wavelengths.
The surface roughness of the photocathode, as shown in the high roughness image in Figure 7a, is modeled theoretically. This increased roughness enhances electron accumulation on the active surface, further promoting the reaction. The generation rate of H2 gas is demonstrated to be 35 µmol/h·cm2, as illustrated in Figure 7b using Equation (1). This indicates that the MnO–MnO2–P1HP/P1HP photocathode is a promising electrode for H2 production from seawater, particularly when activated by heavy metals. The combination of these factors makes this photocathode highly effective for water splitting and H2 gas generation under various light conditions, and Table 2 provides this promising behavior relative to other previous studies.
| Photoelectrode | Electrolyte | Jph (mA/cm2) |
|---|---|---|
| Polypyrrole/graphene oxide [35] | Sewage water | 0.1 |
| Mn(IV) oxide/Mn(IV) sulfide/poly-2-amino-1-mercaptobenzene [36] | Sewage water | 0.26 |
| Cr2S3-Cr2O3/poly-2-aminobenzene-1-thiol [37] | Sewage water | 0.017 |
| Polypyrrole/NiO [38] | Sewage water | 0.11 |
| Poly-O-aminothiophenol/intercalated iodide composite [39] | Red Sea water | 0.12 |
| Poly-3-methyl aniline/graphene oxide [40] | Sewage water | 0.09 |
| MnO–MnO2–P1HP/P1HP (this work) | Sewage water | 2.5 |
4. Conclusions
A MnO–MnO2–P1HP nanocomposite featuring a distinctive pentagonal morphology has been synthesized and thoroughly characterized. XPS is used to identify the peaks associated with the different oxidation states of manganese oxides, while XRD confirms the material’s crystalline structure. Additionally, FTIR revealed changes in bond elongation within the P1HP network following the incorporation of manganese oxides. The nanocomposite consists of large pentagonal particles, ranging in size from 500 to 600 nm, and smaller particles around 20 nm. Optical analysis indicates that the material exhibits strong absorption across a broad range of the optical spectrum, with an estimated bandgap of 1.8 eV.
The MnO–MnO2–P1HP composite was deposited onto a P1HP substrate, forming a MnO–MnO2–P1HP/P1HP photocathode, which has been specifically engineered for H2 gas production using Red Sea water as the electrolyte or a synthetic equivalent free from heavy metals. The performance of the photocathode was evaluated using a three-electrode cell, where the Jph compared to the Jo serves as a measure of the photocathode’s sensitivity. In natural seawater, Jph was found to be −2.55 mA/cm2, with a Jo of −1.6 mA/cm2. When tested with artificial seawater, these values decreased slightly to −2.3 mA/cm2 and −1.4 mA/cm2, respectively. The photocathode demonstrated high sensitivity across different light energies ranging from 3.6 to 1.7 eV, with a maximum Jph of −2.50 mA/cm2 at 3.6 eV, which reduced to 2.25 mA/cm2 at 2.3 eV. Owing to its crystalline nature and cost-effectiveness, this MnO–MnO2–P1HP/P1HP photocathode shows significant potential for converting Red Sea water into H2 gas.
Ethics Statement
This study does not include any human or animal studies.
Conflicts of Interest
The authors have no conflicts of interest.
Author Contributions
Mohamed Rabia: experimental and writing. Asmaa M. Elsayed and Maha Abdallah Alnuwaiser: writing, funding, supervision.
Funding
Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2025R186), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2025R186), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




