Techno-Economic and Sustainability Assessment of a Novel Waste Heat Recovery of Carbon Black Plant Integrated With a Steam Plant
Abstract
This study investigates carbon black (CB) production challenges, including high energy usage and waste of heat sources, by proposing a waste heat energy recovery concept to increase the sustainability of this energy-intensive and environmentally impactful process. The research involves a novel integration of a steam power plant (STP) with an industrial CB plant (CBP) using Aspen Plus simulation software. Comparative exergetic performance analyses of the system were conducted with this tool, while the Engineering Equation Solver (EES) was used to evaluate the exergoeconomic modelling of the plant. Additionally, environmental sustainability indicators were determined. The integrated plant system delivered CB capacity of 1817 kg/s, converted 98.03% of CB feedstocks with a purification value of 99.25% and produced 195 MW of electricity, significantly improving plant efficiency. The overall energy and exergy efficiencies for the integrated system are computed as 98.75% and 80.40%, respectively, with the STP contributing to the overall plant improvement. About 50% of the produced exergy was destroyed, with the CB combustor accounting for 48% of the combined plant exergetic destruction. Despite a substantial waste–exergy ratio from CBP, the integration of the STP increased the system’s exergetic sustainability index (ESI) by 18%. The exergoeconomic analysis highlighted the highest cost of destruction in the combustor and evaluated the evaporator as the least exergoeconomic factor driver. Components with potential exergetic and cost destruction improvements were identified. In conclusion, integrating power generation units with CB production plants can markedly reduce thermal heat waste in the CBP and enhance integrated plant environmental performance.
1. Introduction
Tyres, rubber, plastics, paints, colour pigments, inks [1] and polymer nanocomposites [2] are just a few of the numerous industrial processes that use carbon black (CB) as a raw material for toughening and reinforcing nanoparticles [3]. It is also used as an alternative material to absorb electromagnetic interference (EMI) [4] and decrease its effects [5]. The use of vegetable-based CB as a food colouring [6] is common, as evidenced elsewhere [7–10]. However, producing CB is a high-temperature, energy-intensive process that often results in greenhouse gas (GHG) emissions, which harm the environment [11]. Previous studies in the field have focused on production procedures and properties of CB, the effects of CB on different substances, the distinguishing features between CB and other substances from carbon and its applications. For instance, Karmankar [10] explored the CB manufacturing process from organic compounds and identified the CB manufacturing processes to be through incomplete combustion of hydrocarbons and thermal cracking [12].
An early attempt to reveal the conditions required for optimising the production of CB was conducted by Witt [13]. He studied the process of CB formation and determined how parameters such as reactor temperature, residence time and feed material drop size affect the properties of the CB formed. He observed that the formation of CB consists of three basic steps—nucleation, gas phase and surface reactions, with the last two steps occurring simultaneously. Kumar et al. [14] and Jebur [15] identified the criteria for judging the properties of CB. These criteria include sieve residue, particle size, structure, surface area, surface chemistry, porosity, density, aggregate size, aggregate shape, electronic properties and thermal conductivity [16]. Huang, Yang and Gao [7] studied the effect of the microstructure of CB particles in filled rubber plastics. They simulated the uniaxial tensile behaviour of polymer nanocomposites with CB material of different amounts and particle sizes. Their study revealed the behaviour of CB in polymer reinforcement. The extent to which CB affects the mechanical properties of natural rubber as a base isolation system was presented by Ismail, Mahadi and Ishak [17]. Using five different samples with varying quantities of CB of type N660 [8] as fillers for natural rubber, cured at 1500°C for 23 min in each test case, the tensile strength, hardness and resilience of the resulting natural rubber [18] were determined. The study revealed that the tensile strength and hardness of the natural rubber increase with the amount of CB used as a filler. They also observed that resilience decreases with an increase in the amount of CB used as a filler.
Furthermore, Bera, Acharya and Mishra [19] investigated how epoxy material injected with different weight percentages of CB affects the mechanical properties of the resulting epoxy/CB composites. The need to use CB as a filler in plasticized wheat gluten biopolymer (a biodegradable substance used for packing food) was investigated by Das et al. [20]. They observed that 4 wt.% of CB as a filler is the optimal amount required in plasticized wheat gluten polymers [21], leading to a 24% increase in tensile strength, a 28.6% increase in tensile modulus and an increase in toughness. Exploiting transmission electron microscopy and analysis algorithms developed, Singh and Vander-Wal [22] analysed and quantified different CBs at the nanometre scale, revealing differences in their aggregate morphology, particle size and particle nanostructure. The potential relation of the nanostructure to observed material properties was also ascertained. There are some other studies on the structure of CB. Yehliu, VanderWal and Boehman [23] developed an image analysis method [24] to quantify carbon nanostructure. Pawlyta, Rouzaud and Duber [25] carried out a microspectroscopic characterisation of CB [26] as well as examining structural information. Chipara, Chipara and Chipara [27] carried out a review of the spectroscopy of a few carbonaceous materials to reveal the structure of these substances. In another study, Probst and Grivei [28] investigated the electrical properties in addition to the structure of CB. The effect of CB on the mechanical properties of natural rubber when used as a filler in the latter was also investigated by Savetlana, Sukmana and Saputra [29]. It was observed that the agglomeration of the CB aggregate [30] in the resulting composite increases with an increase in the weight percentage of CB used. Abdul Khalil et al. [31] investigated the flexural and thermal properties of composites formed by using CB derived from biomass as a filler in epoxy. Pundlik et al. [32] carried out demineralization of CB obtained from waste tyres through pyrolysis with the aim of reducing the ash content, which limits its applicability. They observed that treating the CB with a mixture of acids is more effective in reducing the ash content compared to using any single acid.
Natural rubber is often reinforced with CB. It can also be reinforced with hybrid rice husk and CB as a filler. The mechanical properties of these hybrid reinforced composites were investigated by Imoisili et al. [33]. They observed an improvement in tensile strength, hardness, compression set and abrasion resistance in the hybrid reinforced composite as the quantity of CB increased. The use of renewable materials for manufacturing CB in renewable and environmental applications has been suggested [34]. Different grades of CB are needed for each of these applications, with furnace black being preferred for rubber reinforcement [35] due to its surface energy impacting mechanical stability [36]. Acetylene black is used for high electrical conductivities in applications like lithium batteries due to its high graphitization and purity of over 99.8%, small surface hydrogen and oxygen groups [36].
The CB process is a major component of the petrochemical industry that generates waste heat and emissions due to its use of high-viscosity mineral oils and raw materials [37]. The process plant has two main points with high levels of waste heat, including a thermal furnace and stack lines. The quantity and quality of CB production are affected by the type of furnace and quenching process [38]. Various approaches are being used to optimize chemical processes and waste heat of cracking furnaces, including thermodynamic and environmental approaches. Mathematical methods are used to simulate thermochemical conditions and investigate postcombustion products [39]. Research has focused on reducing CO2 emissions from thermal cracking furnaces [40] using the life cycle assessment (LCA) method integrated with exergy mapping information. The study investigates correlations between thermochemical factors and process equipment using machine learning methods and computational fluid dynamics (CFD) simulation to optimize heat transfer in furnaces [40]. Waste heat recovery (WHR) is a cost-effective method for optimising waste heat in petrochemical plants to reduce CO2 emissions [41]. The optimal location for WHR is in high-temperature areas where emissions and waste heat are significant [5]. WHR can produce heat and electricity simultaneously by combining it with thermodynamic power cycles, such as the Brayton cycle and organic Rankine cycle (ORC) [42–44]. The adaptability of WHR to different temperature conditions makes it a suitable application for power generation, as it boosts thermal efficiency [12] and is adaptable to various temperature conditions. The characteristics of the CB process plant suggest that optimizing waste heat is a suitable plan for WHR application [45]. The use of steam power plant (STP) can reduce environmental pollution impact in the CB process [46]. Similar to the olefin petrochemical process, investigations for reducing waste heat in these plants are conducted using the same procedure.
1.1. Motivation, Aim, Gaps in Knowledge and Novelty of the Research
The preceding literature review indicates significant progress in the production and applications of CB. As shown in Table 1, there is a notable gap in investigating methods to improve, utilize and optimise the excess waste heat dumps in CB production processes [59]. Specifically, some studies [48], [54] have examined the exergoeconomic and exergoenvironmental performance of energy systems and revealed the presence of substantial exergy destruction in furnace reaction zones, boiler burners and heat exchangers. Their findings showed that the most significant amount of exergetic destruction took place in the reaction zone of the furnace [60], boiler burners and boiler heat exchangers [61]. However, these studies did not consider the integration of CB production with power plants, which could significantly alter the energy profile economic and environmental outcomes of the CB system [62].
| Type | Carbon black | ORC/STP | Energy analysis | Exergy analysis | Capacity | Remark soft line combustor | Ref. |
|---|---|---|---|---|---|---|---|
| Experimental study on carbon black with nanofluid as coolant for PV modules | ✓ | ✗ | ✗ | ✓ | ✗ | Proposed electrical and thermal exergy efficiencies are 16.3% and 4.5%, respectively | [47] |
| Research on the industrial carbon black production | ✓ | ORC | ✓ | ✓ | ✗ | ORC energy and exergy analysis showed higher energy and exergy values at the slow line combustor (SLC) stack than at the hard line combustor (HLC) point from the numerical investigation of WHR conducted | [48] |
| Carbon black production combustible gas utilization | ✓ | ✗ | ✓ | ✗ | ✗ | Tail gas from carbon black combustion in a carbon monoxide (CO) boiler was effectively utilized, leading to good results | [49]; [50] |
| New H2 production method: thermal decomposition of CH4 with carbon black catalyst | ✓ | ✗ | ✓ | ✗ | ✗ | High-efficiency reactor produces commercially interesting carbon black particles through thermal reactions at 1000°C | [51] |
| Study on carbon black yield and off-gas amount in oil furnace process reactor | ✓ | ✗ | ✓ | ✗ | ✗ | High furnace temperature quickly pushes products into chemical equilibrium, resulting in a lower bound on carbon black yield that can be applied to any feedstock, including bio-oil | [52] |
| Techno-economic analysis of carbon black production and emissions | ✓ | ✗ | ✓ | ✗ | ✗ | Efficiency and economics. Using H2 fuel in the carbon black reactor reduces CO2 footprint by 19%, but current prices raise the cost of carbon black by 47% | [53] |
| Carbon black production and application of exergetic assessments | ✓ | ✗ | ✓ | ✓ | ✗ | Reactors are key for exergoenvironmental analysis, while boilers and heat exchangers are major cost and energy loss sources in exergoeconomic assessments | [54] |
| Carbon black nanoparticle preparation from waste tyres | ✓ | ✗ | ✓ | ✓ | ✗ | An 81% yield of CB nanoparticles was obtained, showing good thermal stability. This nanomaterial can help reduce tyre waste without harming the environment | [55] |
| Carbon black production: a cleaner approach | ✗ | ✗ | ✗ | ✗ | ✗ | CB produced from tyre pyrolysis oil has the potential to be a lower CO2 emission feedstock compared to fossil-based fuels | [56] |
| Pyrolysis dehydration study from CB production technology | ✗ | ✗ | ✗ | ✗ | ✗ | Gas dehydration achieved up to 98% with cost-effective dry gas production. Projected cost of dry gas is US $0.162/kg based on analysis | [57] |
| Closed-loop process between rubber tyre manufacturing and carbon black production | ✗ | ✗ | ✓ | ✗ | ✗ | Pyrolytic oil replaced raw and fuel oil for CB production, resulting in a 43.48% decrease in environmental impact and a 94.62% return on investment compared to conventional methods, as shown by a life cycle assessment (LCA) | [58] |
Driven by the need to harness the substantial waste heat energy within the reactor and quench tower streams at the Warri Refining and Petrochemical Company (WRPC) CB plant (CBP) unit in Nigeria, this study was motivated [63]. The research aims to recover and convert the waste heat from CB processing into electric power opportunities. Specifically, it proposes the integration of a STP generation unit into the CB production process. The integrated plant will be modelled and assessed using classical thermodynamic equations, incorporating energy and exergy analyses via Aspen Plus v10 software. At the same time, the techno-economic aspects of this study are examined using the Engineering Equation Solver (EES) software tool.
The novelty of this research lies not only in the unique integration of the plant systems and its comprehensive approach to utilizing exergy, techno-economic and environmental sustainability analyses to examine the integrated CBP and STP production. The objective of this study, therefore, is to identify components and operating parameters that exhibit high exergy destruction and pose environmental risks—areas that have not been thoroughly explored in existing literature for CB production. Additionally, this study introduces the integration of a CB production plant with a STP, leveraging waste heat-to-power generation [64].
2. Methodology
2.1. System Description
The WRPC has a CB processing unit, which serves as the focus of this study [63]. The plant utilizes the thermal oxidative decomposition process via the furnace black method and produces ~9000 metric tons annually. The raw material used is conversion oil (decant oil), the composition of which is presented in Table 2. This study also includes an innovative power plant section integrated with the CB unit that recovers and utilizes the enormous heat dumped from the CBP production.
| Description | Compound | Mole fraction |
|---|---|---|
| Composition of the aromatic oil feedstock [65] |
|
|
| Input data for process simulation |
|
|
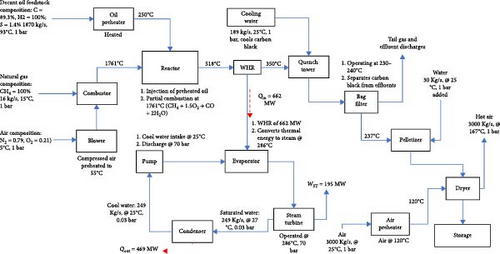
The basic components in a typical CB production plant are the oil preheater, reactor (the heart of the system), bag filter system, pelleting section, drying section, bucket elevator and magnetic separator. The process model reproducibility flowchart and the research methodology flowchart are presented in Appendix Figure A1 and Figure 1. The raw material (decant oil) is supplied through the feedstock section to the oil preheater, where it is heated to about 250°C. The heating is achieved by effluent gases from the reactor, which is fired by natural gas. Temperature control in the preheater is carried out by varying the amount of flow of the oil through the preheater. The preheated oil is sprayed into the reactor. CB is produced in the reactor through partial combustion of the feedstock (decant oil) in inadequate air at a temperature of 1761°C as shown in Equation (1):
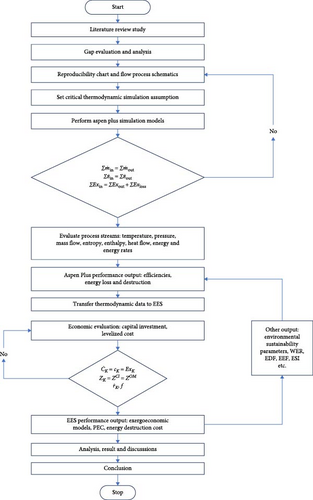
The air temperature for combustion is increased to ~55°C using effluent gases from the reactor. The reactor’s smoke passes through a header after the oil preheater, dissipating some heat into the atmosphere before reaching the quench tower. In the study, this dissipated heat is converted into power generation through a configured power plant utilizing the wasted heat, as illustrated in Figure 2(red broken lines).
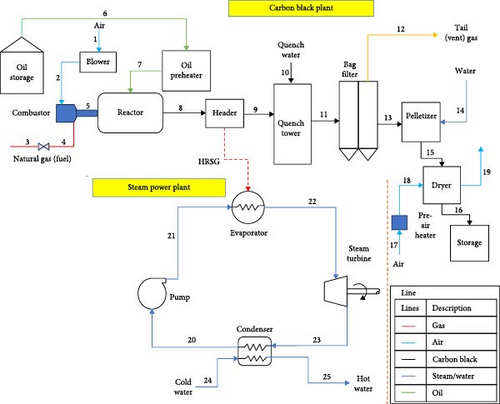
The quench tower utilizes steam from a steam turbine condenser integrated into the CB production line. CB is continuously separated from effluents through a bag filter system divided into compartments with fibreglass filter bags. The system typically operates at 230–240°C. Effluents enter the upper side of the system, and CB accumulates on bag walls, while clean gas goes into the collection header. Accumulated carbon hampers production, leading to compartment cut-offs. CB is transferred to the pelletizing section using recirculated off-gases through a pneumatic blower. CB is pelleted, dried and passed through a magnetic separator to remove impurities before storage in silos. The integrated plant schematic is illustrated in Figure 2.
2.2. Modelling and Analysis
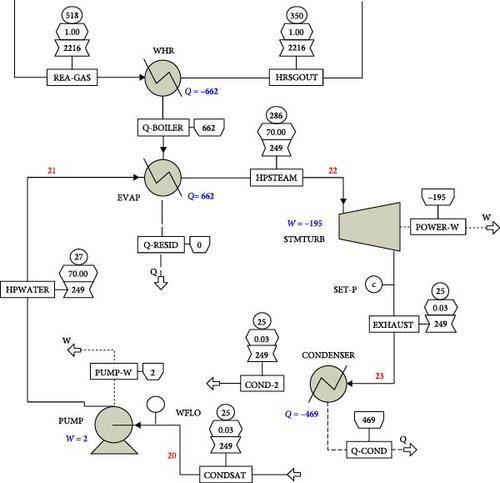
Aspen Plus v10 simulation software has been used for modelling and developing the plant’s thermodynamic data from each stream of the model. In the stream section, the ideal property of Aspen Plus v10 is selected, which sets the activity coefficient for the liquid phase to 1, the equation of state (EOS) to the ideal gas law, and estimates the molar volume of liquids using the Rackett model. The mixture is considered strongly ideal and contains liquid content with hydrogen, not entirely made of hydrocarbons. In the steam power process section, the IAPWS-95 package is chosen for accurately modelling the thermodynamic properties of water and steam under various conditions, including temperature, pressure and phase (liquid, vapour or gas). These guidelines provide precise calculations for properties such as density, enthalpy, entropy and specific heat capacity of water and steam. The data in Table 3, which is extracted from a standalone STP, are being utilized as input data for modelling the integrated STP in this model. As presented, a mixture of low-pressure methane gas and charged air is combusted in the combustor (COMBUSTR) to release CO-rich combustion gas into the reactor [66], as shown in the model schematic in Appendix Figure A1. Other products of the stoichiometric combustion process from the combustor are CO2 and H2O which are produced in the reactor where the heated feedstock is sprayed into the reactor mixture at a temperature of 518°C. A WHR generator is used to recover thermal energy from the reactor outlet in a counter-current heat exchanger to energize cool water to a temperature of 286°C to produce steam for electric power generation via a steam turbine. This recovered thermal energy was used to generate 195 MW of electricity. The CB product is produced in the quench vessel (QUENCH-W) where water at 25°C is added to wash the product prior to arrival at the filters (BACKFILT) compartment where the tail gas in the stream is released for other process utilities. The solid gathered on the walls of the backfilter is produced in the pelletizer (PELLETIZ) unit where additional water is added to drop the stream temperature to about 237°C in a process that causes the CB to develop into pellets. These wet CB pellets are produced in the dryer (DRYER) where a blower is used to discharge hot air over the pellets at 120°C to allow dried pellet production into storage.
| Parameter | Value |
|---|---|
| Heat recovery steam temperature (°C) | 518 |
| HP steam temperature (°C) | 286 |
| Pump discharge pressure (bar) | 70 |
| Steam turbine discharge pressure (bar) | 0.03 |
|
|
| Water flowrate (kg/s) | 250 |
- Note: [63].
2.3. Thermodynamic Assumptions
The design data for the analysis include material flow, operating temperatures and pressures, capacity of components and the material flow type, as well as energy flows where necessary. These input data facilitated the simulation of the system to obtain the thermodynamic properties in the system. The assumptions are categorized as follows:
2.3.1. Steam System Assumptions
- •
The natural gas used is 100% methane delivered at 9 bar and 15°C from the process plant.
- •
Air at 25°C is modelled as a 21% oxygen and 79% nitrogen molar mixture and is dry. Twenty percent excess oxygen is added to achieve complete gas combustion [67].
- •
There are no pressure drops in the heaters or between components [68].
- •
Water at 25°C is converted to steam at 286°C with no superheat [69].
- •
Reference environment: temperature, To = 298.15 K, and pressure, Po = 1.013 bar [70].
2.3.2. CB Stream Properties
- •
The mixture is strongly ideal and has liquid content with hydrogen; therefore, it is not entirely made of hydrocarbons.
- •
The methane gas is modelled as methane, ethane and propane [71].
- •
The cycle is simulated at steady-state conditions [72].
-
The combustion process occurs near atmospheric conditions [73].
- •
Processes in pumps and the steam turbine are isentropic [72].
- •
Steam is modelled as pure water [71].
3. Energy and Exergy Model Analysis
3.1. Reactor and Steam Boiler Modelling
The exergy at points 5 and 8 is physical exergy, while the exergy at point 8 is both physical and chemical. Accordingly, the exergy at point 7 is represented by Equation (21). Equation (19) calculates the chemical exergy component arising from variations in the concentration of chemical reactions within a gaseous mixture compared to the surrounding environment [88] as presented in Equation (22)
Terms in Equation (22) represent the mole fraction of fuel constituent xi and the chemical exergy of the fuel constituents, . The physical exergy at these points can be obtained using the relationship [89, 90] shown in Equation (23). Equation (24) through Equation (26), which represent the exergy of fuel, product and exergy efficiency, are calculated according to the definition provided by Mohammedi et al. [91].
3.2. Steam Boiler and Quench Tower Modelling
As demonstrated, Equation (29) defines the enthalpy balance in the steam boiler [87]. The enthalpies at stream points 21 and 22 for the working fluid are obtained using Equation (30) or any other property combination. The exergy balance is also written as Equation (31). The terms in Equation (31) represent the exergies at stated points and the exergy destruction, ExD,Boiler. The exergy of heat is also represented with ExQ. The exergy terms at points 21 and 22 can be calculated using the general relationship in Equation (32) because it is simpler to compute their specific heats at the specified conditions for most state points in the CBP. Conversely, the exergy terms at these points can also be determined using the general syntax provided [94, 95].
In the quench tower model, the effluent gases are sprayed with water to further cool them before reaching to the pelletizing section. The amount of water spray depends on the required temperature drop. Assuming an x% drop in temperature is needed, the resulting temperature of the water and effluent gases mixture is computed using Equation (33). A summary of the exergy balance expressions, exergy of fuel and product for all components is presented in Table 4:
| Components | Exergy balance | Fuel exergy | Product exergy | Exergy efficiency |
|---|---|---|---|---|
| ST turbine | Ex22 = Ex23 + ExWT + ExD,TURB | Ex22 − Ex23 | WST | |
| ST condenser | Ex23 = Ex20 + ExQ,CND + ExD,CND | Ex23 − Ex20 | ExQ,CND | |
| ST pump | WP | Ex20 − Ex21 | ||
| ST evaporator | Ex21 + ExQ = Ex22 + ExD,EVAP. | ExQ,Evap | Ex22 − Ex21 | |
| CB reactor | Ex5 + Ex7 = Ex8 + ExD,RCT | Ex5 + Ex7 | Ex8 | |
| CB combustor | Ex4 + Ex2 = Ex5 + ExD,CMBST | Ex4 + Ex2 | Ex5 | |
| CB air preheater | Ex17 = Ex18 + ExQ,AIRHTR + ExD,AIRHTR | Ex17 − Ex18 | ExQ,AIRHTR | |
| CB oil preheater | ExQ,OILHTR | Ex7 − Ex6 | ||
| CB valve | Ex3 = Ex4 + ExD,HD | Ex3 | Ex4 | |
| CB quench tower | Ex9 + Ex10 = Ex11 + ExD,qt | Ex9 + Ex10 | Ex11 | |
| CB bag filter | Ex11 = Ex12 + Ex13 + ExD,bf | Ex11 | Ex12 + Ex13 | |
| CB pelletizer | Ex13 + Ex14 = Ex15 + ExD,pt | Ex13 + Ex14 | Ex15 | |
| CB dryer | Ex15 + Ex18 = Ex16 + Ex19 + ExD,dryer | Ex15 − Ex16 | Ex19 − Ex18 |
4. Exergoeconomic Modelling
The passage discusses the use of exergoeconomic modelling in developing models for system costing, employing standard methods. Exergoeconomic analysis is explained as a tool for evaluating the techno-economic performance of energy conversion processes based on exergy [96]. The analysis aims to determine the cost per unit exergy of a system’s products. The general exergoeconomic balance for an exergy conversion system is introduced as per the work of Bejan and Tsatsaronis [76]. Models for the costing of the system based on exergoeconomic analysis are presented in line with standard methods. Exergoeconomic analysis assists in establishing the techno-economic performance of energy conversion processes on an exergy basis and determines the cost per unit exergy of the products of a system [76]. The relationship between the general exergoeconomic balance equation and an energy conversion [76] is illustrated in Equation (34). This expression can be further explained using the concept of exergy of product and exergy of fuel [97], as shown in Equation (35).
SV and PWF represent the salvage value and PWF, respectively.
4.1. Purchase of Equipment Cost
The purchase cost of equipment and plant components is expressed as a function of the operating parameters to compute the cost rates associated with plant usage. Expressions for the purchased equipment cost (PEC) are uniquely stated for the components in line with the schematic of Appendix Figure A2. A summary of the cost equations and corresponding auxiliary expressions is shown in Table 5. To evaluate the PEC estimates, the total investment cost of the integrated plant in 1994 was obtained from the WRPC manual [63]. In this work, the current investment cost in 2022 was estimated using the Chemical Engineering Plant Cost Index (CEPCI), where CEPCI in 1994 was 368.1 and CEPCI in 2022 is 816 [102]. The estimated cost of a CBP in Warri in 2022 can, therefore, be evaluated using the following equation: The present cost of the integrated plant is calculated [103] using Equation (40). This approach allows for a comparative assessment of cost escalation over time, reflecting changes in economic conditions and industry standards:
| Components | Exergoeconomic balance | Auxiliary equation |
|---|---|---|
| ST turbine | ||
| ST condenser | Nil | |
| ST pump | Nil | |
| ST evaporator | Nil | |
| CB reactor | Nil | |
| CB combustor | ||
| CB blower | ||
| CB air preheater | ||
| CB oil preheater | Nil | |
| CB valve | Nil | |
| CB quench tower | Nil | |
| CB bag filter | ||
| CB pelletizer | Nil | |
| CB dryer |
4.2. Plant Exergoeconomic Parameters
The exergoeconomic analysis of the plant is necessary to determine the exergoeconomic parameters of the plant as defined in the equations in Tables 5 and 6. These include the cost of exergy destruction, ; component exergy efficiency, ψk; cost rates of components, ; component exergy destruction rates, ExD,k; and the relative cost difference of the components, . The cost of exergy destruction [111] and the relative cost difference [112] are obtained with the expressions in Equations (41) and (42). The remaining exergoeconomic parameters have been discussed in Section 2:
| Component | Cost function (PEC) | Cost of product | Cost of fuel | Refs. |
|---|---|---|---|---|
| ST turbine | [104] | |||
| ST condenser | [105] | |||
| ST pump | [106] | |||
| ST evaporator | [107] | |||
| CB reactor | [108] | |||
| CB combustor | [108] | |||
| CB blower | [106] | |||
| CB air preheater | [105] | |||
| CB oil preheater | [109] | |||
| CB valve | 114.5ṁ3 | [104] | ||
| CB quench tower | 280.3|ṁ|0.67 | [110] | ||
| CB bag filter | 280.3|ṁ|0.67 | [110] | ||
| CB pelletizer | 280.3|ṁ|0.67 | [110] | ||
| CB dryer | [109] | |||
4.3. Cost of Exergy Destruction and Exergetic Improvement Potential
4.4. Environmental Sustainability Indicators
Environmental sustainability indicators are exergy-based indices that comparatively evaluate the performance of energy conversion systems based on exergy efficiency, useful system output and the environmental impact of such systems resulting from thermodynamic irreversibilities. These environmental sustainability indicators are expressed as follows:
Waste exergy ratio (WER): The WER quantifies the degree of cumulative thermodynamic irreversibilities in a plant with respect to the available external exergy input to the system. For thermal power plants and boilers, the available external exergy input is the chemical exergy of the fuel used. The overall waste exergy is determined by combining both the destroyed and the lost exergy within the system [88], as denoted in Equation (45). The WER is obtained mathematically as the overall exergy waste (or destruction) for the system divided by the total exergy input [113], as expressed in Equation (46).
Exergy destruction factor (EDF): The EDF is a crucial parameter that signifies the reduction in the positive impact of the engine on exergy-based sustainability. This factor can be determined by calculating the ratio of exergy destruction to the overall exergy input, as outlined in Midilli, Kucuk, and Dincer [116].
5. Results and Discussion
The simulation results are presented in line with the developed methodology. The results were obtained using Aspen Plus with data extracted from the plant manual and other thermodynamic assumptions to streamline the simulation process and properties of the working fluids used in the study. The Result section is illustrated using the detailed schematic diagram obtained in Aspen Plus, as shown in Appendix Figure A2. Charts and tables have also been created, where necessary, to provide a comprehensive overview of the findings.
5.1. Thermodynamic Performance and Energy Analysis Indices of the Plant
The performance analysis of the plant is shown in Appendix Table A1. The indices considered were the heat transferred by the components, the quantity of power requirements and the amount of dry carbon produced by the plant. The system has a CB production capacity of 1817 kg/s using a decant oil feedstock of 1870 kg/s and hot flue gas of 346 kg/s combusted in the reactor. The composition of the CB from the simulation shows it has 89.3% carbon, 9.3% hydrogen and 1.4% sulphur (Table 2). Furthermore, it is important to note that the CB process is entirely a low-pressure process occurring at atmospheric pressure. Aromatic oil, which is the feedstock utilized in this model, was initially preheated in storage to about 93°C and later heated at 250°C, generating an exergy flow rate of 128.4 MW to get the aromatic oil to the desirable temperature required to minimize reactor work and allow combustor heat to enter the reactor. Preheater air is also required to enhance the efficient combustion process in the combustor. This blower, operating at 10.17 MW, was used to convert ambient air at 25°C into preheater air at 55°C, further helping the CB system ensure optimal energy utilisation. Due to the high energy content of the flue gas from the reactor, a significant amount of heat released from the CB system has been recovered to power a steam turbine as shown in the T–Q performance chart in Figure 3.
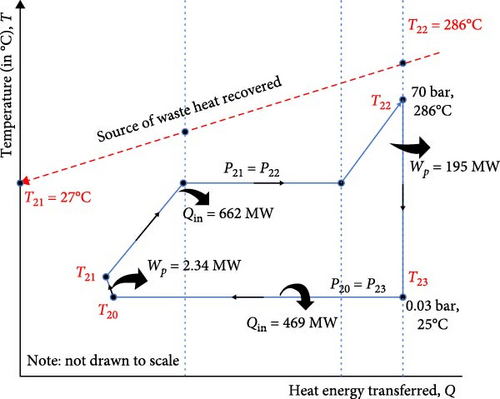
The heat energy released at 518°C was sufficient to produce 662 MW of thermal energy which was recovered through WHR techniques to produce 195.03 MW of electricity for the plant at a steam temperature of 286°C in Figure 3A. The installed pump consumed about 2.34 MW of electricity to deliver 249 kg/s of water at 70 bar to maintain pressure in the steam turbine. A significant pressure drop in the steam turbine occurred to generate electricity, utilizing only about 30% of the 662 MW of thermal energy recovered from the CB reactor. The rest of the thermal energy is dissipated via a condenser into the environment. Without digressing from the focus of this work, it is important to note that the T–Q diagram in Figure 3B aims to provide a clear and concise way to analyse and optimize the heat exchangers (evaporator and condenser) behaviours in the heat recovery process by visually representing the temperature profiles of the working fluids as they transfer heat [70, 117]. As presented in Figure 3B, T21 represents a rising temperature gradient ranging from 27 to 286°C to reach the latent heat absorption point at the steam turbine inlet at a constant temperature, T22. At the turbine discharge, the temperature, T23, drops as the working fluid tends to saturate at the condensation temperature, T20, of 25°C in the pump inlet stream. This T–Q diagram effectively shows the relationship between the temperature gradient of the working fluid and the waste heat transferred from the CBP to the STP plant. It also highlights how waste heat is utilized in the evaporator and rejected in the condenser. This aids in illustrating the heat recovery efficiency, identifying the potential system improvements and optimising the design and operation of the heat exchanger in the STP.
5.2. Exergy Efficiency of the Plant
This work focused on the physical exergy efficiency because it largely considered the efficiency associated with the conversion of mechanical or thermal energy within the integrated plant system. A higher physical exergy efficiency could indicate better performance. The thermal efficiencies of the CB and the STPs were evaluated. It was observed that the CB thermal efficiency was significant at a combustor exergy efficiency of 67.4%. This is an improved plant because, unlike a typical CBP where the combustor heat is dumped to regulate the product specification, in this study, the dumped heat is recovered to energize the power plant, hence improving the thermal efficiency of the CB component of the integrated plant. On the other hand, the STP like most others delivered only 29.12% thermal efficiency, which is also due to over 70% of thermal energy being released to the surrounding at the power plant condenser. The combined plant (CB and the steam power) overall performances were evaluated as standalone as well as overall plant. The overall energy and exergy efficiency of the integrated plant are shown in Figure 4 as 98.75% and 80.40%, respectively.
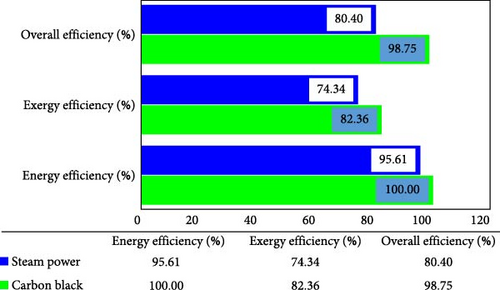
On the other hand, the energy and exergy efficiencies of the individual plants are also given as CBP (100% and 82.36%) as well as STP (95.61% and 74.34%), respectively. The exergy performance of the individual components of the integrated plant has also been examined, and the component behaviour relative to energy utilization was observed. Other than the exergetic performance of the components of the system, Appendix Table A2 also presents other performance metrics of the plant components including the fuel and product exergies, exergetic destruction, exergetic ratio and improvement potentials of the components of the system.
In Figure 5, the Grassmann diagram illustrates the exergy balance for the integrated CB and STP. The main contributors to exergetic gains are the methane gas combustion (803.35 MW) and the blower air compression (10.17 MW). Significant irreversibilities are associated with the combusted gas cooling across the plant. The rational efficiency of this plant accounts for losses due to irreversibilities, including the adiabatic combustion process (143.53 MW, 17.6%), heat transfer over a finite temperature differential in the evaporator (62.64 MW, 24.1%) and exergy dissipation through tail gas (93.84 MW, 22.9%) and the dryer (75.73 MW, 18.5%). Since the combustor is modelled adiabatically, reducing its irreversibility is challenging. However, irreversibilities related to heat transfer and dissipation can be reduced through effective design improvements and better process integration.
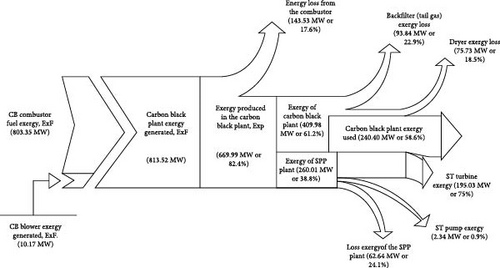
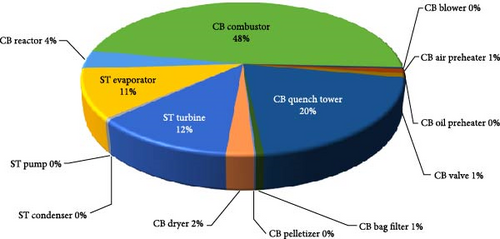
The component with the most exergetic destruction in the integrated plant is the combustor with 261.78 MW, hence the equipment with the most improvement potential. Obviously, the high-temperature gradient from the partial combustion process and the chemical mix of elements during the combustion process are the causes of the large exergetic destruction in the combustor. It is, therefore, important to focus on the differential temperature reduction in the design of the combustor to eliminate the huge destruction of exergy as well as the design of the component to reduce its potential for improvement. The quench water component and the steam turbine are the next most exergetically destroyed component in the system due to the rapid cooling with water at 25°C to the quench water vessel of 350°C and the rapid pressure drop inside the steam turbine from 70 to 0.03 bar. It is observed that the most exergetically destroyed components are also those that have the highest improvement potentials. Figure 6 describes the exergetic destruction of the system in a pie chart pictorial view which documents the percentage destruction of the component exergy relative to the total exergetic destruction of 549.27 MW. It is observed that the combustor alone contributed 48% to the total exergetic destruction of the system. From Figure 6 chart, it is seen that quench water, steam turbine and the evaporator in addition to the combustor contributed 91% of the total exergy these components have destroyed in the plant system.
5.3. Results of Exergoeconomic Analysis
A summary of the exergoeconomic results is shown in this section. The cost streams were obtained and linked to the driving parameters of the system at the component level. The significance of the components in cost formation is depicted through the combination of the component-related cost rate, Żk, and the cost of exergy destruction . This is equivalent to the total investment cost rate of a component, denoted as presented in Appendix Table A3. External costs to the system were not estimated, and they comprised mainly air and water, as well as CH4 gas and feedstock oil (decant oil) that were used for firing the combustor and reactor, respectively. These evaluated costs are a function of the irreversibility expressed in these components in Section 3.0. Therefore, Appendix Table A3 details an overview of the economic and exergetic performance of the components of the integrated plant. This exergoeconomic analysis facilitates the understanding of cost formation processes and the equivalent computation of the cost rate of the product and fuel, the determination of the cost implication of exergetic destruction and the cost parameters performance such as the exergoeconomic factor of the integrated plant. The initial capital investment, Żk, for the combustor, is uniquely the most significant initial investment with a considerable annualized cost of $1,005,000 and plays a significant role with a cost per hour of $13,317.00. As anticipated, the maximum value of corresponds to the combustor equipped with a burner zone, which has an exergoeconomic factor, f, of 96%, hence, a relative cost difference, ṙ, of 4%. This high value underscores the exergoeconomic significance of this unit, indicating that considerable attention should be given to this component to improve the overall economic performance of the system, as presented in Figure 7. The combustor destroyed exergy cost, , is $599.58 per hour, combined with a low exergoeconomic factor, indicating that the cost rate of destroyed exergy is lower than the cost rate of capital investment.
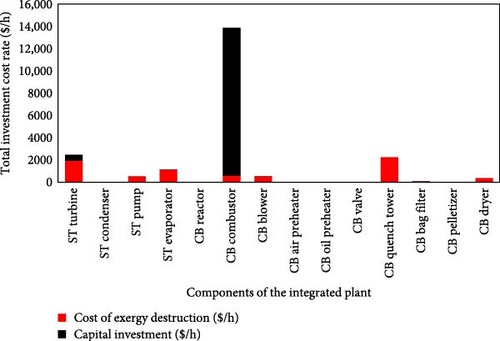
However, it is evident that significant improvements to the combustor performance are infeasible because all the sources of irreversibility, such as combustion, large temperature differences and mixing, are inherent to this unit. The steam turbine with an exergoeconomic factor, f, of 22% and exergetic efficiency of 75% is notably the next highest contributor to the initial capital investment rates and indicates a substantial upfront cost for the crucial component with a high levelized cost per hour at $541.60, emphasizing its impact on hourly operational expenses in the plant. This f value indicates that the purchasing cost of steam turbine is higher than the cost of the destroyed exergy within this component. Therefore, replacing it with a cheaper steam turbine is recommended, even if it comes at the expense of thermodynamic second law efficiency. Table 6 outlines additional exergoeconomic variables that aid in devising strategies for cost reduction. The blower and air heater also incur a high annualized cost that reflects their contribution to the operational expenses of the plant, while the condenser and oil heater demonstrate the opposite; these components have revealed the substantial lower cost of exergy destruction. Hence, there are improved exergetic efficiencies. Thus, these components are the primary contributors to cost formation within the process. On the other hand, an exergoeconomic factor of nearly 1% is observed in both the pump and the quench water vessel, which indicates that the costs of destroyed exergy are significantly close to the capital investment cost. This is further confirmed by the low exergy efficiency of the pump. Improving the exergetic performance of the pump or quench water vessel, even at a higher purchasing cost, can significantly enhance the overall economic performance of the integrated plant.
The cost due to the incorporation of a steam boiler has been accounted for by the evaporator. All cost streams attributed to exergy products are slightly less than the fuel streams and demonstrate the economic viability of the plant. Since the exergy-related costs are directly related to the quantity of exergy destruction, the cost of exergy destruction for the reactor can be affected by employing novel technologies for converting decant oil to CB. A major improvement in the reduction in exergetic cost will be required for the evaporator as it had one of the least f values of 13% compared to the other major components in the CBP. The CB blower demonstrated a high average cost per fuel and product exergy, which indicates significant economic investment, while the near-zero destroyed exergy, ExD, and cost rate of exergy destruction, , also imply efficient performance. The pump in the STP also demonstrated high costs per fuel, , and product exergy, , suggestive of significant economic input. However, while the blower shows a high dependency and functionality due to its substantial capital investment, , with a high exergy efficiency, ψ, the steam pump, on the other hand, displays moderate exergetic destruction with associated extremely low capital investment as well as low exergy efficiency that needs to be improved. The oil preheater, valve, air heater and condenser all indicate moderate to negligible costs per fuel and product exergy, which indicates a cost-effective operation. They express a minimum cost scenario due to small-to-moderate exergetic destruction in the components. However, while the valve and the air heater both show indications of high capital investment, the oil preheater and the condenser demonstrate some extremely low capital investment. As shown in Table 7 and Figure 7, it should be noted that improving the performance of individual components may have a deteriorating impact on the overall exergetic and exergoeconomic performance of each component. Therefore, recommendations to enhance the economic performance of each component should be achieved through comprehensive component design. Attempting to improve individual components does not necessarily lead to enhanced performance for the entire integrated plant system. A summary of initial investment (PEC) and levelized capital cost rate are calculated by converting CEPCI 1994 cost (in USD) to 2022 (in USD) for the components of the system, as shown in Table 7. The cost analysis was done using the EES platform. The high points indicated that the ST turbine and the combustor both contribute about 91% of the total cost attributed to the plant, with the ST turbine and combustor associated with 73% and 18%, respectively, to the investment cost. Though having a relatively low cost per unit of energy, it contributes significantly to the overall cost, indicating its critical role in the plant.
| Plant components | Purchase equipment cost (PEC) ($) ∗ | Levelized cost per year ($/year) ∗ | Levelized cost per hour ($/h) ∗ |
|---|---|---|---|
| ST turbine | 23,005,469.0 | 4,088,000.0 | 541.60 |
| ST condenser | 13,052.0 | 2319.0 | 0.31 |
| ST pump | 280,213.0 | 49,791.0 | 6.60 |
| ST evaporator | 24,002.0 | 4265.0 | 0.57 |
| CB reactor | 299,409.0 | 53,202.0 | 7.05 |
| CB combustor | 5,656,155.0 | 1,005,000.0 | 13,317.00 |
| CB blower | 749,810.0 | 133,234.0 | 17.65 |
| CB air preheater | 358,820.0 | 63,759.0 | 8.45 |
| CB oil preheater | 8344.0 | 1483.0 | 0.20 |
| CB valve | 137.0 | 24.4 | 0.00 |
| CB quench tower | 529,950.0 | 94,167.0 | 12.48 |
| CB bag filter | 262,060.0 | 46,566.0 | 6.17 |
| CB pelletizer | 149,860.0 | 26,629.0 | 3.53 |
| CB dryer | 354,430.0 | 62,979.0 | 8.35 |
However, efficient resource allocation that prioritizes investments in components such as the steam turbine and combustor will be required due to their significant impact on both initial and ongoing operational costs. Strategic planning, investment prioritization and potential optimizations to ensure plant financial performance over its operational life would be helpful in reducing the overall project cost over its life cycle. It is also indicated in Table 7 that most components presented a moderate cost per fuel and product exergy, suggesting a considerable economic input and substantial cost rate of exergy destruction, that is, combustor, quench water vessel and the steam turbine. The component with moderate cost rates of exergetic destruction (reactor, back filter and dryer) and the low exergy destruction with associated low-cost rates (evaporator and pelletizer) presented various degrees of capital investment cost as well as low moderate or high component investment rates which could indicate no, little or high potential for improvement. In summary, the plant exergoeconomic analysis highlighted the efficiency, economic input and potential areas for improvement in each component’s operation. The values in each column offer insights into the overall exergoeconomic dynamics of the plant.
5.4. Results of Environmental Sustainability Analysis
This analysis evaluates the environmental impact of the integrated plant to ensure that this model meets today’s needs and does not compromise the capabilities needed for the future by understanding the environmental aspects and potential consequences associated with the operation of this plant. The exergy-based environmental sustainability analysis is presented in Table 8. As widely recognized, CB tail gas stands out as the primary by-product in the CB industry, constituting ~30% to 40% of the total energy expended in CB production. This tail gas is a notable source of air pollution, characterized by its low calorific value and polluting nature. Harnessing the full potential of CB tail gas not only presents an opportunity to conserve energy and minimize consumption but also offers a means to mitigate pollution in the industry.
| Environmental indicator | CBP | CBT + STP |
|---|---|---|
| Waste exergy ratio (WER) | 0.201 | 0.231 |
| Exergy efficiency (%) | 0.824 | 0.804 |
| Exergy destruction factor (EDF) | 0.522 | 0.511 |
| Environmental effect factor (EEF) | 0.244 | 0.287 |
| Exergetic sustainability index (ESI) | 4.106 | 3.479 |
This paper presents the exergetic assessment and exergetic sustainability parameters of the CBP and the integrated CBP and STP configurations, otherwise tagged as CBP + STP as shown in Table 8. Prerequisite to determining sustainability parameters of these plants, the exergy parameters of the components needed to have been determined as presented. Furthermore, to compare the different energy systems based on their efficiency and environmental footprint, the ESI matrix is employed in the selection of more sustainable options. Exergetic sustainability results are presented in Figure 8 and Table 8 to understand how each of the sustainability indicators including, WER, exergy efficiency, EDF, EEF and ESI affects each of the plant configurations. It is observed that the STP inclusion in the CB cycle slightly increased exergy efficiency from about 74.3% to 80.4%, whereas a decrease in exergy efficiency is observed in the CBP by 2.4%.
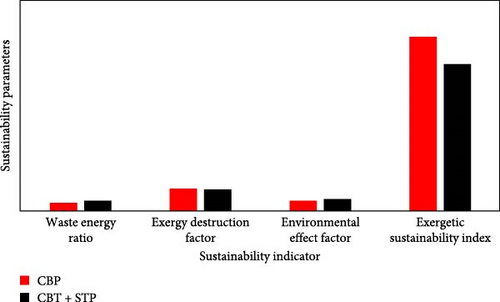
The improvement in the STP evidently showed an 8.2% cascaded improvement in the EDF. However, there is a fractional increase in the waste–exergy ratio of about 13%, which is twice the rate of exergetic efficiency increase. This indicates that the exergetic losses or waste from the CBP are not recovered. Otherwise, most of the tail gas that constituted this waste could have been used as fuel in the STP or for other district heating purposes. However, due to the power contribution from the steam turbine, the system’s ESI decreased by 0.627 units, which is about an 18% increase from a very high sustainability index value of 4.106. It is important to note that the exergetic sustainability values are desired to be above unity. However, the decrease in the sustainability index with the integrated plant is due to the enormous thermal energy released by the condenser. Otherwise, the integrated system had the capacity to increase the ESI by further utilising the condensate dissipated heat streams for other needs, including district water heating and bottoming ORCs. As shown in Figure 8, the CBP clearly showed substantial evidence of environmental impact, while the CBT + STP has shown and contributed a much-reduced impact. Therefore, the integration of the STP in the CBP is worthwhile for increasing the plant exergetic efficiency and WER, as well as being effective in overall ESI improvement.
The high ESI in Table 8 confirms the presence of emissions in the integrated plant. Most prevalent are the emissions in the tail gas released from the bag filter consisting primarily of CO, CH4 and NOx compounds, which are comparable to the impurity levels in flue gases from coal-fired power plants [118]. Carbon dioxide is the primary GHG on earth, absorbing and radiating heat from the planet’s surface. CO2 accounts for two-thirds of the total warming influence of all human-produced GHGs [119]. The emission factors for carbon dioxide from CB production in the literature range from 1.90 to 5.25 kg of CO2 per kg of CB (kgCO2 per kgCB) [120]. These estimates encompass a wide variety of plant configurations, feedstock compositions and product types. Emissions from the CB production process originate from the combustion of decant oil and natural gas used in the reactor. This process produces a tail gas, which can be utilised to generate steam for electricity or sold. The composition of these emissive gases varies depending on the grade of CB manufactured.
Table 9 compares the performance parameters of this integrated plant with those of similar and conventional plants reported in the literature. It is observed that the parameters of this work are consistent with the conventional data and perhaps most data in the literature for each key component of the CBP system. More importantly, the table compares the molar concentration of the tail gas composition in this work to those found in the literature. However, only about 0.01% of CO2 is contained in the tail gas produced compared to the conventional 1.5–3.9 mol concentration in the literature. The low carbon concentration in this model may be due to the excess 20% air used in methane gas combustion in the reactor. To minimize emissions in a CB production process, several strategies can be employed. This may include the use of cleaner fuels such as natural gas instead of furnace oil and the implementation of advanced combustion technologies that ensure more complete fuel burning, as well as optimizing process efficiency, such as improving heat recovery systems and reducing energy losses, all of which processes have been adopted to lower overall emissions in this work. However, the integration of carbon capture and storage (CCS) and the utilization of the tail gas produced during the process to generate steam and electricity can both reduce the environmental impact, but unfortunately, this could be the objective of another future study.
| Variables | Type | Rosner et al. (2024) | Conventional specification | This work | References |
|---|---|---|---|---|---|
| Carbon black yield | Input | 55 wt.% | 40–65 wt.% | 82 wt.% | [120] |
| Natural gas fuel flow | Input | 14.0 kmol NG/tonneCB | 13.2–17.9 kmol NG/tonneCB | 1 kmol propane/1.87 tonnes/s CB | [65] |
| Air consumption factor | Input | 52 mol% | 30–80 mol% | — | [53] |
| Reactor heat loss | Input | 2%-LHVNG | 1–2%-LHVNG | Adiabatic | [121] |
| Air preheat temperature | Input | 225°C | 150–250°C | 150°C | [53, 65] |
| Fuel preheat temperature | Input | 600°C | 400–800°C | — | [65] |
| Reactor temperature | Output | 1588°C | 1200–1900°C | 1761°C | [65] |
| Quench water flow rate | Input | 5 m3/tCB | N/A | 189 kg (s) | N/A |
| Reactor outlet temperature | Output | 790°C | 500–900°C | 518°C | [65] |
| Heat recuperation outlet temperature | Outlet | 540°C | At least 400°C | 518°C | [53, 121] |
| Baghouse inlet temperature | Output | 231°C | 200–280°C | 237°C | [65, 121] |
| Tail gas composition | Output | — | — | — | — |
|
— |
|
|
|
[65] |
6. Conclusion
- •
The CBP has a production capacity of 1817 kg/s using a feedstock of 1870 kg/s decant oil from a STP.
- •
The exergy efficiencies of the CBP and CBP + STP are 74.3% and 80.4%, respectively, with the efficiency improvement coming from the power output of the STP.
- •
The thermal efficiency of the STP was found to be 29%.
- •
The model converted 98.03% of the feedstocks and produced CB with a purification value of 99.25%.
- •
The waste heat recovered from the CBP is about 662 MW, causing the stream temperature to drop from 518 to 350°C at the inlet of the quench water vessel. This heat is used to generate steam in the power plant evaporator, producing ~195 MW of electricity in the steam turbine. The mass flow rate of the steam turbine is 249 kg/s, dissipating 469 MW at the condenser, with pump work requirements of 2.34 MW. The oil and air preheaters consume heat flow rates of 355 MW and 289 MW, respectively, to enhance the CB processing.
- •
The exergy of the product, that is, the CB, is obtained as 44.85 MW, with a total exergy of fuel from the decant oil and methane gas produced at 813.52 MW. The CB exergy efficiency obtained is 82.36%. However, with an additional 195 MW of electric power from the steam turbine, the overall system efficiency is up to 80.4%
- •
Similarly, the STP delivered energy and exergy efficiencies of 95.61% and 74.34%, respectively. The overall plant efficiency is calculated as 95.31%.
- •
Due to the high operating temperature and the partial combustion of the feedstock oil in the reactor, significant exergetic destruction is observed in the combustor. Other components affected by exergetic destruction include the quench water, steam turbine and the CB dryer, with the combustor contributing 48% of the total exergy destruction in the integrated plant system.
- •
The WER from the CBP is 20.1%, while the combined plant relative to the total generated exergy is 23.1% (i.e. 13% of incremental exergy destruction).
- •
It is observed that both plants have similar EDFs, indicating that a comparable proportion of the available energy is destroyed in the processes of each plant, with CBP having a slightly higher EDF.
- •
Conversely, the environmental impact factor is lower for CBP compared to the combined plant. This suggests that CBP has a lesser environmental impact relative to the exergy destroyed, whereas CBP + STP has a slightly higher environmental impact.
- •
Meanwhile, the CBP demonstrates a higher ESI compared to the combined plant (CBP + STP), with an ESI value of 4.106 compared to 3.479. This represents an ~18.02% higher sustainability for CBP. Consequently, CBP is more efficient in energy utilization with a lower environmental impact.
- •
The cost of capital investment in the combustor is the highest, amounting to about $13,317.00 per hour in the plant, while the cost of exergy destruction is the highest in the quench water vessel.
- •
A major improvement for reducing the exergetic cost will be required in the evaporator as it had the least exergoeconomic factor of 0.05% compared to the other major components in the CBP or the STP plants.
Since the study was conducted using operational data, performing performance analysis through sensitivity analysis was not feasible. Therefore, a study that thermodynamically links the various processes in the entire plant would be beneficial as it would help optimize the plant and analyse the sensitivity of the different processes on plant output.
Nomenclature
-
- Ex:
-
- Exergy rate (MJ/s or MW)
-
- Ė:
-
- Energy rate (MJ/s or MW)
-
- h:
-
- Specific enthalpy (kJ/kg)
-
- IP:
-
- Improvement potential (%)
-
- m:
-
- Mass flow rate (kg/s)
-
- Q:
-
- Rate of heat (MW)
-
- P:
-
- Pressure (kPa)
-
- s:
-
- Specific entropy (kJ/kg K)
-
- W:
-
- Work rate, power (kW)
-
- x:
-
- Mole fraction of fuel constituent
-
- C:
-
- Constant
-
- R:
-
- Ideal gas rate
-
- Ċ:
-
- Exergy cost rate ($/h)
-
- CP:
-
- Specific heat (kJ/kg K)
-
- e:
-
- Specific exergy (kJ/kg)
-
- :
-
- Standard chemical exergy rate of the ith component
-
- f:
-
- Exergoeconomic factor
-
- I:
-
- Irreversibility (kW)
-
- s:
-
- Specific entropy (kJ/kg K)
-
- T:
-
- Temperature (°C)
-
- Ẇ:
-
- Work rate (MW)
-
- Ż:
-
- Capital investment ($/h).
Greek Symbols
-
- ε:
-
- Exergy or second law efficiency (%)
-
- h:
-
- Energy or first law efficiency (%)
-
- j:
-
- Specific flow exergy (MJ/kg)
-
- ψ:
-
- Exergy efficiency (%)
-
- η:
-
- Energy efficiency (%).
Subscripts
-
- D:
-
- Destruction/damaged
-
- in:
-
- Inlet
-
- i:
-
- Inflow
-
- e:
-
- Exit
-
- f:
-
- Fuel
-
- p:
-
- Product
-
- l:
-
- Loss
-
- is:
-
- Isentropic
-
- j:
-
- Successive number of elements
-
- k:
-
- Components
-
- mech:
-
- Mechanical
-
- out:
-
- Outlet
-
- 0:
-
- Reference state
-
- ex.qt:
-
- Resulting mixture of quench water
-
- Q:
-
- Hat transfer
-
- PHY:
-
- Physical
-
- CHM:
-
- Chemical
-
- P:
-
- Pressure.
Abbreviations
-
- COND:
-
- Condenser
-
- EES:
-
- Engineering equation solver
-
- PM:
-
- Pump
-
- ST:
-
- Steam
-
- VAP:
-
- Vaporizer
-
- CV:
-
- Control volume
-
- WRPC:
-
- Warri Refining and Petrochemical Company
-
- EOS:
-
- Equation of state
-
- HRSG:
-
- Heat recovery steam generator
-
- KE:
-
- Kinetic energy
-
- PE:
-
- Potential energy
-
- RCT:
-
- Reactor.
Disclosure
The employer played no role in the manuscript’s writing, editing, approval or decision to publish.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ayeyemi Ajegunle: conceptualization, methodology. Peter Aigba: methodology, writing–review and editing. Olusegun D. Samuel: project administrator, supervisor, final draft. Joseph Oyekale: supervisor, writing–review and editing. Benjamin U. Oreko: formal analysis, visualization. Christopher C. Enweremadu: visualization, validation, writing–review and editing. Prabhu Paramasivam: resources, visualization, software. Larry Orobome Agberegha: investigation, formal analysis, writing–review and editing. H. Fayaz: methodology, data interpretation.
Funding
The research presented in this manuscript did not receive specific external funding. The study was conducted as part of the employment of the authors at their respective institution.
Acknowledgments
Prabhu Paramasivam thanks Mattu University, Ethiopia, for their extended support on this publication process.
Appendix A
| Streams | From | To | Temperature (°C) | Pressure (bar) | Mass flows (kg/s) | Specific enthalpy (kJ /kg) | Specific entropy (kJ/kg K) | Heat flow (MW) | Specific exergy (kJ/kg) | Exergy flow rate (MW) |
|---|---|---|---|---|---|---|---|---|---|---|
| AIR-IN | INLET | BLOWER | 25.00 | 1.01 | 329.72 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 |
| AIR-OUT | BLOWER | COMBUSTR | 55.47 | 1.31 | 329.72 | 30.84 | 0.17 | 10.17 | 23.76 | 7.83 |
| AROM-OIL | INLET | PREHXOIL | 93.00 | 1.01 | 1869.86 | 61.73 | 0.19 | 115.43 | 6.32 | 11.81 |
| AUX-OUT | AIR-HITA | DRYER | 120.00 | 1.00 | 3000.00 | 96.38 | 0.43 | 289.14 | 11.60 | 34.80 |
| AUXAIR | INLET | AIR-HITA | 25.00 | 1.01 | 3000.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 |
| COMBGAS | COMBUSTR | REACTOR | 1760.91 | 1.00 | 345.76 | 187.15 | 2.43 | 64.71 | 1566.30 | 541.57 |
| CONDSAT | CONDENSR | PUMP | 25.00 | 0.03 | 249.11 | 15864.32 | 9.06 | 3952.02 | 0.00 | 0.00 |
| DRYPROD | DRYER | OUTLET | 167.04 | 1.00 | 1817.29 | 127.61 | 0.35 | 231.90 | 24.68 | 44.86 |
| EXHAUST | STMTURB | CONDENSR | 25.03 | 0.03 | 249.11 | 13981.18 | 2.74 | 3482.91 | −0.02 | 0.01 |
| FUELGAS | VALVE | COMBUSTR | 15.00 | 1.31 | 16.04 | 4667.21 | 5.23 | 74.87 | 40.45 | 0.65 |
| GAS-OUT | DRYER | OUTLET | 167.04 | 1.00 | 3000.00 | 144.47 | 0.55 | 433.41 | 25.24 | 75.73 |
| HPSTEAM | BOILER | STMTURB | 285.83 | 70.00 | 249.11 | 13198.29 | 3.61 | 3287.88 | 1043.80 | 260.02 |
| HPWATER | PUMP | BOILER | 27.28 | 70.00 | 249.11 | 15854.93 | 9.02 | 3949.68 | 0.08 | 0.02 |
| HRSGOUT | HRSG | QUENCH-W | 350.00 | 1.00 | 2215.62 | 115.66 | 0.87 | 256.26 | 139.34 | 308.73 |
| LPGAS | INLET | VALVE | 15.00 | 9.00 | 16.04 | 4667.21 | 6.23 | 74.87 | 337.86 | 5.42 |
| OIL-OUT | PREHXOIL | REACTOR | 250.00 | 1.00 | 1869.86 | 251.49 | 0.61 | 470.25 | 68.68 | 128.42 |
| PEL-OUT | PELLETIZ | DRYER | 234.36 | 1.00 | 1817.29 | 206.99 | 0.51 | 376.17 | 54.12 | 98.36 |
| Q-WAT-IN | INLET | QUENCH-W | 25.00 | 1.01 | 189.16 | 15864.32 | 9.06 | 3000.90 | 0.00 | 0.00 |
| QWAT-OUT | QUENCH-W | BACKFILT | 237.10 | 1.00 | 2404.78 | 1354.45 | 0.36 | 3257.16 | 81.74 | 196.56 |
| REA-GAS | REACTOR | HRSG | 517.73 | 1.00 | 2215.62 | 183.04 | 1.29 | 405.54 | 312.03 | 691.34 |
| SOLIDOUT | BACKFILT | PELLETIZ | 237.10 | 0.95 | 1787.29 | 210.47 | 0.52 | 376.17 | 55.57 | 99.31 |
| TAILGAS | BACKFILT | OUTLET | 237.10 | 0.95 | 617.49 | 5884.04 | 0.07 | 3633.33 | 151.98 | 93.84 |
| WATLIQIN | INLET | PELLETIZ | 25.00 | 1.01 | 30.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Appendix B

| Equipment | Components | Fuel exergy (MW) | Product exergy (MW) | Exergy destroyed (MW) | Exergy destruction (%) | Exergy destruction ratio yD (%) | D (%) | Exergy efficiency (%) | Improvement potentials |
|---|---|---|---|---|---|---|---|---|---|
| ST turbine | STMTURB | 260.02 | 195.03 | 64.99 | 24.99 | 6.04 | 11.83 | 75.01 | 16.24 |
| ST condenser | CONDENSR | 0.02 | 0.01 | 0.01 | 70.56 | 0.00 | 0.00 | 29.44 | 0.01 |
| ST pump | PUMP | 2.34 | 0.02 | 2.32 | 99.20 | 0.22 | 0.42 | 0.80 | 2.30 |
| ST evaporator | BOILER | 260.01 | 202.68 | 57.33 | 22.05 | 5.33 | 10.44 | 77.95 | 12.64 |
| CB reactor | REACTOR | 691.34 | 669.99 | 21.35 | 3.09 | 1.98 | 3.89 | 96.91 | 0.66 |
| CB combustor | COMBUSTR | 803.35 | 541.57 | 261.78 | 32.59 | 24.33 | 47.66 | 67.41 | 85.31 |
| CB blower | BLOWER | 10.17 | 7.83 | 2.33 | 22.95 | 0.22 | 0.42 | 77.05 | 0.54 |
| CB air preheater | AIR-HITA | 39.75 | 34.80 | 4.95 | 12.46 | 0.46 | 0.90 | 87.54 | 0.62 |
| CB oil preheater | PREHXOIL | 128.75 | 128.42 | 0.33 | 0.26 | 0.03 | 0.06 | 99.74 | 0.00 |
| CB valve | VALVE | 5.42 | 0.65 | 4.77 | 88.03 | 0.44 | 0.87 | 11.97 | 4.20 |
| CB quench tower | QUENCH-W | 308.73 | 196.56 | 112.17 | 36.33 | 10.43 | 20.42 | 63.67 | 40.75 |
| CB bag filter | BACKFILT | 196.56 | 193.16 | 3.41 | 1.73 | 0.32 | 0.62 | 98.27 | 0.06 |
| CB pelletizer | PELLETIZ | 99.31 | 98.36 | 0.95 | 0.96 | 0.09 | 0.17 | 99.04 | 0.01 |
| CB dryer | DRYER | 133.16 | 120.59 | 12.57 | 9.44 | 1.17 | 2.29 | 90.56 | 1.19 |
| Total | — | 1075.86 | 865.02 | 549.27 | 72.64 | 51.05 | 100.00 | 80.40 | 107.64 |
| Plant component | Cost of fuel exergy, ($/GJ) | Cost of product exergy, ($/GJ) | Exergy destroyed, ExD (MW) | Cost of exergy destruction, ($/h) | Capital investment, ($/h) | Exergy efficiency, ψ (%) |
Component investment rates, ($/h) | Exergoeconomic factor, f (%) | Relative cost difference, ṙ (%) |
|---|---|---|---|---|---|---|---|---|---|
| ST turbine | 8.33 | 11.93 | 64.81 | 1943.67 | 541.60 | 75.01 | 2485.27 | 0.22 | 0.78 |
| ST condenser | 0.01 | 0.00 | 0.01 | 0.00 | 0.31 | 29.44 | 0.31 | 1.00 | 0.00 |
| ST pump | 63.33 | 70.62 | 2.31 | 527.35 | 6.60 | 0.80 | 533.95 | 0.01 | 0.99 |
| ST evaporator | 5.55 | 6.23 | 0.19 | 3.79 | 0.57 | 99.97 | 4.36 | 0.13 | 0.87 |
| CB reactor | 0.18 | 5.55 | 22.37 | 14.79 | 7.05 | 96.76 | 21.84 | 0.32 | 0.68 |
| CB combustor | 0.63 | 7.81 | 263.95 | 599.58 | 13317.00 | 67.14 | 13916.58 | 0.96 | 0.04 |
| CB blower | 63.33 | 67.10 | 0.00 | 0.00 | 17.65 | 100.00 | 17.65 | 1.00 | 0.00 |
| CB air preheater | 0.00 | 0.27 | 4.95 | 0.00 | 8.45 | 87.54 | 8.45 | 1.00 | 0.00 |
| CB oil preheater | 0.00 | 0.00 | 0.33 | 0.00 | 0.20 | 99.74 | 0.20 | 0.99 | 0.01 |
| CB valve | 0.00 | 0.01 | 4.77 | 0.01 | 0.00 | 11.97 | 0.02 | 0.19 | 0.81 |
| CB quench tower | 5.55 | 7.83 | 112.41 | 2245.20 | 12.48 | 63.59 | 2257.68 | 0.01 | 0.99 |
| CB bag filter | 7.83 | 7.96 | 3.41 | 96.04 | 6.17 | 98.27 | 102.21 | 0.06 | 0.94 |
| CB pelletizer | 7.96 | 8.05 | 0.95 | 27.21 | 3.53 | 99.04 | 30.74 | 0.11 | 0.89 |
| CB dryer | 8.05 | 10.39 | 12.53 | 363.00 | 8.35 | 90.58 | 371.35 | 0.02 | 0.98 |
Open Research
Data Availability Statement
The data used to support the findings of this study are included in the article. Should further data or information be required, these are available from the corresponding author upon request.




