Effects of Gamma Ray Irradiation on Physicochemical Characteristics and Leaching Behaviors of Spent Radioactive Anion Exchange Resin
Abstract
Gamma ray irradiation of spent anion exchange resins resulted in significant physicochemical changes. The poly(styrene-divinylbenzene) (PS-DVB) backbone underwent oxidation, leading to the formation of various oxygen bonds. Damage to the functional group was confirmed by the decomposition and oxidation of the quaternary ammonium group. Oxygen in the air was grafted on PS-DVB backbone at 300 kGy. Both grafting and crosslinking occurred simultaneously at 500 kGy but more active crosslinking reactions. In contrast, at 700 kGy, degradation became predominant over grafting and crosslinking. Thermal analysis showed elevated decomposition temperature and increased residual products, indicating highly crosslinking reactions with escalating radiation dose. The substantial release of cobalt ions and organic substances is observed in the irradiated anion exchange resins. The disposal of spent resins in a radioactive waste facility carries a substantial risk of leaching complexing agents and complexed radioactive isotopes, underscoring the importance of radioactive waste management to minimize environmental hazards. The insights gained from this study are crucial for informing the development of effective risk mitigation strategies and ensuring the safe, long-term containment of radioactive materials in disposal sites.
1. Introduction
Nuclear power plants require a periodic decontamination process to eliminate radioactive isotopes. Typically, decontamination agents are applied in excess, generating a large amount of radioactive waste. To manage such waste, most of organic decontamination agents should be decomposed, and the remaining is finally removed using anion exchange resins [1–3]. At the end of 2020, 441 operational nuclear power plants exist in the world, with a total capacity of 392.0 GWe. As nuclear power plants consume about 5–7 m3/reactor/year of ion exchange resins, a tremendous amount of spent radioactive ion exchange resins is generated and subsequently disposed of in a radioactive waste disposal facility. For long-term safety, radioactive waste must be isolated from human beings and the environment, meeting disposal facility standards even after 300 years or more [4–8].
The management of spent resins represents a critical challenge in nuclear power plant operations, requiring sophisticated treatment protocols to ensure both safety and regulatory compliance. These resins, essential components in maintaining water chemistry within nuclear facilities, accumulate significant radiological contamination during their operational lifetime, necessitating careful handling and disposal procedures. The treatment process comprises a comprehensive 10-stage protocol, developed through decades of industry experience and international collaboration. This systematic approach begins with detailed characterization of the spent resins’ radiological and chemical properties, followed by strategic segregation to optimize subsequent treatment [9]. Volume reduction through established techniques such as drying and mechanical compaction serves to minimize the environmental footprint [10]. The protocol then progresses through crucial stages of stabilization [11], solidification [12], and encapsulation [13], before implementing rigorous storage [14] and transport [15] procedures aligned with international safety standards. The final phases of this process emphasize proper waste classification [16], regulatory adherence, and continuous monitoring [17], creating an integrated framework that effectively manages radiological hazards while ensuring environmental protection. This comprehensive methodology reflects the nuclear industry’s commitment to safety and environmental stewardship while acknowledging the necessity of maintaining compliance with evolving regional and international regulations [18, 19].
The effects of gamma ray radiation on anion exchange resins can result in various physicochemical changes and their leaching behaviors. Noted changes included the resin becoming darker, a distinctive smell of free amines, the release of trapped water, weight reduction, diminished exchange capacity, the generation of radiolytic gases, and the emission of water-soluble acidic and basic substances. Degradation of the resin matrix was accelerated in the presence of water and oxygen followed by grafting and crosslinking reactions. The alterations that occurred under radiolysis were determined by the chemical composition and leaching behaviors [3, 11, 12]. The damage to anion exchange resins caused by gamma ray irradiation is reported to be more severe than that of cation exchange resins. In addition, anion exchange resins with quaternary ammonium group are severely damaged by irradiation at doses exceeding 100 kGy. The ion exchange capacity (IEC) may be affected by radiation-induced chemical and structural changes. The exposure to gamma rays caused direct radiolysis of these resins, leading to the breakdown of functional groups and the creation of radical products, or to an indirect radiolytic effect through interactions with the resins and their degradation products. This can impact the resin’s ability to selectively bind and release ions during the exchange process [8–17]. Several investigations involve experimental studies using various analytical techniques to assess the chemical, structural changes, and leaching behaviors of anion exchange resins by gamma ray irradiation. Despite extensive research for several decades, there is still an insufficient understanding of the physicochemical changes caused by high-dose gamma ray irradiation [16–22]. In this study, leaching tests on irradiated resins were conducted with an emphasis on understanding the potential release of contaminants under conditions simulating groundwater infiltration. Unlike conventional leaching tests, this research focuses on the behavior of resins subjected to structural degradation caused by irradiation.
This study aims to investigate the effects of high-dose gamma radiation on the physicochemical changes and leaching behaviors of spent anion exchange resins. Given the widespread use of anion exchange resins in nuclear facilities, it is important to assess how they are affected by exposure to gamma radiation. Specifically, the investigation focused on the alterations of spent resins when subjected to high-dose gamma rays exposure in the radioactive waste disposal facility. The findings of the study highlighted the potential risks associated with storing irradiated spent resins in radioactive waste disposal facilities, particularly in scenarios where groundwater infiltration might occur. This research is crucial for developing strategies to mitigate the risks of contaminant release and to ensure the safe long-term disposal of radioactive spent resins.
2. Materials and Methods
2.1. Gamma Irradiation of Anion Exchange Resins
To investigate the physicochemical alterations of anion exchange resins by gamma ray irradiation, a commercial nuclear grade anion exchange resin Amberlite IRN78 was purchased from Rohm and Haas Company. The supplier-provided physicochemical properties are shown in Table 1. To simulate the conditions of anion exchange resins contained in a radioactive waste container, about 5.0 g of anion exchange resins was delivered into a test tube in an exposed state to air and irradiated by gamma rays using a 60Co (Nordion, Canada) source at the Korea Atomic Energy Research Institute. Gamma ray irradiation was carried out under ambient temperature and pressure conditions with a dose rate of 10 kGy/h. The resin underwent irradiation at doses of 0, 300, 500, and 700 kGy. Anion exchange resins, particularly those containing quaternary ammonium groups, undergo severe degradation when exposed to radiation doses exceeding 100 kGy. In fact, all amine-functionalized resins (primary, secondary, tertiary, and quaternary) demonstrate poor radiation resistance even at low doses. Anion exchange resins generally showed inferior radiation resistance compared to cation exchange resins when exposed to doses up to 1000 kGy [23].
| IRN 78 | |
|---|---|
| Ionic form | OH-form |
| Matrix | Gel |
| Copolymer | Styrene-DVB |
| Particle size (μm) | 630 ± 50 |
| Ion exchange capacity (eq/L) | ≥1.2 |
| Moisture content (%) | 44–60 |
| Operating pH | 0–14 |
| Max. operating temperature (°C) | 60 |
2.2. Water Uptake
2.3. IEC
2.4. Analytical Methods
Physicochemical changes of anion exchange resins under gamma ray irradiation were analyzed by infrared spectrum (IR), field emission scanning electron microscopy (FE-SEM), energy dispersive spectroscopy (EDS), and thermogravimetric analysis (TGA). IR analysis was performed using an ALPHA (Bruker, USA) instrument to investigate the chemical structure changes of the ion exchange resin. To compare the changes caused by gamma ray irradiation, the IR spectrum was normalized using the wavenumber of 1460 cm−1 (CH3 stretching), which showed almost no change in spectrum. FE-SEM analysis using Merlin Compact (Carl Zeiss, Germany) was used to analyze the surface changes of the ion exchange resin, and EDS analysis was performed using AZtec Energy X-MaxU (Oxford, England). TGA was analyzed using a thermogravimetric analyzer (SDT Q600, Waters, USA) under air atmosphere at a heating rate of 10°C/min over a temperature range of 25–800°C. After the leaching test of the ion exchange resin, the leachate was analyzed for cobalt ions and organic compounds to analyze the radioactive isotopes leaching characteristics of the irradiated resins. The leached cobalt ions were quantified using an atomic absorption spectrometer (AAnalyst 400, Perkin Elmer, USA), and the concentration of leached organic compounds was analyzed using a total organic carbon analyzer (TOC-LCPH, Shimadzu, Japan).
2.5. Leaching Experiments
To simulate the spent resins where radioactive isotopes and organic chelating agents are adsorbed, a solution of 1.6 mM CoSO4 and 3.2 mM EDTA was prepared, and Co2+–EDTA was formed by mixing them. Subsequently, 30.0 g of IRN78 was introduced for ion exchange. To prevent a sudden change in pH during the adsorption process, the mixture was slowly stirred using gentle agitation for 1 day. The leaching experiments were carried out on anion exchange resins irradiated at a dose of 700 kGy to focus on the leaching behaviors of radioactive isotopes and organic complexing agents. These tests were conducted directly on the ion exchange resin itself, not on the solid phase, following a modified leaching test method based on the ANSI 16.1 standard by directly performing the leaching test on the ion exchange resin. The conditions for the leaching experiments are shown in Table 2. The column reactor for leaching test was fabricated with an inner diameter of 15.0 mm and a length of 400.0 mm. The irradiated resins were packed into the column. Prior to the experiments, it was washed with deionized water to remove cobalt ions, EDTA, and other contaminants physically adsorbed or present on the surface of resins. The washing solution was exchanged with fresh deionized water over one to two cycles. After washing, to simulate porosity condition in a radioactive waste disposal facility’s artificial barrier, the leaching tests were initiated by exchanging a leaching solution of 2.1 × 10−2 M Ca(OH)2 (pH 12.5). The leaching tests were conducted for 5 days, with exchanges of a fresh leaching solution at 24-h intervals. The temperature of both the washing and leaching solutions was maintained at 25°C. The volumes of the deionized water used for washing and leaching were adjusted to be 10.0 mL per unit weight of the packed ion exchange resins (0.01 L/g).
| Ca(OH)2 solution | Packed resin (g) | Number of wash | Leaching time (day) | ||
|---|---|---|---|---|---|
| pH | Conc. (M) | Flow rate (L/h) | |||
| 12.5 | 2.1 × 10−2 | 0.3 | 25.0 | 2.0 | 5.0 |
2.6. Statistical Analysis
For EDS and leaching experiments, five test samples at each condition were collected and analyzed (n = 5). One-way analysis of variance (ANOVA) was conducted to investigate the effects of gamma ray irradiation on the physicochemical characteristics of irradiated resins. Post hoc multiple comparisons were performed using Bonferroni test when necessary, with statistical significance set at a p-value below 0.05.
3. Results and Discussion
3.1. IR Spectrum
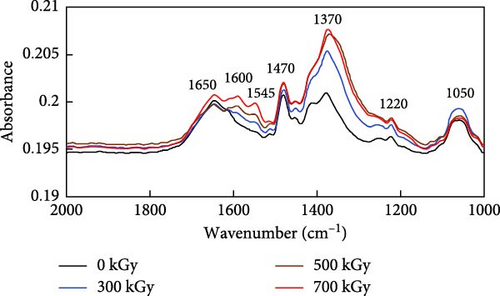
The IR spectrum changes of anion exchange resins by gamma ray irradiation are shown in Figure 1. The anion exchange resins is frequently used for the removal of organic decontamination agents in nuclear power plants [1–3]. When spent resins are stored in an exposed state to air, they are subjected to changing their physicochemical properties due to direct effects of gamma ray irradiation and/or indirect effects of radical reactions. The decomposition of poly(styrene-divinylbenzene) (PS-DVB) was confirmed by the observation of anhydride CO─O─CO stretching at 1050 cm−1, vinyl ether C─O stretching at 1220 cm−1, and alcohol O─H bending at 1370 cm−1. Furthermore, the IR spectrum analysis revealed the presence of N─O stretching of the nitro compound at 1545 cm−1, indicative of oxidation of the quaternary ammonium group. C═C stretching of the conjugated alkene was observed at 1600 and 1650 cm−1. The conjugated alkene bond originally exists in unirradiated anion exchange resins, but as gamma ray irradiation increased, the absorbance of each wavenumber gradually increased (0.1983 at 0 kGy, 0.1989 at 300 kGy, 0.1995 at 500 kGy, and 0.200 at 700 kGy). This showed oxidative degradation and saturation bonding of the irradiated resins. The alkane’s C─H bending of the PS-DVB methylene group was also observed at 1470 cm−1 [25, 26].
3.2. FE-SEM and EDS
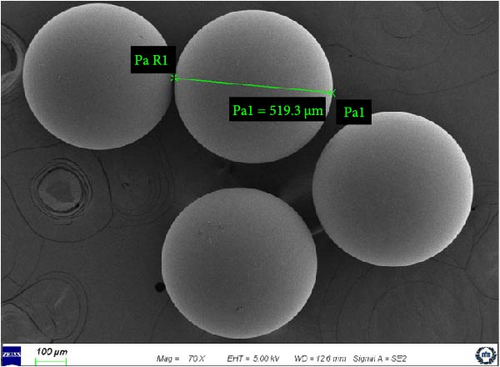
The surface modifications of anion exchange resins by gamma ray irradiation are shown in Figure 2. Prolonged exposure to gamma rays caused deleterious effects on the resin surface such as indentations and scratches. Unlike cation exchange resins in our previous study, physical cracks and surface changes in anion exchange resins occurred [27]. Cation exchange resins showed higher stability than anion exchange resins up to 1000 kGy absorbed dose [23]. The quaternary ammonium group in resins were particularly susceptible to degradation. The resins with primary, secondary, tertiary, or quaternary amine groups commonly exhibited low resistance to irradiation even at low absorbed doses. This degradation generated a distinctly pungent odor of amine groups such as mono-, di-, and tri-methylamines [6, 20, 21]. However, the diameter of the irradiated resins remained stable at ~500 μm with negligible changes. In the supplier-provided information, the diameter of virgin resins was stated to be 630 ± 50 µm, but the diameter was at ~500 μm in the actual FE-SEM analysis.



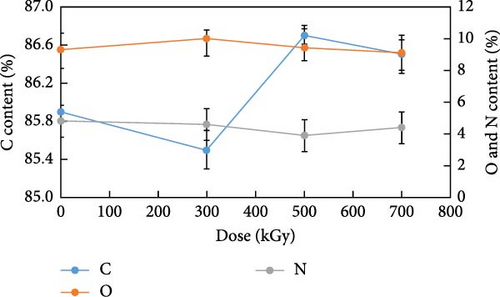
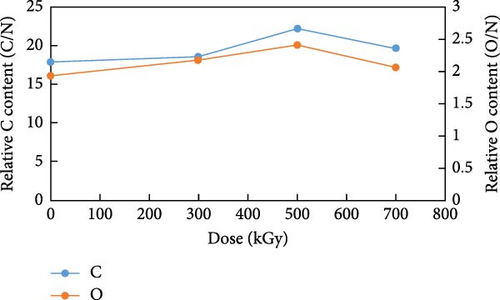
The alterations in elemental composition of anion exchange resins by gamma ray irradiation are presented in Figure 3. EDS analysis showed that the relative elemental ratios of carbon, oxygen, and nitrogen were changed. The relative carbon content increased to 85.9, 85.5%, 86.7%, and 86.5% at doses of 0, 300, 500, and 700 kGy, respectively (p < 0.005). In contrast, the relative oxygen content exhibited the opposite pattern, showing 9.3, 10.0%, 9.4%, and 9.1% with an increasing irradiation dose (p < 0.005). For the relative nitrogen content, a slight decrease was observed, with values of 4.8, 4.6%, 3.9%, and 4.4% at doses of 0, 300, 500, and 700 kGy, respectively (p < 0.005). The data provided by EDS showed the relative elemental composition in the irradiated resins. To accurately analyze changes in elemental composition, the relative change of carbon, oxygen, and nitrogen content at each dose must be analyzed. To detail the chemical composition changes in the irradiated resins, each elemental content was divided by the nitrogen content at each dose. The changes in carbon and oxygen content relative to nitrogen content are presented in Figure 3b. The carbon content relative to nitrogen was 17.9, 18.6, 22.2, and 19.7 times high at doses of 0, 300, 500, and 700 kGy, respectively. The relative carbon content increased up to a dose of 500 kGy but decreased at 700 kGy. The increase in the relative carbon content was more pronounced at 500 kGy compared to 300 kGy. The oxygen content relative to nitrogen was 1.9, 2.2, 2.4, and 2.1 times high as the gamma irradiation dose increased. The relative oxygen content increased at 300 kGy and continued to increase at 500 kGy. However, at 700 kGy, it decreased similarly to the carbon content. At lower radiation doses, grafting was the predominant reaction. At 300 kGy, the relative increase in oxygen content compared to carbon content indicated grafting of oxygen in the air. This phenomenon was attributable to the generation of oxygen groups such as carboxyl, carbonyl, and hydroxyl groups [27]. At 500 kGy, the relative increase in carbon content was more significant than that of oxygen. These are formed by the reaction of PS backbone carbon radicals, along with decomposition reactions facilitated by hydroxyl radicals. The G(S)/G(X) of PS is noted to be 0.02 at 30.0°C. The G value is a measure of the chemical yield resulting from radiation exposure. G(X) is crosslinking events, while G(S) is chain scissioning reactions. It is widely acknowledged that crosslinking reactions for PS are more vigorously activated than degradation reactions [28–30]. In contrast, at 700 kGy, degradation became predominant over grafting and crosslinking. This led to a decrease in both relative carbon and oxygen content. The change of relative carbon and oxygen element at each dose compared to lower dose is shown in Table 3. The relative carbon content exhibited an increment of 3.9% at 300 kGy relative to the unirradiated resins, followed by a subsequent increase of 19.6% at 500 kGy compared to the 300 kGy dose. Similarly, the relative oxygen content demonstrated increases of 12.2% and 10.9% at 300 and 500 kGy, respectively, relative to their preceding doses. These distinct compositional changes suggested the predominance of grafting reactions at 300 kGy, while crosslinking reactions appeared to dominate at 500 kGy. A notable decrease in both relative carbon and oxygen content was observed when the absorbed dose increased from 500 to 700 kGy, indicating the prevalence of degradation reactions at this dose level. These findings suggested that prolonged irradiation exposure could result in significant structural deterioration of the spent resins, resulting in potential release of radioactive isotopes in a radioactive waste disposal facility. Nitrogen was considered to be present in the quaternary ammonium group (−N(CH3)3). The amine groups of the irradiated resins gradually decreased as the C─N bonds were degraded and the quaternary ammonium group was oxidized. Traboulsi et al. [26] showed the detachment of −N(CH3)3 groups and the breakdown of C─N and N─H bonds from the irradiated resins first, whereas PS-DVB backbone were little damaged. Considering the cleavage of C─N bonds and the generation of N─O bonds, it can be explained why both relative carbon and oxygen content severely decreased compared to nitrogen content at 700 kGy. Also, the nitrogen content was inversely proportional to the crosslinkage of resins [31]. It is reasonable to concluded that grafting and crosslinking of resins were followed by degrading of the quaternary ammonium group first and subsequently PS-DVB backbone.
| Element | Change of relative carbon and oxygen element (lower dose → higher dose) | ||
|---|---|---|---|
| 0 kGy → 300 kGy | 300 kGy → 500 kGy | 500 kGy → 700 kGy | |
| C | 3.9% | 19.6% | −11.6% |
| O | 12.2% | 10.9% | −14.2% |
Several investigators showed indirect methods to estimate radiation-induced crossliking of polymer with the data of the gel content [32], oxygen uptake rate [33], and specific surface area and nuclear magnetic resonance spectra [28]. To deduct the crosslinking of the irradiated resins from the data of gel content, the irradiated resins were dissolved in tetrahydrofuran. However, as they were not dissolved at all in solvent, it was not possible to show the crosslinkage of the irradiated resins. Thus, in this study, some physicochemical changes of the crosslinked resins such as relative elemental content, release of trapped water, diminished IEC, and weight loss were provided [6, 20, 21, 28, 33, 34].
3.3. Water Uptake and IEC
The changes in water uptake and IEC of anion exchange resins by gamma ray irradiation are shown in Table 4. After gamma ray irradiation, the water uptake decreased with increasing gamma irradiation dose. Especially, when irradiated at 500 kGy, the water uptake decreased the most, which was consistent with previous research that water uptake decreased due to radiation-induced crosslinking reactions in polymer [32–36]. When irradiated at 700 kGy, the resin matrix was degraded, and the decrease of water uptake was prominent more. Benavides et al. [35] showed the similar results with radiation-induced PS-co-AA ion exchange membrane. Water uptake and IEC of the irradiated membrane were severely decreased with gamma irradiation dose. Bance-Soualhi et al. [36] stated that the water uptake and swelling decreased in proportion to the crosslinkage in radiation-grafted anion exchange membranes. Sittiwong et al. [37] showed the importance of crosslinking reactions in hydrogels for drug release. The amount of drug release in poly(vinyl alcohol) hydrogels depended on swelling and weight loss, which decreased with higher crosslinked hydrogels. The lower crosslinked hydrogels showed looser networks allowing more water diffusion and swelling.
| Dose (kGy) | ||||
|---|---|---|---|---|
| 0 | 300 | 500 | 700 | |
| Water uptake (%) | 51.8 | 44.3 | 28.6 | 24.1 |
| Ion exchange capacity (eq/L) | 1.6 | 1.5 | 1.0 | 0.7 |
Similar to water uptake, IEC decreased with increasing gamma irradiation dose. When irradiated at 500 kGy, IEC also decreased the most due to the radiation-induced crosslinking [32, 33, 36]. When irradiated at 300 kGy, IEC of the irradiated resins was 1.5 eq/L that was similar to the conditions without gamma ray irradiation. This may be because the grafted oxygen provided electron donors. On the other hand, when irradiated at 700 kGy, IEC decreased by less than 50% compared to that without gamma ray irradiation. As anion exchange resins were irradiated at a high dose exceeding 500 kGy, a large amount of quaternary ammonium functional group was degraded [38].
3.4. TGA
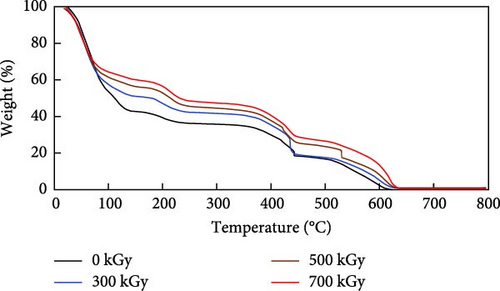
The results of the thermal gravimetric analysis of anion exchange resins by gamma ray irradiation are shown in Figure 4. The decomposition of the quaternary ammonium group in the resins was distinctly observed by TGA. Typically, the quaternary ammonium group is decomposed at 180–320°C and PS-DVB at 400–600°C. As shown in Figure 4, the TGA curves dropped three times with temperature. Weight loss due to moisture evaporation occurred at 100–150°C, which accounted for 40%–60% of the total weight loss. Quaternary ammonium group was decomposed at around 180–320°C, accounting for a weight loss of 50%–60%. PS-DVB was decomposed at 400–600°C. To examine the weight loss data more closely, the weight loss percentages at temperature ranges for moisture evaporation, quaternary ammonium group decomposition, and PS-DVB decomposition are calculated in Table 5. As irradiation dose increased, the weight loss percentages for moisture evaporation decreased. The water uptake of the irradiated resins decreased with increasing irradiation dose. In contrast, the weight loss percentages for quaternary ammonium group decomposition increased with increasing gamma irradiation dose. The weight loss was much severe at 500 kGy. This showed that the decrease in nitrogen content (C─N bond) correlated with the weight loss observed from the decomposition of the quaternary ammonium group (−N(CH3)3) at 180–320°C. A noticeable weight loss accounting for quaternary ammonium group was observed at 500 kGy. Ezzeldin, Apblett, and Foutch [34] stated that the nitrogen content was inversely proportional to the crosslinkage of the resins. Conversely, the most significant weight loss due to PS-DVB decomposition occurred at 300 kGy, a consequence of oxygen grafting. The weight loss of resins irradiated at 500 kGy was similar to that of unirradiated resins.
| Temperature range (°C) | Dose (kGy) | |||
|---|---|---|---|---|
| 0 | 300 | 500 | 700 | |
| 0–150 | 57.2% | 48.7% | 43.5% | 39.8% |
| 180–320 | 7.3% | 9.8% | 12.4% | 13.1% |
| 400–600 | 33.3% | 36.3% | 36.1% | 33.9% |
| Total | 97.8% | 94.8% | 92.0% | 86.8% |
The resistance to radiation decomposition was partially increased, but most of the resins were completely oxidized above 600°C. The onset decomposition temperature of the quaternary ammonium group and PS-DVB in the anion exchange resin is shown in Table 6. The onset decomposition temperature of the quaternary ammonium group increased from 178.4 to 194.7°C at doses of 0, 300, 500, and 700 kGy, respectively. Similarly, the onset decomposition temperature of PS-DVB also increased from 505.6 to 582.4°C at doses of 0, 300, 500, and 700 kGy, respectively. In general, anion exchange resins are severely damaged by gamma ray irradiation. However, in this study, the onset decomposition temperature of the quaternary ammonium group and PS-DVB also increased with the gamma irradiation dose. The quaternary ammonium group is known to be converted to nitric acid, which promotes the decomposition of the irradiated resins. Moreover, considering that the C─N bond is relatively weak compared to other molecular bonds, an increase in the onset temperature of TGA alone does not conclusively indicate an enhancement in the radiation-induced resistance of the anion exchange resin [38, 39].
| Dose (kGy) | ||||
|---|---|---|---|---|
| 0 | 300 | 500 | 700 | |
| –N(CH3)3 | 178.4 | 184.8 | 192.5 | 194.7 |
| PS-DVB | 505.6 | 546.9 | 591.7 | 582.4 |
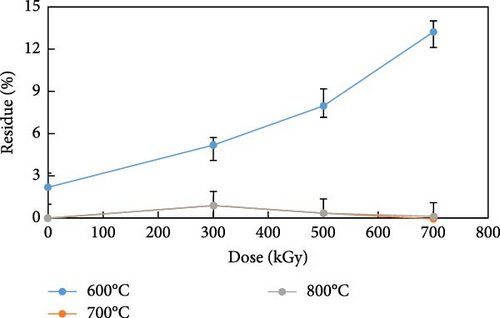
The thermal decomposition residue of the anion exchange resin is shown in Figure 5. The thermal decomposition residue at 600°C increased from 2.2% to 13.2% consistent with the change in the onset decomposition temperature. As shown in Table 6, the onset decomposition temperature of anion exchange resins increased up to about 600°C with the gamma irradiation dose. As the gamma ray irradiation dose increased, the onset decomposition temperature and the residue increased at 600°C. This showed the irradiated anion exchange resins were grafted and crosslinked, and the onset decomposition temperature increased up to about 600°C, resulting in the decomposition residue of them increased with dose. However, above 700°C, most of the resins was completely oxidized and observed to be less than 1.0% regardless of the gamma irradiation dose. The thermal decomposition residue of the anion exchange resin over the onset decomposition temperature could be minimized.
3.5. Leaching Behaviors
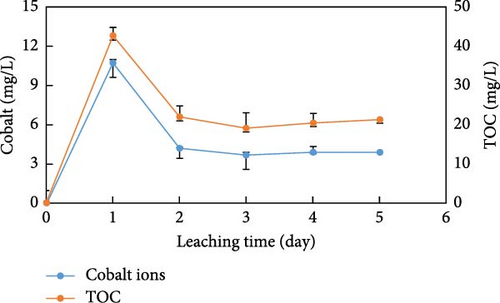
The changes in pH, concentration of cobalt ions, and TOC in the leachate from the irradiated anion exchange resins are shown in Table 7. Substantial release of cobalt ions and organic substances occurred during the washing resins. The concentration of leached cobalt ions reached 5.7 mg/L after the second wash, which was 8.9% of the initially adsorbed cobalt ions. This showed a significant leaching of cobalt ions from the irradiated resins. Similarly, the concentration of leached organic substances was elevated to 80.2 mg/L after the first wash. Although the concentration significantly decreased in the second wash, it still reached 9.7 mg/L. This suggested that the irradiated resins were considerably susceptible to gamma ray irradiation, and the presence of ion exchanged EDTA and Co2+–EDTA complexes significantly enhanced the leaching of cobalt ions and organic substances. Following the wash, the leaching experiments were carried out using a Ca(OH)2 solution. The leaching characteristics of cobalt ions and TOC from the irradiated anion exchange resins are shown in Figure 6. The cobalt ion was continuously released from the irradiated resins. The average concentration of cobalt ions reached 5.3 mg/L for 5 days, and the cumulative release of cobalt ions increased with the elapsed leaching time. Simultaneously, the average TOC concentration reached 25.1 mg/L for 5 days, showing an increase in the leached organic material proportional to the leaching time. Like the washing resins, the leaching tests also revealed a significant release of cobalt ions and TOC at the beginning of the leaching time. This indicated that the physicochemical properties of the irradiated resins were severely changed by gamma ray irradiation. The pH of the leachate increased to 12.2 after 5 days due to hydroxide ions from the quaternary ammonium group and leaching solution, and no precipitation of cobalt ions occurred. Therefore, it was inferred that the observed leaching results were released from the irradiated resins. As the resins were irradiated, the diffusion of solutes within the resin pores slowed down. This phenomenon resulted in excluding some exchangeable ions from the resins, a phenomenon known as size exclusion. The degree of swelling, weight loss, and mesh size of the polymer decreases with increasing irradiation dose. High irradiation dose forms chemical bonds between polymer chains and creating narrow network structures [40–42]. As irradiation dose increases, the resin becomes more rigid due to these interconnected bonds. Consequently, the diffusion of solutes through the irradiated resins slows down. The high irradiation dose impedes the movement of molecules, making it harder to traverse the resin. Additionally, the gamma ray irradiation to the resins led to a decrease in the sorption rate, attributed to the reduced free solvent content within the resins. The charge density of functional group played a role in ion exclusion efficiency, where highly irradiated resins resulted in stronger exclusion phenomena [43–45]. Consequently, the spent anion exchange resin waste in a radioactive waste disposal facility poses a high risk of leaching in the form of complexing agents and complexed radioactive isotopes.
| pH | Co2+ (mg/L) | TOC (mg/L) | ||
|---|---|---|---|---|
| Wash | 1st | 9.3 | 34.2 ± 0.5 | 80.2 ± 2.1 |
| 2nd | 7.2 | 5.7 ± 1.2 | 9.5 ± 0.4 | |
| Leaching time (day) | 1 | 10.5 | 10.7 ± 0.2 | 42.6 ± 3.2 |
| 2 | 10.9 | 4.2 ± 0.3 | 22.0 ± 2.2 | |
| 3 | 11.5 | 3.7 ± 0.2 | 19.1 ± 2.8 | |
| 4 | 11.8 | 3.9 ± 0.2 | 20.5 ± 3.9 | |
| 5 | 12.2 | 3.9 ± 0.4 | 21.3 ± 2.4 | |
| Average concentration of leachate | 5.3 | 25.1 | ||
4. Conclusions
High-dose gamma ray radiation adverse affected the physicochemical changes and leaching behaviors of spent anion exchange resins. Gamma ray irradiation induced various oxidation bonds in the PS-DVB backbone. Oxygen was grafted onto the PS-DVB backbone at 300 kGy. At 500 kGy, radiation-induced crosslinking occurred predominantly. However, degradation reactions overwhelmed grafting and crosslinking at 700 kGy. Thermal analysis showed an increase in decomposition temperature and residual decomposition products with escalating radiation dose. This showed the occurrence of highly crosslinking reaction in the irradiated resins. In the leaching experiments, substantial release of cobalt ions and organic substances was observed. The decomposition and oxidation of the quaternary ammonium group caused to release EDTA and Co2+–EDTA complexes. As the crosslinkage of resins increased, the decrease of water uptake and IEC become more pronounced, directly impacting the concentrations of cobalt ions and TOC in the leachate. The experimental results underscore significant risks associated with the storage of spent resins in radioactive waste disposal facilities, especially in circumstances where groundwater ingress could facilitate contaminant release.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Seung Joo Lim: investigation, methodology, project administration, writing–original draft, writing–review and editing. Wang-Kyu Choi: conceptualization, methodology. Mansoo Choi: data curation, validation. Seonbyeong Kim: investigation, resources. Sang-Hun Lee: formal analysis, data curation, writing–review and editing.
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy (RS-2023-00236918).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




