Progress in Sodium-Ion Batteries: A Focus on Phosphate-Based Cathodes and Their Interaction With Electrolytes
Abstract
Sodium-ion batteries (SIBs) are attracting interest from both the scientific and commercial sectors due to their unique approach, which contrasts lithium-ion batteries (LIBs). SIBs employ easily accessible and low-cost salt as a primary resource, making them environmentally viable and cost-effective. Researchers have focused on enhancing the performance of cathode, anode materials, and electrolytes in the SIB field, recognizing the urgent need to compete with LIB technology. The paper will concisely address current progress in polymer-based electrolytes and cathode structural materials for SIBs, providing an in-depth review of the developing technology.
1. Introduction
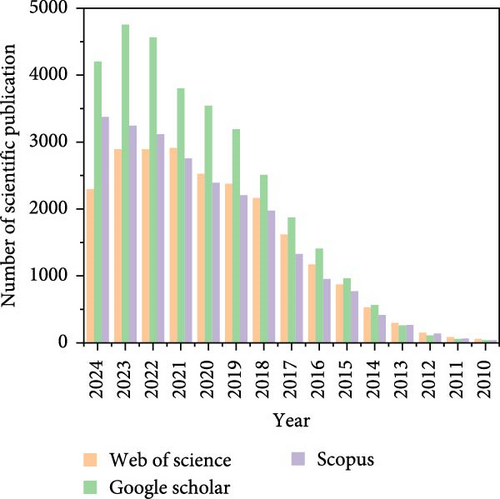
The growing demand for energy storage devices has led to accelerated advancements in the development of innovative energy storage systems that remain cost-effective and environmentally friendly. These systems are being developed as alternatives to existing lithium-ion batteries (LIBs), which are costly and prone to high temperatures [1–6]. Since the rapid expansion of portable devices, and electric vehicle industries, as well as concerns regarding the scarce supply of lithium, significant enhancements are urgently required in the energy storage field. Adopting alternative electrode materials is expected to reduce dependency on lithium resources [7–13]. Sodium (Na) is an abundant substance that occurs in the Earth’s crust. It is a potential replacement for lithium since it has a similar reaction mechanism and is readily available. Na is especially commonly found in seawater, where it exists in large quantities as dissolved salts [2, 14, 15]. As a result, sodium-ion batteries (SIBs) have received significant interest due to distinct safety advantages and the abundant availability of Na resources, lower power density, improved longevity, and similar reaction behavior to LIBs [16–18]. The mechanism by which SIBs function is fascinating due to the reversible movement of sodium ions (Na+) between the anode and cathode. During discharge, the device is powered by the passage of electrons through an external circuit. At the same time, the key players in this process are the Na ions transported from the anode (which is typically carbon-based) to the cathode through the electrolyte. During recharge, the battery is recharged by the return of electrons through the circuit. This is accomplished by driving Na ions from the cathode to the anode with an external power source. The fundamental components of redox reactions at the anode and cathode are the insertion and extraction of Na ions. SIBs are promising candidates for large-scale energy storage not only due to the abundance of Na but also due to their low cost. The widespread availability of Na makes it an attractive option for significantly lowering battery expenses, providing a reassuring economic aspect to their potential. Several studies have focused on electrode material development using different approaches to improve battery efficiency [19–25]. The battery research communities have focused on advancing the development of cathode materials that exploit polyanionic compounds. Phosphate is a highly desirable material for cathodes among these compounds due to its affordability, outstanding efficiency, and improved electrochemical potential. These materials are widely recognized as high-capacity cathodes. Several drawbacks impede the extensive commercial utilization of these materials, such as limited strength, inadequate storage stability, and slow kinetics. Some proposed solutions have gained significant traction in addressing the limitations associated with polyanionic materials and are currently being extensively employed [26]. Much attention has been focused on polyanionic compound-modified cathode materials that have a higher theoretical capacity, such as NaFePO4 [27–29], NaVPO4F [30–33], Na3V2 (PO4)3 (NVP) [34–39], and Na3V2 (PO4)2F3 (NVPF) [36, 40–43]. Significant attention has been gained as a result of their improved theoretical capability [15]. Li et al. precisely presented the fabrication of hollow amorphous NaFePO4 with a nanosphere structure via the hard template technique. This material had excellent storage capabilities. The cathode material NVP has a structure that acts as a super-ion conductor for Na. It has gained considerable interest, owing to its abundant interstitial channels that facilitate the movement of Na+ ions. Cao et al. [44] conducted a significant investigation whereby they successfully obtained a three-dimensional (3D) covering resembling graphene on NVP nanoflakes. This was accomplished using a one-step, solid-state reaction in a molten hydrocarbon. The cathode material obtained, consisting of NVP nanoflakes covered with graphene, displays remarkable electrochemical characteristics. The reversible capacity of the material is nearly equal to the theoretical limit, measuring 115.2 mA h g−1 when measured at a rate of 1 C. Additionally, it demonstrates higher rate capacity, achieving a capacity of 75.9 mA h g−1 at a rate of 200 C. Furthermore, it exhibits excellent cyclic stability, retaining 62.5% of its capacity over 30,000 cycles at a rate of 50 C. Furthermore, the cycling stability of the SIB was satisfactory, with a capacity retention rate of 77.1% throughout 200 cycles at the same current density of 0.1 A g−1. Hard carbon has been studied in great detail for SIBs; however, because of an irregular solid electrolyte interface (SEI) layer that limits reversible Na insertion and removal, hard carbon experiences an initial capacity loss that affects capacity retention over cycling [45]. According to reports, their structures significantly influence the flow of Na+ ions in olivine-structured the distinctive pathway for Na+ transfer [46]. Lin et al. [47] synthesized Na2MnPO4F/C nanocomposite materials using the spray drying technique. These materials demonstrated initial discharge capabilities of 140 and 178 mA h g−1 at 30 and 55°C [47]. The main aim has been to investigate cathode materials for SIBs that are both cost-effective and durable, as these batteries are crucial for achieving efficient energy storage on a significant scale. Ren et al. [48] successfully tackled the challenge by employing electrospinning and subsequent pyrolysis to produce hierarchical carbon-decorated Na4Fe3 (PO4)2P2O7 nanofibers. Using an innovative design in the study resulted in the creation of a cathode that demonstrates a significant reversible capacity of 118 mA h g−1 when operated at a rate of 0.2 C. In addition, the cathode under consideration has remarkable cycling stability, because it maintains 79.6% of its initial capacity after it undergoes 10,000 cycles at a rate of 10 C. Furthermore, the observed performance of the system is remarkable; when discharged at a rate of 20 C, it attains a specific capacity of 64 mA h g−1. Fang et al. [49] employed a freeze-drying route to fabricate different manganese (Mn)-doped NVP/C composite cathode materials for Na-ion batteries. The findings from the modified cathodes demonstrate a capacity of around 107 mA h g−1 at 1 C and maintain a capacity of 78 mA h g−1 at 30 C [49]. It is of greatest significance to emphasize that oxides operating voltage, stability, and energy density are significantly impacted by several parameters, including structural characteristics, Na concentration, and the specific transition metals present in their composition. A steady voltage range of around 4.2–4.5 V versus Na+/Na is frequently observed in electrolytes and is regularly used in literature, using organic carbonate- or ether-based solvents. This voltage limitation further restricts the performance of cathode materials [50]. The issue of inadequate electrical conductivity is a substantial challenge in the efforts to improve the practical effectiveness of cathodes based on polyanions. Several approaches have been utilized to tackle this problem. The incorporation of carbon-based materials, like graphite, carbon nanotubes (CNTs), reduced graphene oxide (rGO), and cation substitution, has been exploited (e.g., Mn, Ni, Mg). Nevertheless, the current endeavors have not yet fulfilled the criteria necessary for extensive utilization in the business community [18, 51–57]. Zhang et al. [58] demonstrated a scalable sol–gel technique for fabricating cathode materials made up of Ni-doped NVP/C composites. The findings revealed that these Ni-doped NVP/C cathodes displayed improved rate performance compared to their initial samples, a significant advancement in the field of energy storage. This research could potentially lead to the development of more efficient and cost-effective SIBs for commercial use, thereby addressing the pressing need for high-performance energy storage solutions. Zhou et al. [59] introduced a novel approach in their research, proposing a single-step solid-state reaction technique to synthesize carbon-encapsulated Na4MnV (PO4)3 nanorods on a large scale. In SIBs, the NMVP/C composite serves as the cathode material to address the problems associated with insufficient electrical conductivity and poor ion diffusion kinetics. The composite material demonstrates an impressive rate capacity of 58.3 mA h g−1 at 5 A g−1 and displays exceptional cyclic stability, maintaining 85.1% of its capacity after undergoing 1200 cycles at 1 A g−1. The ex-situ X-ray diffraction (XRD) investigation demonstrates the presence of highly reversible phase transitions, emphasizing the exceptional crystal reversibility and structural stability seen throughout the electrochemical cycling process. This research could pave the way for the development of more stable and long-lasting SIBs, thereby contributing significantly to the advancement of energy storage technologies. Chen et al. [60] effectively synthesized vanadium-modified hard carbon submicron spheres, showcasing an anode material with a notable capacity at low potentials and excellent cycle stability. The HC/VC-1300 (vinylene carbonate) electrode exhibits outstanding performance in Na storage, with a capacity of 420 mAh g−1 at a current density of 50 mA g−1, and a favorable ability to maintain high performance even at a current density of 1 A g−1. In a separate investigation, Zhou et al. [61] presented a noteworthy discovery about the remarkable Na+ storage capabilities of Na3V2 (PO4)1.95(SiO4)0.05O2F (referred to as NVPOFSi0.05). This achievement was accomplished by partially substituting PO43− ions in NVPOF with SiO44−. A reversible two-electron redox process is facilitated by adding SiO44− to the crystal lattice, which improves structural integration and leads to a higher average discharge voltage and higher energy density, respectively. As a result, it can be shown that the NVPOFSi0.05 cathode exhibits a significant enhancement in its high operational capacity, with a noted capacity of 75.5 mA h g−1 at 30 C. Furthermore, it showed little capacity degradation when subjected to long-term cycling at 10 C for a total of 1000 cycles. The cell, which combines the NVPOFSi0.05 cathode with a hard carbon anode, has a significant energy density of 280 W h kg−1 and 92.3% of the capacity was retained after 300 cycles at 5 C, demonstrating exceptional long-term cyclability. This research could lead to the development of high-energy-density SIBs for use in a wide range of applications, thereby significantly advancing the field of energy storage. Amid the SIB system, electrolytes are important for the device’s performance and abide by the obligation of ionic transport. It is observed that cycling performance, capacity, applied temperature range and battery system safety are extremely persuaded by the selective electrolytes [62–64]. Investigators have explored various formulations of polymer electrolytes, including gel-polymer electrolytes (GPEs), which serve as substitutes for conventional liquid electrolytes (LEs). Extensive research has been prompted by polymer electrolyte benefits, such as enhanced safety, decreased weight, and simplified production [65, 66]. It is reported that polymer-based electrolytes are considered to be a good alternative to liquid-based electrolytes due to their promising feature like good electrochemical windows, excellent thermal stability, and minimized electrolyte solution leakage issues [67, 68]. Research has centered on the modification of the polymer matrix and the integration of diverse additives to improve electrochemical stability and ionic conductivity. The endeavor to develop innovative polymer electrolyte de-signs is apparent, to resolve obstacles and maximize functionality to facilitate the incorporation of SIBs into a wide range of applications [69, 70]. Solid polymer electrolytes (SPEs) disclose low ionic conductivity while they demonstrated an adequate solution to replace LEs [71–74]. The SPEs are investigated for all-solid-state batteries related to easy fabrication, flexibility, and connection over an electrode/electrolyte interface [74–76]. On the other side, solid electrode electrolyte displayed poor electrochemical performance [77, 78]. Researchers made tremendous efforts to develop GPEs to be used in Na-ion batteries that will provide higher ionic conductivity and flexibility [79–81]. The most abundant substance is water which is comparing fresh water (3%) and saline water (97%). The sea water contains numerous resources like ions and salts, that is, 35 g of salt/liter [82–84]. The resources present in the saline water resulted in the development of purification plants in many parts of the world [85]. The recovery of the resources will benefit economically to the society and avoid discarding. It was calculated that the market value of the elements such as Na, Ca, Mg, and K after being extracted from the desalination plant in Saudi Arabia, could be $US18 billion per year [86]. Due to the low cost of the resources, that is, Na, considerable efforts have been utilized on Na-ion batteries as well as it has a comparable reaction mechanism as of LIBs [87–102]. Various issues related commercialization of SIBs, the size difference between Na and Li ions, during charge/discharge volume change of SIBs is greater than LIBs which affects cycle stability and power capability [90, 91, 103]. These challenges highlight the need for further research and innovation in energy storage, particularly in developing novel materials and electrolytes that can address these issues and improve the performance and applicability of SIBs. Figure 1 represents the number of scientific publications on SIBs through the sources Web of Science, Google Scholar, and Scopus (keyword) over the last 14 years.
In summary, this review paper significantly improves the field of SIBs across several vital details: This comprehensive study offers an in-depth analysis of NaFePO4, focusing on several production processes and their corresponding electrochemical implications. This study provides valuable insights into sustainable energy storage and tackles the many issues associated with developing high-performing SIBs. This paper offers inclusive scrutiny of the production process and electrochemical properties of NVP, focusing on the advantages of customization, examples of which include carbon coating, particle size optimization, and doping. This investigation focused on the electrochemical efficacy, stability, and cycle retention of NVPF using fluorine-based compounds. The research demonstrates that applying carbon coating and composite designs improves performance in these areas. This observation implies a boost in the rate whereby the charge is transferred. This study investigates the potential of GPEs derived from phosphate mixes, focusing on their battery performance attributes. The NVP/GPE/Na cells exhibit remarkable capacity and retention after prolonged cycling. This study showcases improved ionic conductivity and increased cycle stability. This investigation aims to further understand cathode, electrolyte, and GPE in the context of SIBs. By exploring these components, this study seeks to identify techniques that might enhance the efficiency of SIBs and contribute to the development of secure energy storage technologies. The findings presented in this study, a result of thorough and meticulous research, make a valuable contribution to the continuing endeavors aimed at enhancing the efficiency and sustainability of SIBs. These advancements offer significant potential for the development of energy storage systems with a substantial effect, instilling confidence in the thoroughness of the research and the reliability of the findings.
2. Cathodes
Considerable attention is presently directed toward researching potential electrode materials for SIBs, including a wide range of compounds such as oxides, phosphates, and fluorophosphates. Each electrode material has its unique advantages and disadvantages in energy storage applications. In this section, we will discuss the primary significance of each category.
2.1. Phosphates
2.1.1. Na Iron Phosphate
Zhang et al. [104] fabricated a mesoporous Na2FePO4F@C composite from mesoporous FePO4 as a SIB cathode material. The synthesis involved a two-step method to create mesoporous amorphous FePO4 and Na2FePO4F@C (M-NFPF@C) composites. Initially, 0.02 mol of (NH4)2Fe (SO4)2 and ammonium dihydrogen phosphate (NH4H2PO4) were dissolved in deionized water. The NH4H2PO4 solution was gradually mixed with the (NH4)2Fe (SO4)2 solution with stirring. Next, 4 mL of 30 wt% H2O2 was added as an oxidizer, followed by 2 h of stirring, yielding FePO4·3H2O. The precursor was dehydrated at 400°C for 24 h to form FePO4. In the second step, M-NFPF@C was synthesized by ball-milling CH3COONa, NaF, and FePO4 with citric acid, then sintering at 600°C [104]. The resulting electrode exhibited a high reversible capacity (114 mAh g−1 at 0.1 C) and superior rate capability (42 mAh g−1 at 10 C), showing promise for energy storage applications. In a different study by Kim et al. [28], maricite NaFePO4, an initial cycle 142 mA h g−1 capacity was dispensed. The findings of this study significantly contribute to the understanding of NaFePO4 and its potential in SIBs, particularly its unique advantages in energy storage technologies, such as its amorphous structure and consistent voltage variation profiles. The outcome of the findings demonstrates highly impressive cycling stability, with negligible capacity deterioration observed even after 200 cycles [28].
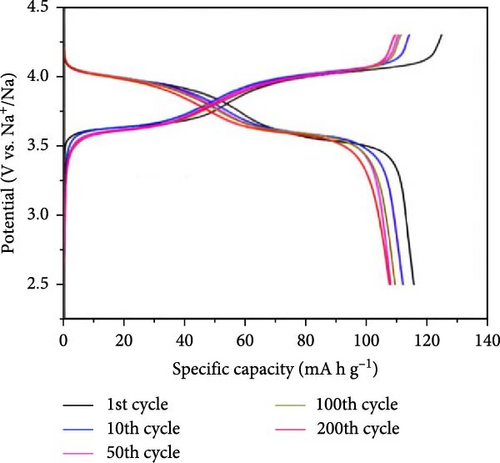
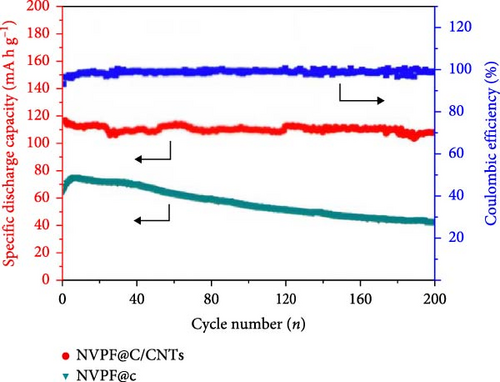
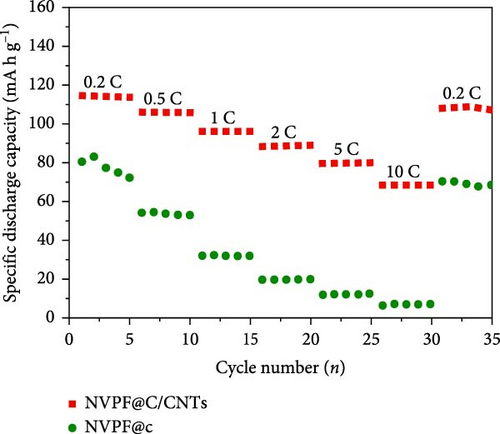
A one-step solvothermal method was employed by Qin et al. [105] to create NVPF@C/CNTs nanocomposites of NVPF@C/CNTs. When comparing NVPF@C with NVPF@C/CNTs, it was noted that the latter displayed a reasonable performance rate and a larger specific discharge capacity. Specifically, NVPF@C/CNTs achieved a discharge capacity of 107 mA h g−1 for 200 cycles at 0.2 C and 75 mA h g−1 for 500 cycles at 5 C. The study focused on the impact of carbon coating, nanostructure, and a 3D conductive network on the performance of the electrode in SIBs (Figure 2). Wang et al. [106] conducted comparative scrutiny on electrode materials of amorphous and crystalline FePO4. The results suggest that the amorphous material demonstrates outstanding electrochemical efficiency, such as improved longevity, rate capability, and capacity. The discharge capacities of amorphous FePO4 were estimated as 179 and 168 mA h g−1, respectively, when subjected to current densities of 20 and 50 mA g−1. Two crucial assumptions support the claim that amorphous materials are more desirable to crystalline FePO4. Amorphous materials have the potential to enhance the movement of Na ions and accommodate volume changes during the process of charging and discharging.
Additionally, the nanosized particles and porosity of amorphous materials promote the passage of ions and electrons, while the large surface area resulting from the nanoscale effect ensures an electronically active surface that remains unaffected by distribution regulations. In a separate study, Boyadzhieva et al. [107] reported that the oxidation of nanosized NaFePO4 occurred at around 280°C in the presence of oxygen. The preparation involved the use of the phosphate-formate method, along with the inclusion of carbon modifications using ball milling. This technique is used to improve the electrochemical efficiency of the materials. Eventually, the maricite NaFePO4 is not electrically active. However, pretreatments such as amorphization, doping, and oxidation can transform it into an electrically active cathode material [107]. The electronic conductivity and capacity of NaFePO4 are not up to the theoretical valve and are strongly reliant on carbon combination and composite materials fabrication in nanoscale methods [108]. The olivine NaFePO4 particles ~80 nm in size coated with carbon deliver around 120 mA h g−1 at a specific capacity and retain nearly 90% after 100 cycles at 0.1 C [15]. In a different study, Zheng et al. [109] examined triphylite-NaFePO4 and mari-cite-NaFePO4 redox reactions. The investigations demonstrated that NaFePO4 exhibits a noticeable charge reduction for oxygen ions during the charging process, but LiFePO4 shows less loss for oxygen ions. Moreover, it is noted that the Bader charges are less than 0.1 e/oxygen, indicating that the oxygen exhibits inadequate electrochemical activation [109]. This section has thoroughly examined the investigation into NaFePO4, encompassing a complete analysis of several synthesis techniques and their influence on electrochemical efficacy. Regardless of the obstacles encountered in attaining the theoretical capability, this assessment contributes to the continuous efforts to advance high-performing SIBs. The findings of this study present opportunities for addressing the need for sustainable energy storage solutions and furthering the practical implementation of NaFePO4-based cathodes in the field of next-generation energy storage systems.
2.1.2. Sodium Vanadium Phosphate (NVP)
Research into the feasible usage of NVP for the cathode material in SIBs has been driven by the increasing need for effective and environmentally friendly energy storage alternatives. With the growing worries over the environmental effect and availability of resources, the need for dependable energy storage technologies has escalated. SIBs have emerged as a viable replacement for conventional LIBs, displaying significant potential. The compound NVP, characterized by its NASICON crystal structure, notable capacity, and desirable thermal stability, emerges as a convincing candidate for use as cathode materials in SIBs. The main focus of this investigation is to enhance our awareness of cathode materials based on NVP, optimize their electrochemical properties, and make significant contributions to the advancement of SIBs with superior performance. Researchers attempt to address the issues of reversibility, ion diffusion, and volumetric changes during cycling by investigating diverse synthesis processes and integrating novel techniques, including carbon coating and doping. The primary objective is to enable the seamless incorporation of cathodes based on NVP into actual conditions, hence advancing the adoption of sustainable energy storage alternatives.
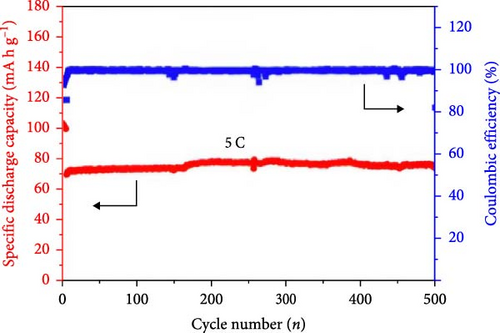
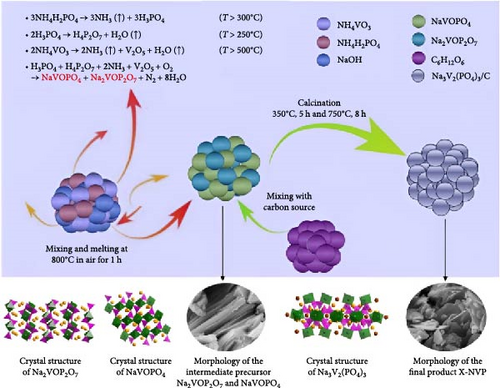
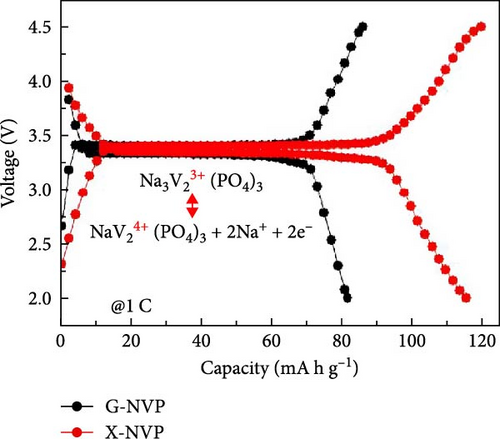
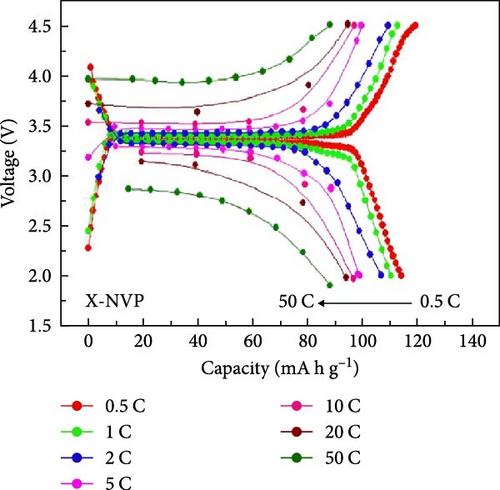
Several cathode materials were previously examined for SIBs. Among them, NVP has received significant interest due to its NASICON structure, higher capacity of approximately 118 mA h g−1, favorable thermal stability, and a high possible level of around 3.4 [98, 110]. Meligrana et al. [111] studied the production of NVP/CNF electrodes using electrospun carbon nanofibers. The researchers specifically focused on optimizing the stabilization and carbonization procedures to enhance the efficacy of the electrodes. NVP, a highly attractive material for cathodes for Na+ technology, has stimulated the exploration of an alternate strategy. The fabricated NVP/CNF electrodes had exceptional cycling performance and demonstrated high C-rate capability, surpassing 200 cycles with no degradation [111]. Zheng et al. [112] presented a two-step process for the development of the resulting products. Initially, NaOH, NH4VO3, and NH4H2PO4 were precisely mixed in a 3 : 2 : 2 as part of the synthesis process. The resulting mixture was placed in a muffle furnace and reacted for 1 h at a temperature of 800°C. The reaction yielded an intermediate product with a green color, indicating the presence of V4+. Subsequently, a procedure known as ball milling was used for around 12 h to incorporate sucrose powder, the carbon source, into the intermediate outcome. The product of the ball milling process was then subjected to thermal treatment in an electric oven at a temperature of 80°C overnight. The specimen underwent sintering at a temperature of 350°C for 5 h to eliminate the presence of water and ammonia. Ultimately, calcination was conducted for 8 h at 750°C in an argon gas environment, forming the composite materials. On the other hand, a one-step method was used to synthesize the carbon-coated NVP materials using a solid-state reduction procedure for comparison. Sodium hydroxide, NH4H2PO4, and ammonium metavanadate (NH4VO3) were added to the mixture, along with an appropriate quantity of sucrose powder. The precursors were homogeneously combined using ball milling for approximately 12 h. The resulting product from the ball milling process was subjected to drying in an oven at a temperature of 80°C for one night. The specimen underwent sintering at a temperature of 350°C for 5 h. Subsequently, the calcination process was commenced at a temperature of 750°C for 8 h in an atmosphere of argon to produce the G-NVP composite. Scheme 1 depicts the mechanism for the formation of X-NVP. The discharge capacity reaches 117 mA h g−1 at 0.5C and then slightly degrades after 250 cycles at 2C. However, an 80 mA h g−1 capacity was measured at 50C, and even after 3000 cycles, the reverse capacity retention remains at 78% (Figure 3). The X-NVP has excellent stability and rate performance when operating at a lower temperature of 20°C [112]. Duan et al. [113] adopted a hydrothermal and sol–gel process to synthesize NVP nanoparticles, yielding a capacity of 104.3 mA h g−1 at 0.5 C and 94.9 mA h g−1 at 5 C and maintaining 96% of its capacity after 700 cycles. The advancement of SIBs has encountered challenges including minimal reversibility, lethargic ion diffusion, and substantial fluctuations in volume. Hydrothermally assisted self-assembly, subsequent in situ crystallization, and N-doped carbon coating comprised the practical method that Cao et al. [114] utilized to produce novel hierarchical porous microspheres (NVP/C-MSs). This methodology guarantees strong structural integrity by employing Na oleate as a structure-directing agent and a Na source. It reduces the extent of volume deformation that may occur during recurrent Na+ extraction and insertion procedures. The half-cell exhibits noteworthy performance characteristics, including a capacity of 116.3 mA h g−1 at a rate of 0.5 C, a capacity of 99.3 mA h g−1 at a high rate of 100 C, and exceptional cyclic stability for more than cycles at 20 C [114]. Salehi et al. [115] studied synthesizing porous NVP/C composite materials employing a cost-effective and scalable sol–gel approach. The structure of the composite materials was modified by adjusting the concentrations of CTAB and glycine, which impacted their electrochemical performance. This modification was carried out in addition to the chemical processing of the materials. The discharge rate of the NVP/C composite materials decreased from 113 to 99 mA h g−1 after 200 cycles at 1 C, while maintaining a capacity retention of 88.1%. Additionally, the composite materials demonstrated remarkable performance at high discharge rates, delivering a discharge capacity of 96 mA h g−1 at a rate of 10 C. This can be attributed to their distinctive structure, conductivity, and morphology [115]. The synthesis of NVP/C through a solution-based technique, aiming at improving Na-ion diffusion, resulted in a capacity of 61.5 mA h g−1 at 40C [116]. Gečė et al. [117] effectively produced and examined the electrochemical characteristics of mixed phosphate–pyrophosphate compounds, namely Na7V4(PO4)(P2O7)4, Na4Mn3(PO4)2P2O7, and Na4Fe3 (PO4)2P2O7. The compounds Na4Fe3(PO4)2P2O7 and Na7V4(PO4)(P2O7)4 exhibited outstanding performance in organic electrolytes but experienced degradation in “water-in-salt” aqueous electrolytes. The compound Na4Mn3(PO4)2P2O7 showed low reactivity in organic electrolytes and negligible reactivity in “water-in-salt” electrolytes, which can be because of breakdown mechanisms [117]. Using the ball milling method, Jian et al. [16] synthesized NVP carbon-coated composites by coating NVP materials with a 6 nm carbon layer. The composites exhibited initial charge and discharge capacities of 98.6 mA h g−1 and 93 mA h g g−1, respectively. Furthermore, these materials retained 99% of their capacity after performing 10 cycles. According to their study, improving the particle size to maximize surface area and modifying the carbon concentration in the composite materials can improve the performance of the cathode. In an alternative report [118], a two-step methodology involved a simple chemistry process and heated pretreatment to synthesize nanosized NVP particles coated with carbon embedded into a porous carbon framework as a cathode material. The composite materials of C/NVP/pC outperformed the cathodes of Li, demonstrating enhanced charge/discharge properties, allowing for a 6-second cycle at 22 A/g current density and 44 mA h g−1 specific capacity (Figure 4).
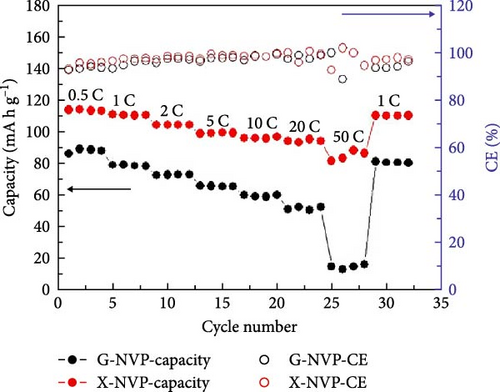
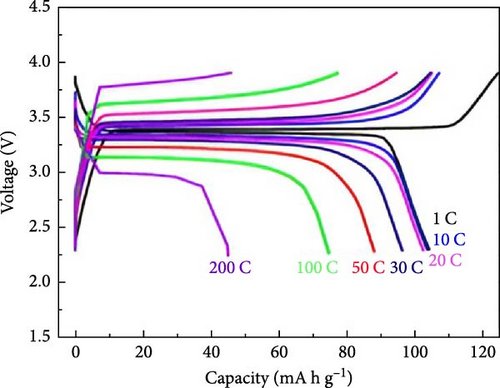
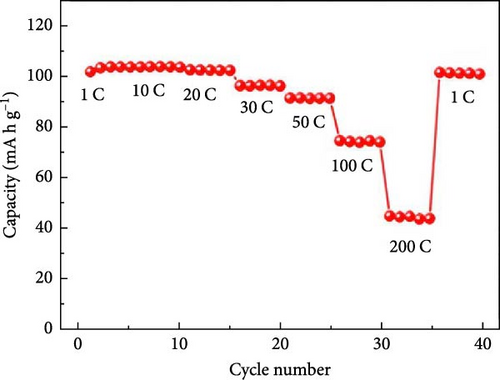
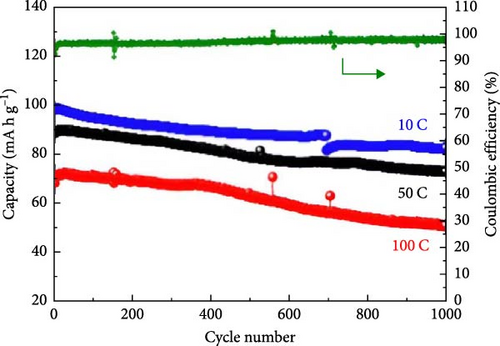
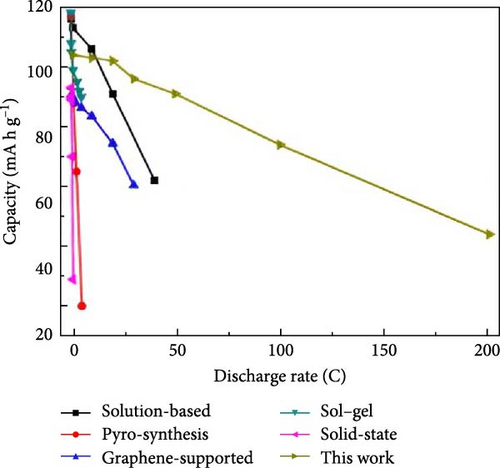
It is reported that NVP/C composites were prepared via (i) ball milling, (ii) spray drying, and successive calcination process. The prepared composite materials displayed consistency when shared with stable counter electrodes and attained 1500 cycles with 80% retention at 100 mA/g [119]. Zheng et al. [34] investigated Li ion doping to enhance the intrinsic properties of NVP. It was observed that when the doping ratio is lower (x ≤ 0.1), NVP-Li ions tend Na2, while at a higher ratio (i.e., x ≥ 0.1) upholding Na1 and Na2 sites due to repulsion (Figure 5) [34].
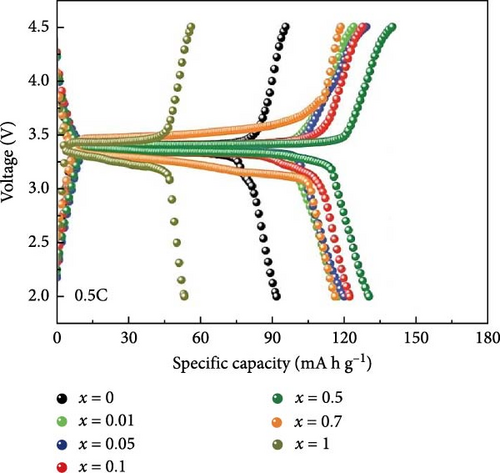
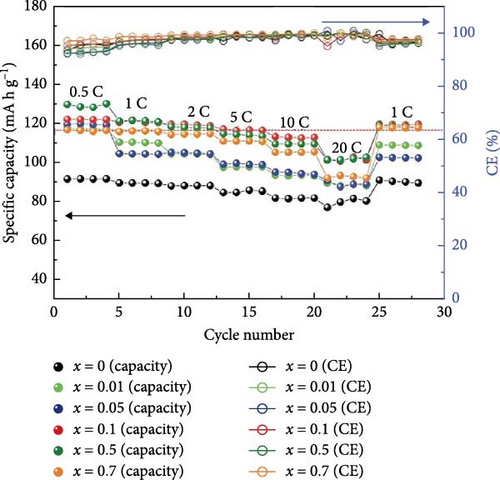
Chen et al. [52] performed a specific study describing their research results on improving Na3+xV2–xMIIx (PO4)3/C composite cathode materials. They accomplished this by using a scalable sol–gel technique and incorporating various portions of Co- and Cu- (x—0, 0.01, 0.03, 0.05). The investigation concluded that doping elevated conductivity, which greatly enhanced the electrochemical performance of the doped materials. Moreover, composite materials like Na3.01V1.99Co0.01 (PO4)3/C and Na3.03V1.97Cu0.03(PO4)3/C showed significant initial discharge capacities of 116 and 114 mA h g−1 at 0.5°C, respectively [52]. Wang et al. [110] investigated the effect of Br and N doping on the electrochemical characteristics of NVP/C composite cathode materials. The presence of doping elements induces the production of amorphous carbon on the nanosize articles of NVP. The doped Br/N/a-C/NVP-2 nanocomposite materials demonstrated a specific capacity of 41 mA h g−1.
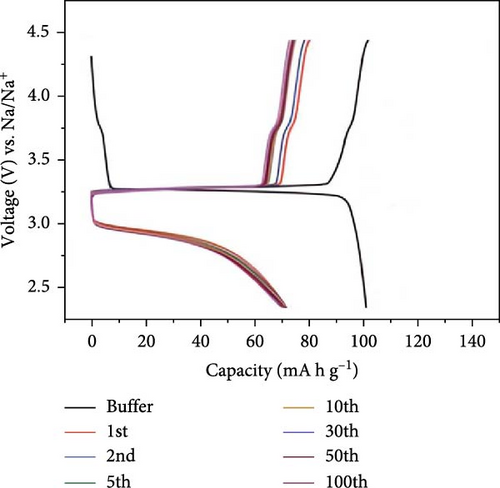
The electrochemical efficiency of NVP can be further enhanced by cationic replacement, as demonstrated by the capacity and cyclic stability enhancement of Mg/Fe substitution [120, 121]. Li et al. [122] showed that the phase structure of Mn-HCF undergoes a transition from rhombohedral to cubic when Sn4+ is introduced. In addition, they noted higher capacity retention of 80.5% after 100 cycles at 240 mA g−1, compared to 54.0% for the Mn-HCF without doping. In a related investigation, the impact of Mn in NVP/C cathode materials was the focus of a doping investigation by Park et al. [123]. Mn is known to strengthen the crystal structure, thereby proving its stability and electrochemical performance. The addition of Mn resulted in a decrease in the band gap and enhanced electron transport compared to NVP. The electrochemical analysis demonstrated that administering Mn to NVP/C enhanced the initial discharge capacity. Particularly, NVMP showed a discharge capacity of 95 mA h g−1, while C/NVMP displayed a higher discharge capacity of 107.4 mA h g−1. Additionally, NVMP composite structural materials, including carbon, showed an impressive discharge capacity of 78 mA h g−1 after 50 cycles at 10C (Figure 6).
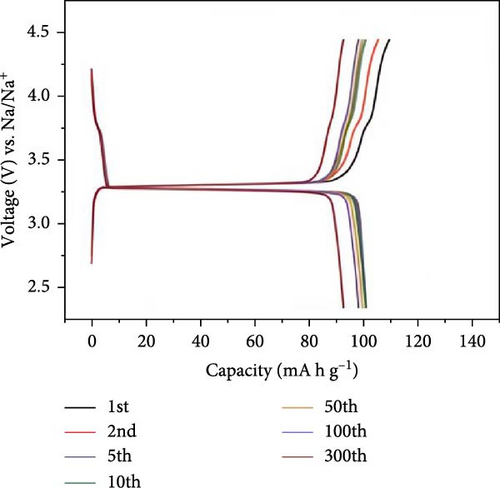
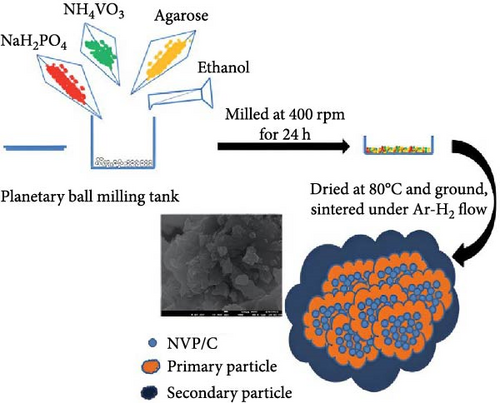
Feng et al. [124] used a solid-phase method to produce cathode materials for SIBs, specifically NVP/C composite. The synthesis of the materials was carried out in a two-step approach: (i) ~1.1 of sodium dihydrogen phosphate (NaH2PO4), NH4VO3 ~ 0.5, and 1.0 g agarose were mixed. (ii) In the next step at 400 rpm, the mixed product was ball-milled for 24 h, and in the final step (iii), two-step calcination treatment was provided at 650°C and 750°C for 6 h and 8 h, respectively, under the environment of Ar/H2 (Scheme 2).
SEM and TEM studies disclosed the framework structure and numerous secondary particles with sizes ranging from 500 to 1000 nm (Figure 7). Additionally, Figure 7d shows the crystallinity of the composite materials, with NVP particles uniformly coated with carbon material measuring around 2–5 nm. The NVP/C composite materials exhibited a significant initial capacity of 116 mA h g−1 following 500 cycles at 1 C, with a capacity retention of 97.3% of its capacity. Furthermore, they maintained 78% capacity retention at 20 C after 7000 cycles [124]. In a critical study, Ling et al. [125] presented a significant 3D hierarchical porous NVP/C structure, which shows enhanced electrochemical performance as a cathode material for SIBs. The cathode materials were produced using a hydrothermal method. The cathode materials were synthesized using hydrothermal methods, milling, and subsequent calcination. The nanocomposite cathode materials demonstrated exceptional electrochemical characteristics, achieving a capacity of 93.3 mA h g−1 after 8000 cycles at 20 C and displaying outstanding performance at high rates [125].
The investigation of Na₃V2 (PO4)₃ (NVP) for SIBs provides critical insights into the optimization of NVP for energy storage. Carbon coating, particle size modification, and doping with elements such as Li, Co, Cu, and Mn have been employed by researchers to improve the conductivity, stability, and efficacy of NVP through a variety of synthesis methods. These improvements address critical challenges in SIB development by enhancing discharge capacity, cycle stability, and rate performance. This research significantly advances NVP-based cathodes, paving the way for their practical use in energy storage. However, it also underscores the need for continued refinement of these materials, as the field of energy storage is constantly evolving.
2.2. Fluorophosphates
Several prominent research groups from around the globe have investigated substances such as Na2FePO4F [126, 127], NVPF [128, 129], NaVPO4F [130, 131], and Na3V2O2x (PO4)2 F3−x [132, 133]. Among them, materials with an NA-SICON-type structure, such as NVP, have received significant interest because of their exceptional ability to store Na+ ions. As a result, they are very desirable as potential materials for the cathode of SIBs. Moreover, Na3V2 (PO4)3F3 (NVPF) has demonstrated superior electrochemical performance, thus emphasizing its potential for diverse battery applications, from portable electronics to grid-scale energy storage [134]. The primary intent of this investigation is to understand the distinctive attributes of fluorophosphate, with the ultimate goal of providing significant knowledge that propels the progress of effective and environmentally friendly energy storage systems.
2.2.1. Fluorine-Based NaVPO4F
Sodium vanadium-fluorophosphate (NVPF) is a unique polyanion molecule characterized by a NASICON structure, which offers a stable 3D framework that allows fast transit of Na+ [135]. Ling et al. [136] devised a carbonization-assisted solvent thermal approach to synthesize NaVPO4F/C composite materials in a single step. The initial process involved the addition of citric acid and NH4VO3 in a 1 : 1 stoichiometric ratio. The mixture was dissolved in 200 mL of double distilled water while stirring at 85°C for 5 h. The gel obtained was subjected to vacuum drying at an oven temperature of 80°C for 12 h. Subsequently, the desiccated precursor transformed into a powdered form and was subjected to sintering at 350°C for 5 h. Later, the product was subjected to heat treatment at 750°C for 5 h in an environment containing argon gas, forming V2O3/C. For 6 h, carbon-coated materials were produced by exposing V2O3/C to ball milling treatment at 200 revolutions per minute. This process resulted in the formation of carbon-coated V2O3/C─B. During the subsequent stage, a mixture of various vanadium sources (V2O3/C, V2O3/C─B, VPO4, and NH4VO3), combined with NaF and NH4H2PO4, in a V/Na/F/P ratio of 1 : 1 : 1 : 1, was introduced into a container made of poly (tetrafluoroethylene). To avoid aggregating together, Na dodecylbenzene sulfonate was employed. The initial mixture was combined with a 1 : 1 of distilled water and ethanol. Then, 70 mL of the resulting mixture was transferred to stainless steel autoclave vessels and heated in an electric oven at 180°C for approximately 18 h. After repeated washings, the final product was stored in a vacuum oven for 12 h at 80°C. Postannealing treatment in the Ar environment was employed to achieve the monoclinic phase of NaVPO4F at 750°C for 8 h. The NaVPO4F/C composite materials demonstrated exemplary performance and cycling stability. The rate performance at 10 C was 75 mA h g−1, whereas the initial capacity of around 133 mA h g−1 reached 0.2 C. The composite materials’s energy density (i.e., 509 W h kg−1) was attained, revealing its high performance. After 1000 cycles, the capacity holding of NaVPO4F/C displayed 77% at 1 C (Figure 8) [136].
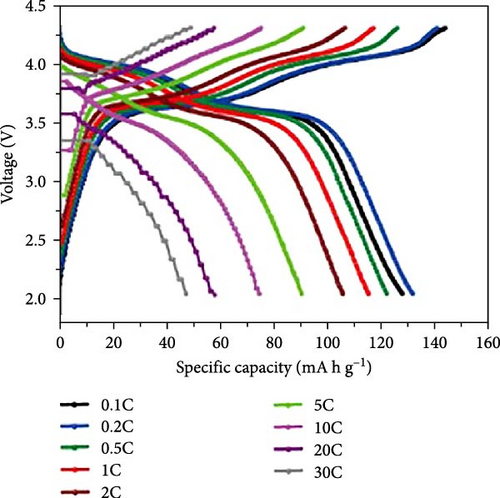
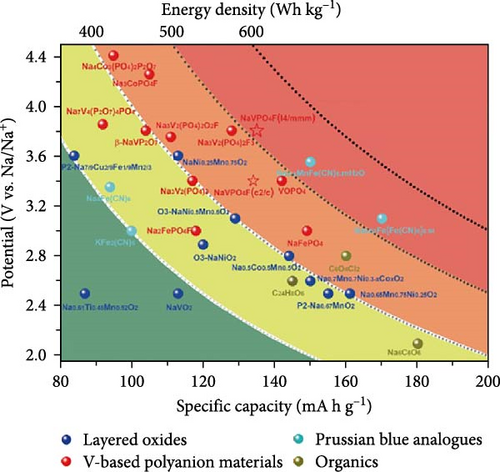
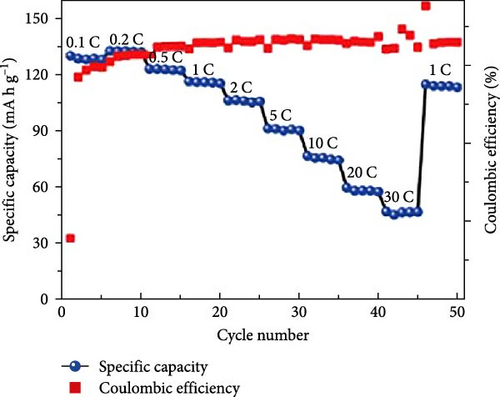
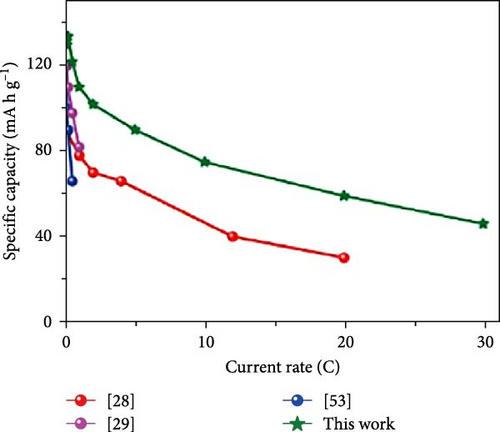
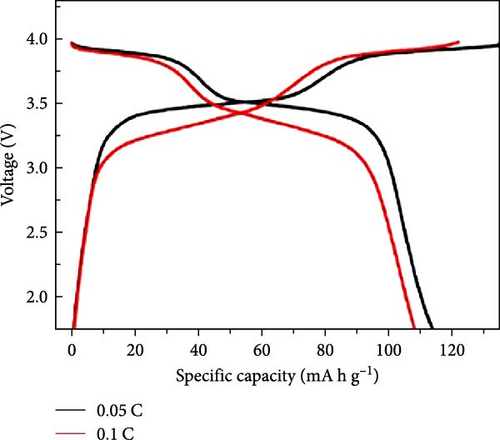
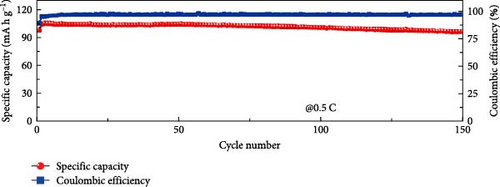
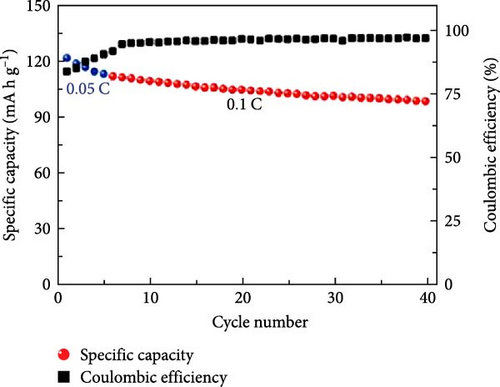
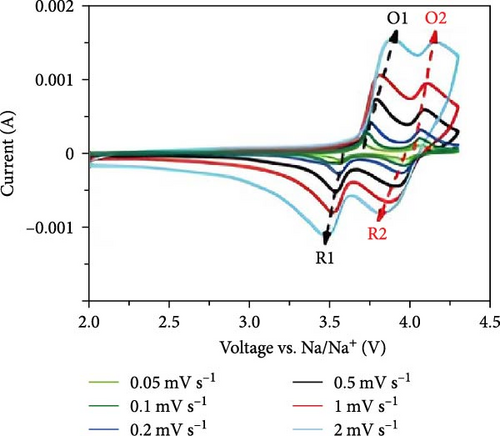

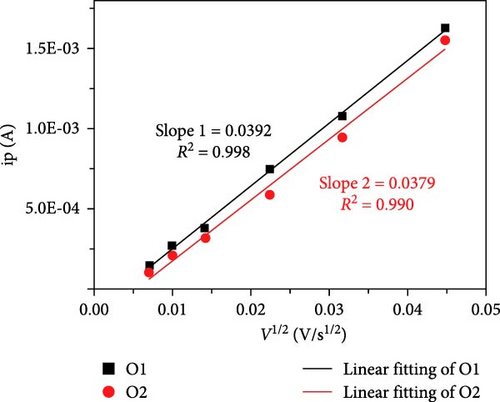
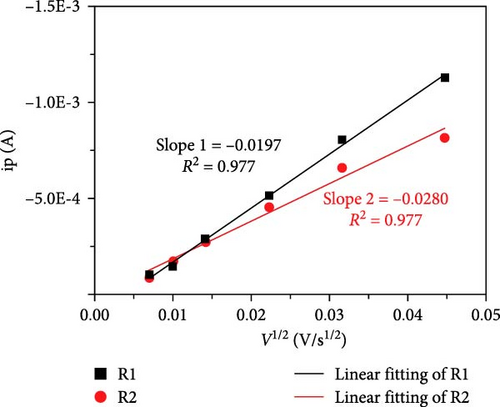
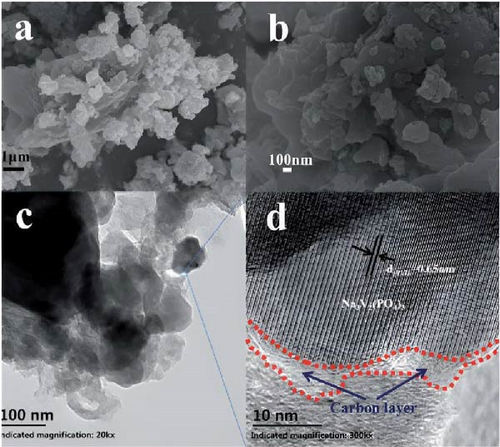
Cheng et al. [137] performed a study where they produced nano-NaVPO4F particles with average particle sizes of around 100 nm attached to rGO sheets using the freeze-drying procedure. TEM examinations yielded valuable insights into the morphology of the composite materials. The uniform crystalline nanoparticles had a size range of 60–90 nm. However, in certain areas, the particle size was found to vary between 100 and 200 nm (Figure 9a), indicating the presence of monoclinic NVPF planes. The composites revealed exceptional electrochemical performance, specifically when used as cathode materials in SIBs. During 3000 cycles, the composite materials exhibited a capacity of 100.0 mAh g−1 at a rate of 10 C and 86.5 mAh g−1 at 100 C. The synthesis technique and exceptional performance of composite materials show great potential for their application as electrode materials in SIBs (Figure 9b). The research conducted on the utilization of fluorine-based NaVPO4F as a cathode material has shown encouraging findings that display its efficacy in SIBs. Carbon coatings and refined synthesis techniques have enhanced electrochemical characteristics, including significant capacity preservation across several cycles. Consequently, fluorine-based NaVPO4F emerged as highly desirable for advanced energy storage purposes. The advancement in comprehending and enhancing the efficacy of cathodes using fluorine plays a vital role in the advancement of battery technologies, which are both practical and sustainable. NVPF is a cathode material that is highly promising for SIBs. This is due to its exceptional electrochemical performance and reliable NASICON structure. Carbon coatings and advanced synthesis methods have further improved its capacity retention and cycling stability, making NVPF a desirable candidate for sustainable, high-performance energy storage applications.
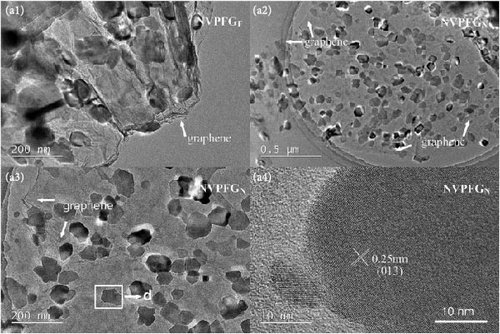
2.2.2. Fluorine-Based Na3V2 (PO4)3F3
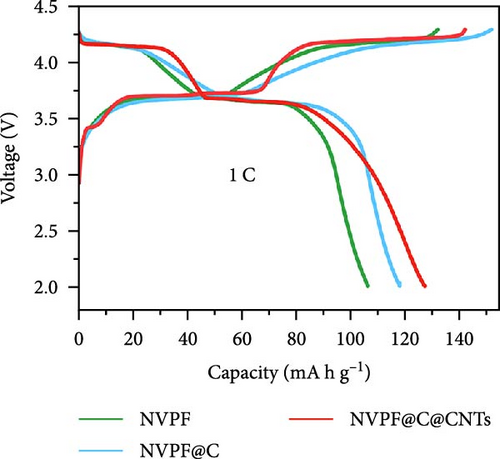
Recent developments have shown that vanadium-based cathode materials for SIBs can be improved by replacing phosphate with fluorine, taking advantage of its strong electronegativity. Gao et al. [138] developed a method to create composite electrode materials by cross-linking a dual-carbon framework with NVPF, improving electric charge transport within the particles. The NVPF@C@CNTs composite demonstrated a remarkable capacity of 129.10 mA h g−1, coupled with exceptional stability. It retained 93% of its capacity even after 1000 cycles at 20 C, as shown in Figure 10 [138]. A separate study addressed a research group’s development of electrode materials for the SIBs. They utilized an easy approach to produce carbon-coated NVPF composite electrodes, with the carbon sourced from polytetrafluoroethylene (PTFE) and fluorine as a substitution agent. The NVPF materials, which are composed of carbon, exhibited exceptional performance in terms of both capacity and stability [139]. In addition, another investigation investigated the synthesis of NVPF/NVP/C composite cathode materials using a carbon thermal reduction process. The hybrid cathode materials were improved in terms of their electrochemical properties, including capacity and stability. They achieved a specific capacity of 83 mA h g−1 at a rate of 100 C and maintained 63.1% of their capacity after 3000 cycles [140]. Thamodaran et al. [141] introduced a single-step process to produce NVPF hierarchical microspheres (NVPF-HMS) as cathode materials for LIBs, which are suitable for use at low and high temperatures. The findings showed a reversible capacity of 119 mA h g−1 at a rate of 0.1 C, along with a capacity of 85 mA h g−1 at a rate of 1 C. Additionally, there was an 80% capacity retention after 250 cycles at a temperature of 25°C [141]. Separate research used the hydrothermal method to synthesize NVPF/C with Mn doping (Mnx+x = 2, 3, 4). The performance of NVPF@C was improved by introducing Mn2+ doping, which resulted in a decrease in charge transfer resistance (Rct) and an increase in Na+ diffusion kinetics. The enhanced electrochemical performance of the improved composite material cathodes depends on structural stability, optimized Mn ions, and homogeneous carbon coatings. The cathode materials, NVM2PF/C, were altered to have optimized valence states. These modified materials showed excellent stability and enhanced rate performance. They had an initial discharge capacity of 116.2 mA h g−1 at a rate of 0.2 C, which reduced to 93.2 mA h g−1 after 100 cycles [142]. In addition, Li et al. [143] displayed effective methods of producing NVPF nanotube particles with a nitrogen-doped carbon layer using a solid-phase process. The thickness of the carbon layer was around 4 nm, as shown in Figure 11 [143]. Using a one-step carbothermal reduction method, carbon-coated NVPF composite cathode materials are synthesized incorporating varying amounts of glucose reagent to synthesize (NVPF/C) with different levels of excess carbon. The NVPF/C composites exhibited outstanding electrochemical characteristics and improved cycling stability, with an initial capacity of 112.3 mA h g−1 at 1 C and retaining 89.7% of its capacity after 100 cycles [144]. Criado et al. [145] adopted the emulsion approach and created a micellar system by using oleic acid and Vaseline oil as surfactants and organic media during the synthesis of C@NVPF. NVPF and NVPF/CNT were fabricated by spray-drying and subjected to a 2-h heat treatment in an Ar environment. Further, a composite material consisting of NVPF and CNT was created by adding CNT to NVPF using a spray-drying technique. The CNT-based NVPF composite showed good electrochemical performance with lithium, reaching capacities of 125 mA h g−1 at C/10 and 103 mA h g−1 at 1 C [146].
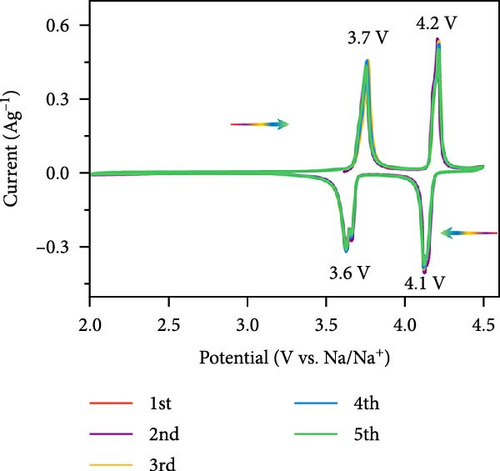

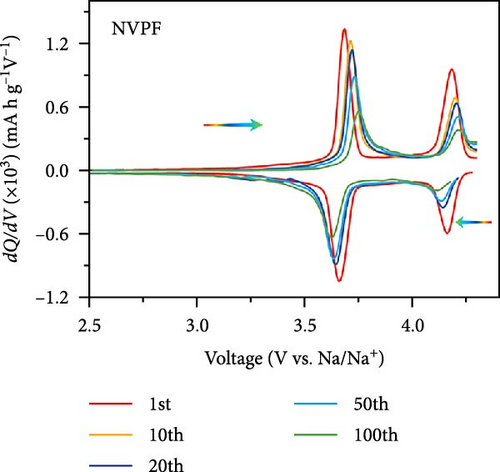
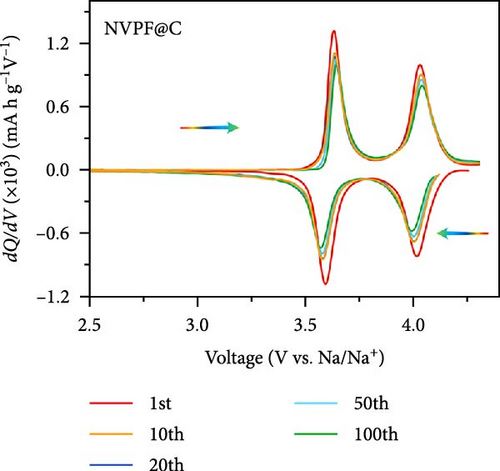
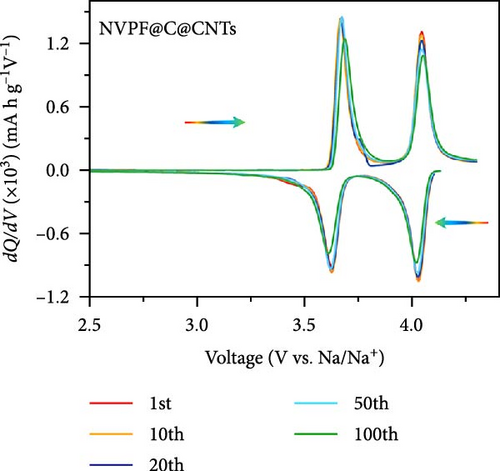
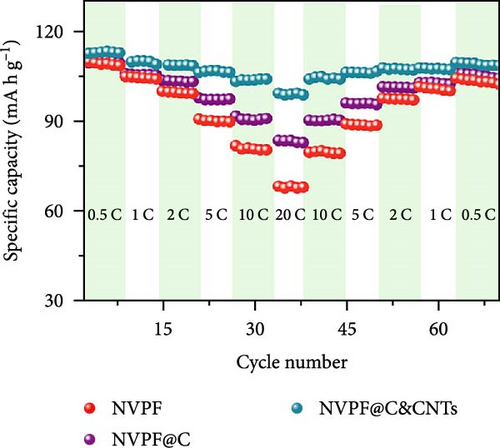
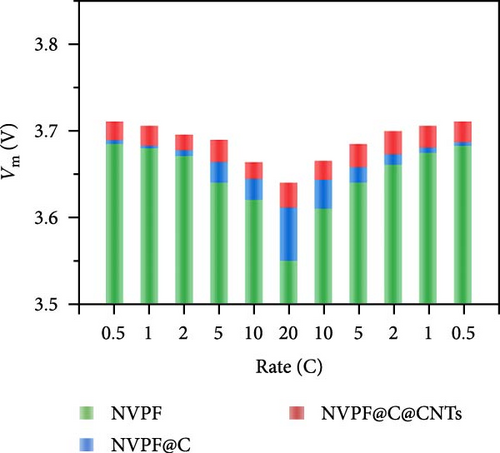
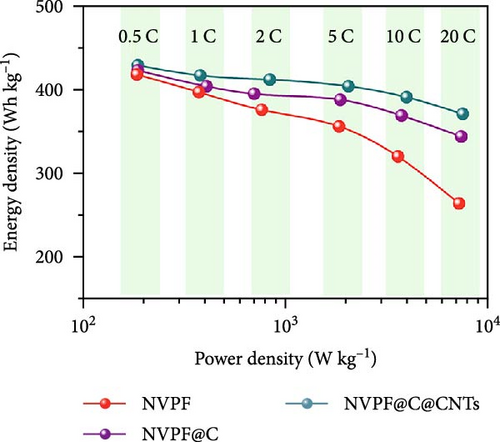
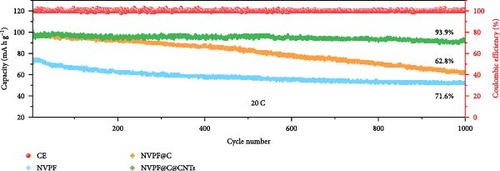
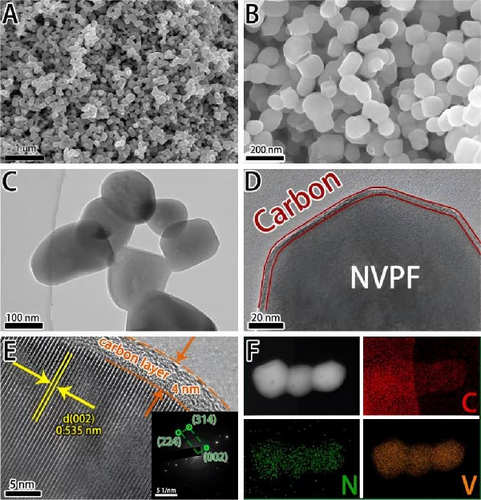
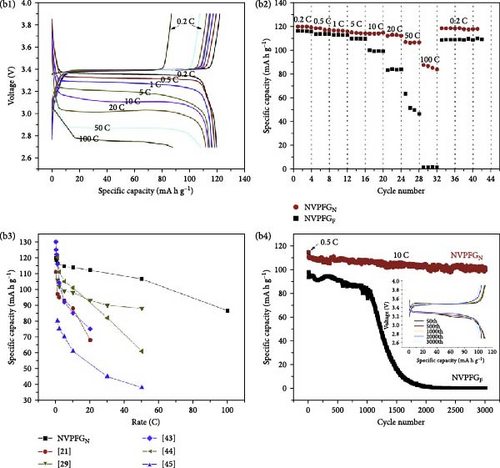
Extensive research on fluorine-based NVPF as a SIBs cathode material shows promising results. Electrochemical performance, stability, and cycle retention have been substantially enhanced by implementing techniques such as carbon coating and composite designs. Charge transfer kinetics and overall battery efficiency are improved by including fluorine, which represents a significant advancement in the development of cathode materials. These findings contribute to the ongoing efforts to create high-performance, sustainable SIBs, bringing us closer to their practical application in future energy storage solutions.
3. Electrolytes
3.1. GPEs
The investigation of GPEs is driven by the need to develop safer and more efficient batteries. The unique approach to electrolyte design involves a specific emphasis on phosphate-based GPEs, which are manufactured via in situ thermal polymerization. These GPEs exhibit excellent levels of ionic conductivity and electrochemical stability, making them a promising choice for SIBs. The many studies conducted on different formulations of GPE highlight their wide range of applications, as they effectively handle important factors such as ionic conductivity, cycle stability, and interfacial properties. GPEs present a potentially advantageous safety resolution for batteries. Although not to the same extent as dry polymer electrolytes, GPEs demonstrate reduced electrolyte discharge, decreased flammability, and enhanced safety compared to their liquid counterparts [147].
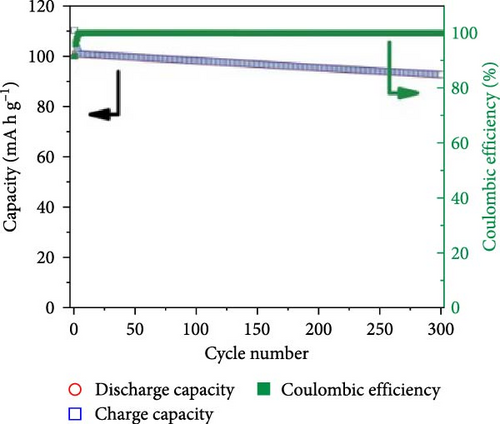
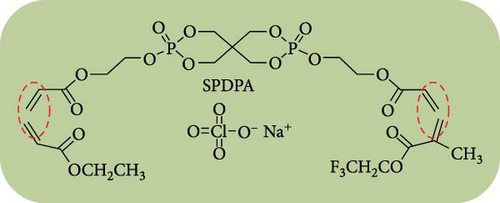
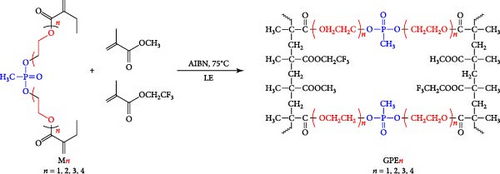
Phosphate-based GPEs, produced via in situ thermal polymerization (Scheme 3), exemplify a novel approach to electrolyte design. The ionic conductivity of these GPEs is remarkable at 6.29 × 10−3 S cm−1, and their electrochemical stability is up to 4.9 V versus Na+/Na. The published work revealed an impressive efficiency of 110.7 mA h g−1 for an NVP/GPE/Na cell operating at a 1 C rate [148]. Surprisingly, 69.2% and 81.8% of the capacity were retained after 4500 and 10,000 cycles at a 5 C rate, respectively (Figure 12). Krishna Jyothi et al. [149] employed poly-acrylonitrile (PAN) in conjunction with dimethyl formamide and ethylene carbonate as plasticizing solvents in Na systems; the PAN was prepared using the solution cast technique. The sample containing 30 wt% NaI demonstrates the maximum conductivity, with activation energy that falls within the range of 0.25–0.46 eV: 2.35 × 10−4 S cm−1 at 303 K and 1 × 10−3 S cm−1 at 373 K, by Arrhenius behavior [149]. In their first attempt to use PAN for GPE in Na systems, Osman et al. [150] compared GPEs based on Li and Na PAN with different salt concentrations. PAN + 26% LiCF3SO3 film has a conductivity of 3.04 × 10−4 S cm−1 (RT), while PAN + 24% NaCF3SO3 film has a conductivity of 7.13 × 10−4 S cm−1 (RT) [150]. In another study, Kim, Chung, and Park [151] investigated various types of porous GPE for SIBs. It was mentioned that GPE in the glass fiber matrix will affect the performance. Optimizing GPE in a glass fiber matrix increases ionic conductivity [151]. PAN-based GPEs were synthesized. By optimizing the Na, polymer, and solvents, the ionic conductivity at room temperature was attained at 4.5 mS cm−1 [152]. Kumar and Hashmi [153] documented the performance of a PMMA-based GPE used in a Na battery, which comprised approximately 4% SiO2 and displayed an exceptional electrical conductivity of 3.4 × 10−3 S cm−1 at approximately 20°C. Recently, Zheng et al. [154] fabricated a monomer with two phosphate groups and flame-reducing significance (Figure 13). A thermal polymerization approach synthesized the GPEs for the SIBs. The GPE applied in NVP cathode materials demonstrated excellent performance in specific capacity, ionic conductivity, and cycling stability compared to liquid-based electrolytes [154].
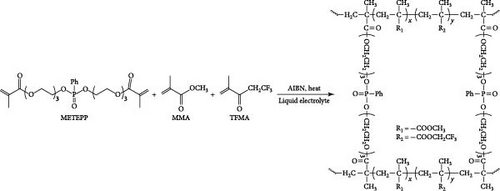
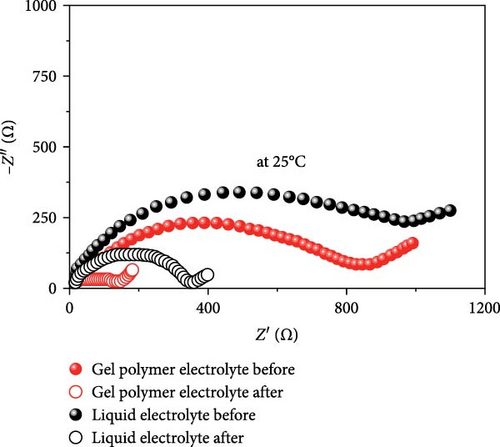
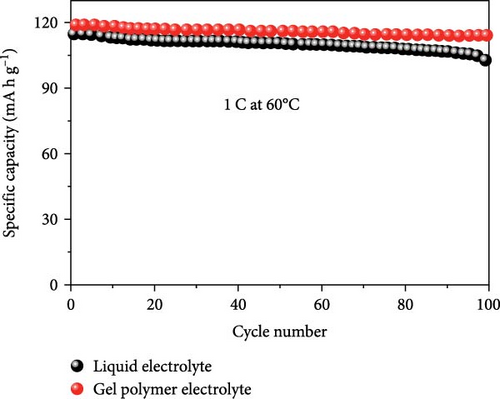
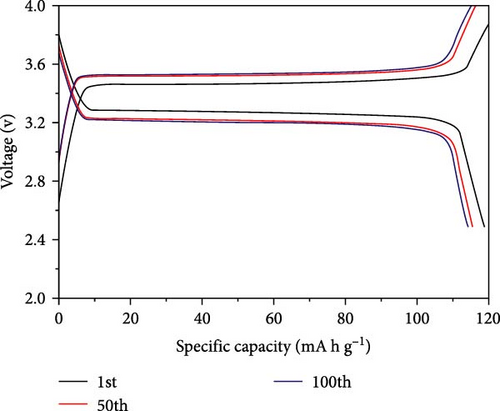
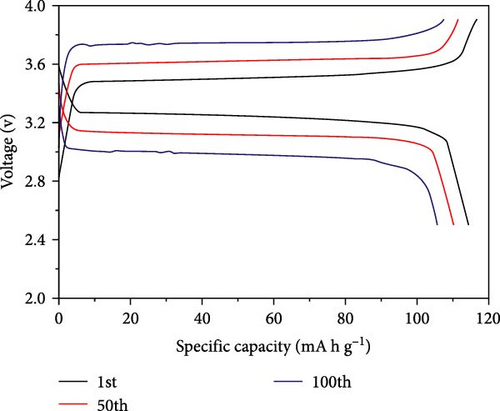

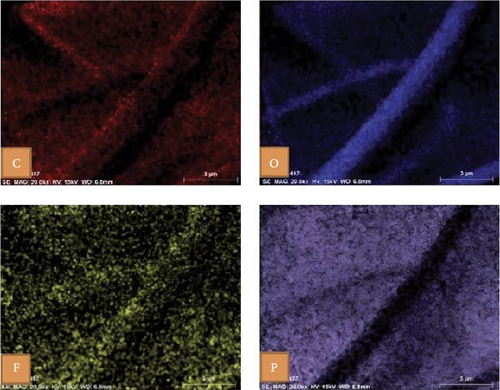
In another example, Bag et al. [155] reported the synthesis of composite polymer electrolytes by SiO2 particles and polyvinylidene fluoride (PVDF). The modified electrolyte revealed the stable electrochemical performance with the NVP electrode. At 0.5 C, the Na-ion fuel cell exhibited a 76 mA h g−1 capacity, while its specific energy was recorded as 126 W h kg−1 [155]. Bella et al. [156] have demonstrated the innovation of novel photopolymerized electrodes for Na-ion batteries. The photopolymer electrolyte provides a lightweight and solid-state architecture, allowing for cost-effective manufacture in many sizes and configurations. Furthermore, they prioritize user safety by being noncorrosive, nonexplosive, and having fewer internal circuits [156]. A GPE based on PVDF was previously reported by Park et al. [157]. At a temperature of 25°C, it had a conductance of 5.1 × 10−4 S cm−1. Tetraglyme was used as a plasticizer, and NaCF3SO3 was used as a salt. Zheng et al. [158] performed a comprehensive study that examined the effects of monomer chain lengths and fabrication processes on the ionic conductivity, interfacial stability, and cycling performance of NVP/GPEs/Na batteries. Using cross-linked GPEs with shorter than enhanced cycle capabilities, thus offering substantial insights for the advancement of GPEs suitable for metal-ion battery applications (Scheme 4).
An electrolyte composed of PVDF-based gel polymer, tetra glyme plasticizer, and Na salt was first described by Park et al. [157]. At 25°C, this electrolyte demonstrated a significant Na ion conductivity. The electrolyte was effectively employed in a Na/GPE/S cell at ambient temperature (RT). Park et al. [159] presented the development of a Na battery GPE utilizing thermoplastic polyurethane (PU) that exhibits remarkable flexibility and high ionic conductivity. When utilized in Na metal electrodes, the GPE exhibited enhanced interfacial characteristics compared to a LE with a polyethylene separator. The PU-based GPE in a Na/NVP cell had a significant initial discharge capacity of 111.7 mAh g−1 [159]. Patel, Chandrappa, and Bhattacharyya [160] achieved remarkable conductivity results of 1.1 × 10−4 S cm−1 by employing a combination of poly (ethylene oxide) (PEO)–NaCF3SO3 with succinonitrile (SN) acting as a plasticizer. By testing different formulations with a wide range of solvents and Na-based salts, Ponrouch et al. [161] could determine the best Na-ion chemistry electrolytes. In Na/hard carbon cells, the EC:PC solvent mixture exhibited the highest level of performance, with a reversible capacity of 200 mA h g−1 and outstanding results for more than 180 cycles. According to the DSC heating curves, NaPF6-EC: PC has excellent thermal characteristics, which makes it an attractive electrolyte for Na-ion batteries [161]. Gao et al. [162] fabricated NVP with a nanofiber network for cathode with PEO/NaClO4/Al2O3 as an electrolyte. The combination reveals that reversible capacity at 0.2 C was 107 and 96 mAh g−1 at 2 C, exhibiting exceptional cycling stability; capacity retention was 87.5% after 1000 cycles at 2 C [162]. Genier and Hosein [163] studied Na+ transport in PEO and poly (tetrahydrofuran) (PTHF) using molecular dynamics simulations, highlighting the effect of oxygen density in individual chains on diffusivity, conductivity, and cation–anion interactions. The outcomes of this investigation demonstrate a distinct form of Na+ transport in PTHF, characterized by a decrease in coordination and an increase in interchain hopping when compared to PEO. These findings contribute to the advancement of our understanding regarding the fundamental aspects of ion transportation in polymer electrolytes, providing significant insights that can be utilized for future applications and enhancements in design. While considerable advancements have been made in understanding the interaction between structure and conductivity in GPEs that transport Na+, a lack of study has been undertaken on GPEs that only facilitate the conduction of a single ion. In general, Na+-conducting GPEs consist of a polymer matrix incorporating a dissolved salt, facilitating the mobility of Na+ and other ionic species. The presence of dual mobility might potentially result in undesirable electrolyte polarization within devices, affecting the overall conduction efficiency. Single-ion conductors offer a notable benefit, as they solely transport a solitary ionic species, which can enhance the effectiveness of electrolytes [164]. To create a highly efficient GPE based on polyacrylonitrile, Lonchakova et al. [165] employed a flexible copolymer known as poly (acrylonitrile-co-methyl acrylate), together with a plasticizer called propylene carbonate and electrolyte salts such as NaClO4 or NaPF6. The electrolytes exhibited ionic conductivities of up to 1.8 × 10−3 S cm−1 and higher cation transference numbers, reaching 0.89. The conductivity demonstrates an Arrhenius pattern, represented by an activation energy of 12–15 kJ mol−1. A GPE was synthesized in a separate investigation using the polymerization of two monomers, 1,3-dioxolane, and trimethylolpropane triglycidyl ether, using NaPF6 as an initiator. Niu et al. [166] reported the hypothetical copolymerization mechanism for the GPE.
The artificially created GPE exhibits enhanced thermal stability and a broad range of operating voltages. Gao et al. produced an economically viable technique that involves in situ radical polymerization using cross-linked PMMA to achieve GPE. Particularly at elevated temperatures, the GPE improves the interface stability between the electrolyte and electrodes in SIBs [167]. GPEs treated with phosphonates demonstrate exceptional electrochemical stability when charged to 4.9 V (vs. Na+/Na) and high ionic conductivity of 6 × 10−3 S cm−1 at ambient temperature. At a rate of 1 C, an NVP/GPEs/Na cell containing a cathode consisting of GPEs and an NVP exhibits a capacity of 110.7 mA h g−1. The GPE cells exhibit remarkable stability under ultra-long-term cycling conditions, as evidenced by capacity retention ratios of 69.2% and 81.8% after 4500 and 10,000 cycles, respectively [148]. Gabryelczyk, Smogór, and Swiderska-Mocek [168] examined the compatibility between a hard carbon anode and a novel Na-conductive GPE using a binary polymer matrix and plasticizer. The properties of the polymer matrix, composed of different mass ratios of polyacrylonitrile and polymethyl methacrylate, were enhanced by incorporating a minute quantity of PMMA. The anion of the ionic liquid significantly impacted the electrochemical properties; systems comprising an ionic liquid containing NTf2-anion demonstrated favorable kinetics for Na+ conduction in a hard carbon anode. The visual representation of temperature-induced changes in the electrolyte size is facilitated by the images depicted in Figure 14, which depict it at different temperatures. The research indicates that these electrolytes may have applications in SIBs [168]. The extensive investigation into GPEs, especially those derived from phosphate mixtures, reveals promising advancements for battery technology. The NVP/GPE/Na cell using a specific GPE demonstrated excellent capacity and impressive retention over extended cycling. Formulations with optimized polymer matrices and tailored monomer designs showed improved ionic conductivity and cycle stability. Customizing GPEs for better flexibility and electrode compatibility could further enhance the practical applications of SIBs. This research significantly contributes to developing safe and efficient energy storage technologies.
3.2. SPEs
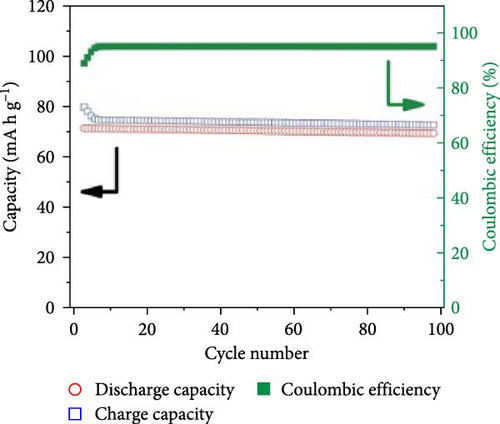
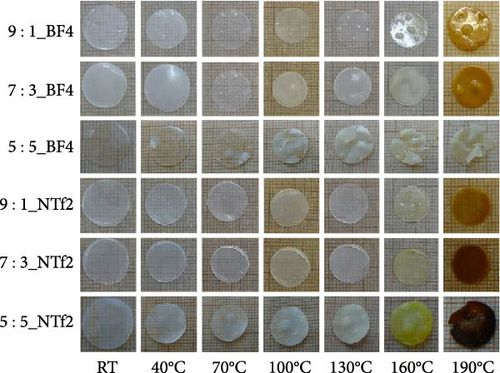
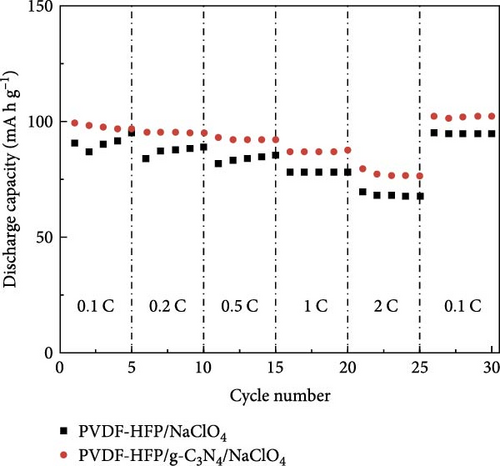
The benefits of polymers, which are long-chain macromolecules that are covalently linked, are their adaptability to many uses, their suitability for mass production, and their simplicity of solution processing [169, 170] Organic compounds provide several advantages over their inorganic substitutes, such as a close electrode–electrolyte interaction, lightweight features, and the ability to be manufactured at low temperatures, in addition to polymeric electrolytes ion-transport properties [171, 172]. Observations indicate Na salts in the SEI are significantly more soluble than their equivalents in a lithium-ion-based SEI. Na2CO3 and NaF, vital components of the Na-derived SEI, demonstrate higher solubility than Li2CO3 and LiF in a lithium-derived SEI [173]. Although ionic self-assembled layers play a role in ensuring the consistent functioning of metal batteries, there are still obstacles to overcome regarding their capacity to conduct ions effectively and retain enough ionic interfaces with the metal anode [174–176]. Polymer electrolytes are divided into GPEs, SPEs, and CPEs. Although GPEs have more excellent ionic conductivity than SPEs, the existence of organic solvents raises serious safety concerns [177]. PEO is a widely researched SPE being extensively examined compared to other options [178, 179]. Adding oligoether units containing ether-oxygen bonds in PEO promotes the fluid movement of segments and the distance between atoms, hence improving the conduction of ions [180, 181]. Thus, the Na ion conductivity of polyvinyl alcohol (PVA), polyvinylpyrrolidone (PVP), copolymers of PVDF, and other linear-chain polymers have been investigated in addition to PEO (Na+) [182–184]. Fenton, Parker, and Wright [185] emphasized the exceptional capacity of PEO to dissolve alkali metal ions, mainly Na and K, solidifying its position as the preferred polymer host in SPEs. The studies conducted by Genier et al. [186], Hashmi and Chandra [187], and Zhang et al. [188] specifically examined the stability of the interface between PEO–NaClO4 and PEO–NaPF6 in all-solid-state cells at a temperature of 80°C. These studies emphasized the need to ensure compatibility between the polymers and metallic Na. The formation of corrosive layers on the metal surface played a vital role in determining their ionic characteristics. Anion selection significantly affected the Na+ to ethylene oxide (EO) ratio, typically from 0.050 to 0.080. Smaller anions, such as PF6, are firmly attached to polymer chains, reducing ionic conductivity. In addition, salt crystallization affected the movement of ions and the consistency of Na deposition [189]. The enhanced deposition of Na in the PEO–sodium trifluoromethanesulfonimide (NaTFSI) system can be attributed to the greater size and spread-out nature of TFSI anions compared to the PEO–NaTFSI system. Shu et al. [190] detailed the exceptional properties of a recently synthesized polymer composite electrolyte PVDF−HFP/g−C3N4/NaClO4. Adding g−C3N4 nanosheets boosted the composite’s conductivity of ions, mechanical characteristics, and electrochemical activity, leading to better Na+ dissociation and a boost in the composite’s contact efficacy with polymers. Using the assembled coin cells in a Na–metal battery using an NVP cathode shows a stable reversible capacity of 93 mA h g−1 over 200 cycles at 1 C and a low polarization voltage of 90 mV (Figure 15). Using a hybrid solid electrolyte (HSE) that conducts Na, Kim et al. [78], demonstrated a new type of flexible solid-state Na battery. A combination of PVdF-HFP polymer and NASICON ceramic powder was evenly distributed in the HSE, and it was produced using a hybrid technique. An ether-based electrolyte was added to the NASICON + PVdF-HFP composite film to solve the problems with the solid–solid interfaces.
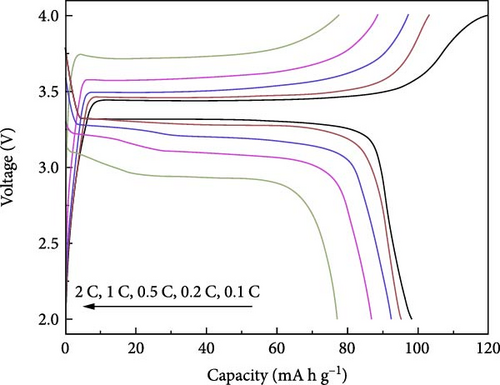
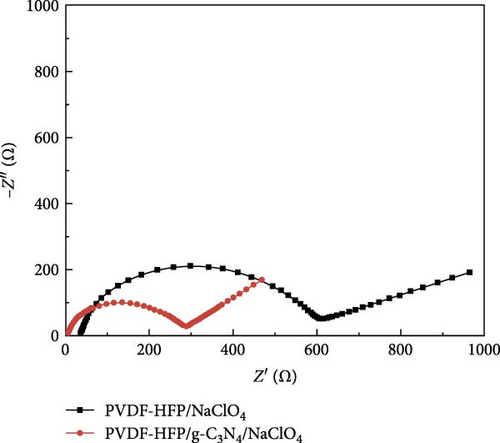
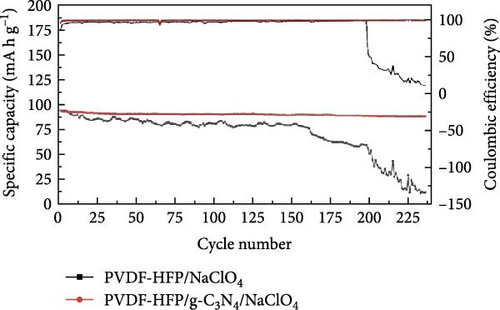
The electrolyte mentioned above, functioning as a HSE for Na+ conduction, exhibited remarkable resistance to shrinkage and demonstrated an ionic conductivity of 3.6 × 10−4 S cm−1. Initially, the discharge capabilities of the half cells reached 330 mA h g−1, using hard carbon as an anode at 0.2 C. Using NaFePO4 as the cathode, a 131 mA h g−1 capacity was achieved at a rate of 0.2 C.
Park et al. [191] achieved a significant breakthrough by introducing PEO as an electrolyte for a high-temperature all-solid Na–sulfur (S) battery. The electrolyte film, comprising a blend of PEO and NaCF3SO3 in acetonitrile, demonstrated consistent and enduring properties. The S cathode, synthesized using S powder, carbon, and PEO, exhibited an orthorhombic S phase and crystalline PEO phase, confirmed by XRD examination. Impedance spectra examination revealed an impressive ionic conductivity of 3.38 × 10⁻4 S cm⁻1 at a temperature of 90°C. Despite initially having a discharge capacity of 505 mA h g⁻1 S, the cell performance decreased rapidly after repeated charge–discharge cycles, stabilizing at 166 mA h g⁻1 S after 10 cycles. SPEs encounter difficulties such as resistance at the electrodes and electrolyte interface, lower ionic conduction than LEs, and significant polarization and capacity degradation issues. Zhou et al. [192] addressed these challenges by inventing a quasisolid-state electrolyte/GPE that demonstrates a significant level of ionic conduction (3.85 × 10³ S cm−1 at 25°C) for Na–S batteries operating at ambient temperature. After undergoing 100 cycles, the Na–S cell utilizing this electrolyte maintained a reversible capacity of 736 mA h g−1, nearly twice the capacity observed in the cell employing LE. The energy density, determined using the mass of S, was roughly 956 Wh kg−1, computed with an average discharge voltage of around 1.3 V. In general, polymers possess flexibility and the ability to be produced in large quantities, which enhances their value in numerous applications. Polymeric electrolytes exhibit effective electrode–electrolyte interaction and the capacity to be produced at low temperatures. The significant solubility of Na salts highlights their crucial role in the advancement of battery technology. Although there are difficulties in conducting ions in metal batteries, current investigations on polymer electrolytes, such as SPEs and HSEs, demonstrate the potential to overcome problems and improve battery efficiency. This progress is paving the basis for future developments in energy storage. Extensive research on polymer electrolytes has revealed their adaptability and mass production potential, which include electrode–electrolyte solid interaction, lightweight properties, and low-temperature processing. Despite challenges, advances in GPEs, SPEs, and hybrid electrolytes are crucial for enhancing battery efficiency, paving the way for sustainable, high-performance energy storage solutions.
3.3. LEs
LEs play a crucial role in SIBs, providing a medium for the transport of Na+ between the anode and cathode during charge and discharge cycles [193]. The overall efficiency, safety, and performance of SIBs depend on the electrolyte [194]. SIBs use LEs, categorized as nonaqueous (organic) electrolytes, ionic liquids, or aqueous electrolytes. Organic LEs pose safety, particularly related to issues like leakage and flammability. Organic electrolytes, extensively employed in batteries, provide an extensive voltage range of up to 4 V. However, this substance is distinguished by safety hazards such as low flash point, toxicity, and weak conductivity [195]. LEs are salt solutions dissolved in water- or nonwater-based solvents or ionic liquids. Solvents play crucial must possess a broad electrochemical stability window, sufficient Na salt solubility, a high dielectric constant, low viscosity, and an extensive liquid range with both low melting and high boiling points [196]. LEs exhibit higher ionic conductivity than other electrolyte types, owing to the positively and negatively charged ions’ free motion in the liquid phase [197]. The conductivity of LEs is a crucial determinant of the efficacy of SIB. Acting as the conductive medium for Na ions during charge and discharge cycles, the ionic conductivity of LE significantly influences the efficiency and speed of ion transport within the battery. Ions influence the ionic conductivity in solution, salts, and solvents, the solubility of salts, interactions between ions, and the rate of side reactions, SEI, or the factors that influence the production of cathode–electrolyte interphases (CEIs), including temperature, aging, dielectric constant, and electrolyte viscosity [198]. The selection of salts, such as NaPF6, NaTFSI, NaDFOB, and NaFSI, influences conductivity, with NaPF6-based electrolytes exhibiting higher conductivity. Including additives such as fluoroethylene carbonate (FEC) and VC alleviates SEI/CEI formation challenges. On the other hand, ester-based and ether-based electrolytes are significant contributors to varying SEI characteristics, and compositions of solvents influence electrode performance. Ionic liquids offer advantages such as being nonflammable and having a shallow vapor pressure, with specific ILs demonstrating enhanced electrochemical performance in SIBs [199–201]. Ester solvents and ether solvents are the predominant types employed in SIBs, demonstrating outstanding performance in the battery. Aqueous electrolytes present an environmentally friendly option but face challenges related to electrode erosion due to water’s narrow electrochemical stability window. Strategies such as highly concentrated electrolytes and water-in-salt type electrolytes aim to overcome these limitations. LEs offer a wide temperature range and display low viscosity. However, the absence of a clearly defined structure necessitates using separators to avoid electrical malfunctions caused by direct contact between the anode and cathode [202]. The use of polyolefin-based separators, however, presents challenges related to poor wettability. Consequently, thicker glass fiber membranes have been employed in SIBs that use LEs, leading to decreased energy densities in volume and weight. To deal with this problem, dip-coating polyolefin separators with ceramic particles are employed. Ultimately, LEs are vital for SIBs, influencing efficiency, safety, and performance. Ionic liquids show potential due to their nonflammable properties. The choice of salts affects conductivity, whereas addition modifications reduce difficulties related to SEI generation. Ester and ether solvents perform well in SIBs, but aqueous choices have difficulties with electrode degradation. Concentrated electrolytes are used to enhance the performance of aqueous electrolytes. Although LEs have broad temperature capabilities, separators are essential to avoid electrical issues. Ceramic coatings improve separator wettability to overcome volume and weight energy density concerns in SIBs. LEs significantly impact SIBs efficiency, safety, and efficacy. Ionic liquids are nonflammable, which enhances safety, while organic electrolytes offer high voltage but pose safety concerns. Aqueous electrolytes are environmentally friendly but suffer from electrode erosion. Advanced separators and concentrated electrolytes are vital for optimizing performance and mitigating challenges.
4. Conclusions
SIBs are attracting significant attention from the scientific and commercial sectors as a viable substitute for LIBs. This research examines the latest developments in cathode materials of the sodium superionic conductor (NASICON) class. These materials possess desirable properties like exceptional ionic conductivity, thermal stability, and strong structural integrity. These materials have several applications, such as SIBs/LIBs, sensors, and electrolytes. Doping techniques can alter the carbon surface by introducing other elements, augmenting salt storage capacity. Various methods, including polymer-based electrolytes, carbon coatings, and material doping, have been investigated to enhance the ionic conductivity of cathode materials. The approaches used to combine different elements, the structure and shape of the materials, and the integration of polymer-based electrolytes are essential aspects that affect the production of cathode materials and their chemical properties. Polymers provide mobility, which is critical in a wide range of applications. Polymeric electrolytes can improve battery technology and set a foundation for future advancements. This review article thoroughly summarizes progress in polymer-based electrolytes and improved cathode materials for SIBs, demonstrating the continuous endeavors to attain effective and environmentally friendly energy storage systems.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, award number (2-17-02-001-0053).
Acknowledgments
This work was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, award number (2-17-02-001-0053).
Open Research
Data Availability Statement
The data that support the findings of this study are available within the article.




