Impact of Phosphide–Phosphate Ratio on NiCoP Catalysts for Hydrogen Evolution in Anion Exchange Membrane Water Electrolysis
Abstract
Alkaline and anion exchange membrane water electrolysis (AEMWE) presents a promising approach for hydrogen production. However, the slow kinetics of the alkaline hydrogen evolution reaction (HER) remains a significant challenge. This study aimed to enhance HER activity by optimizing transition metal-phosphorus compound catalysts, including Ni, Co, NiCo, NiP, CoP, and NiCoP. Their surface structure, crystallinity, electrochemical properties, and HER performance were meticulously studied. Among the catalysts, Ni28Co62P10 exhibited exceptional HER performance, achieving a low overpotential of 48 mV at a current density of −10 mA cm–2 in 1 M KOH. X-ray photoelectron spectroscopy analysis revealed that an optimal 1:1 balance of phosphate to phosphide is critical for achieving efficient HER. These findings emphasize the importance of balancing phosphorus species for optimal alkaline HER catalysis. Moreover, Ni28Co62P10 demonstrated excellent durability, maintaining high performance after 5000 cycles. In AEMWE single-cell tests, the catalyst achieved a cell voltage of 1.88 V at 1 A cm–2, surpassing the performance of Ni/Co-based catalysts from previous studies. The NiCoP-based catalysts in this study presented considerable promise for AEMWE systems, paving the way to the development of more efficient and durable catalysts for hydrogen production and advancing hydrogen-based renewable energy technologies.
1. Introduction
Accelerated global warming due to CO2 emissions from fossil fuel consumption has heightened the urgency for renewable energy development. While renewable energy sources do not generate greenhouse gases, their intermittent characteristic poses challenges for direct integration into the power grid, thereby necessitating stable energy storage systems (ESSs) [1, 2]. Compared to conventional secondary battery-based ESS, hydrogen offers significant advantages, including high energy density, long-term stable storage, and eco-friendliness [3, 4].
Traditionally, hydrogen production for industrial applications has relied on fossil fuel reforming. However, for ESS applications, renewable energy-driven water electrolysis is considered the most viable approach [5, 6]. Based on electrolyte type and operating temperature, water electrolysis can be classified into alkaline water electrolysis, anion exchange membrane water electrolysis (AEMWE), proton exchange membrane water electrolysis (PEMWE), and solid oxide electrolysis. Among these, AEMWE and PEMWE, which utilize polymer electrolyte membranes, have garnered significant research interest owing to their high current density, high-purity hydrogen production, and relatively low operating temperatures compared to other water electrolysis methods [7–10]. PEMWE is associated with high costs primarily due to the need for corrosion-resistant noble metal catalysts, which are required to withstand the harsh acidic operating environment [11, 12]. In contrast, AEMWE offers a significant advantage by operating in a less corrosive alkaline environment, thus alleviating the dependence on expensive noble metals and reducing overall system costs [13, 14]. However, operating in a less corrosive environment does not inherently guarantee rapid electrode reaction kinetics. AEMWE involves a water dissociation step in the cathodic reaction, making the hydrogen evolution reaction (HER) significantly slower than in PEMWE [15].
Thus, achieving an optimal balance between water dissociation and hydrogen/hydroxyl adsorption is critical for efficient alkaline HER [17–19]. Research on alkaline HER catalysts has focused on developing transition metal alloys, transition metal compounds, and strategies to reduce noble metal content [20–23].
MoNi4@MoO3–X exhibits an overpotential of 58.6 mV at –10 mA cm–2 in 1 M KOH, where the Ni component of the Ni-Mo alloy facilitates water dissociation, while the Mo phase plays a pivotal role in hydrogen adsorption, as highlighted in recent studies [22]. Furthermore, Pt2.3%-Fe0.05Ni0.95(OH)2 catalyst effectively reduces the noble metal Pt content to 7.36 μg cm−2. The synergistic interaction between Fe-doped α-Ni(OH)2, which promotes water dissociation, and Pt nanoparticles, which facilitate H recombination, significantly enhances HER activity, achieving an overpotential of 18 mV at–10 mA cm−2 in 1 M KOH [23]. Extensive research has been conducted on transition metal compounds, particularly phosphides, sulfides, and carbides [24–29].
Transition metal phosphides (TMPs) exhibit promising catalytic properties, with Ni2P-MoP@NC optimizing hydrogen adsorption energy (Δ), thus achieving a favorable balance between adsorption and desorption. This optimization also reduces the water dissociation energy barrier, significantly accelerating the Volmer step. As a result, Ni2P-MoP@NC demonstrates an overpotential of 69 mV at –10 mA cm–2 in 1 M KOH [28]. In addition, CoP-400, utilizing P doping, enhances hydrogen adsorption at P-top sites and water dissociation at Co-bridge sites, thereby improving catalytic performance under both acidic and alkaline conditions. This synergy enables CoP-400 to achieve low overpotentials of 113, 154, and 161 mV at −10 mA cm–2 in 0.5 M H2SO4, 1 M KOH, and 1 M phosphate buffer solution, respectively [27]. Moreover, the high electronegativity of phosphorus modified the electronic structure of the transition metal Ni, reducing the absolute values of the Gibbs free energy of hydrogen adsorption (Δ), thereby achieving an overpotential of 59.7 mV at −10 mA cm−2 in 1 M KOH [29].
As aforedescribed, balancing water dissociation and hydrogen/hydroxyl adsorption is crucial for effective alkaline HER catalysis, making research on phosphides significant. Despite the importance of TMPs, limited studies have explored the impact of different phosphorus species, such as phosphide (P3−), phosphite (PO33−), and phosphate (PO43−), on catalytic activity. Phosphate has been reported to act as a proton shuttle in alkaline HER, effectively weakening O–H bonds and stabilizing intermediates formed in the Volmer step, thereby lowering the activation barrier for water dissociation and accelerating the reaction [30–32]. Meanwhile, phosphides enhance the overall electronic conductivity by withdrawing electrons from transition metals because of their high electronegativity, optimizing hydrogen intermediate adsorption energy, and promoting the Tafel/Heyrovsky steps [33–35].
This study aimed to investigate the effect of various phosphorus species, particularly the ratio of phosphate to phosphide, on balancing the two key stages of alkaline HER. While previous studies on NiP and CoP have focused on the metal-to-phosphorus ratio [36–39], this study examined the electrochemical and material properties of bimetallic phosphorus-based compounds and their individual components. The findings were validated not only at the half-cell level but also in a full-cell configuration, affording deeper insights into phosphorus-based catalysts and aiding in maximizing their efficiency in alkaline HER.
2. Experimental
2.1. Catalyst Preparation
Ni, Co, NiCo, NiP, CoP, and NiCoP catalysts were synthesized via electrodeposition using a standard three-electrode setup at room temperature under atmospheric conditions. The catalysts were deposited onto carbon paper (Ballard, Avcarb MGL280) without surface pretreatment, serving as the working electrode with an active area of 1.327 cm2. A graphite rod functioned as the counter electrode, while a saturated calomel electrode (SCE, CHI Instruments, CHI150) was used as the reference electrode.
The electrolyte solution was prepared by dissolving appropriate amounts of NH4Cl (Daejung, 1060–4100), NaH2PO2 · H2O (Yakuri Pure Chemicals Co. Ltd., 51519), CoCl2·6H2O (Daejung, 2570–4405), and NiCl2·6H2O (Wako Pure Chemical Industry, 141–01045) in deionized water (DI water, 18.2 MΩ), followed by stirring for 10 min. Detailed electrolyte compositions are provided in Table S1. Before electrodeposition, the electrolyte was purged with N2 gas for 30 min to remove the dissolved O2. Electrodeposition was performed using three potential cycles between −0.1 and −1.8 VSCE at a scan rate of 5 mV s−1 using a potentiostat (Metrohm, Autolab, PGSTAT302N).
2.2. Material Characterization
The surface morphologies of the synthesized catalysts were analyzed using field emission scanning electron microscopy (FE-SEM; Carl Zeiss, Sigma 300), while their compositions were determined via energy-dispersive X-ray spectroscopy (EDS; Thermo, NORAN System 7). Crystallinity was assessed using X-ray diffraction (XRD; Bruker-AXS, NEW D8-Advance) at a scan rate of 1.5° min−1 over a two-theta range of 5°–80°. The electronic structure of the catalyst surfaces was characterized via X-ray photoelectron spectroscopy (XPS, Thermo-Fisher Scientific, K-alpha+). The spectra were calibrated to the C–C peak in the C 1s region at 284.8 eV, and peak deconvolution was performed using XPSPEAK 41 software.
2.3. Electrochemical Characterization
HER activity was evaluated using cyclic voltammetry over a single cycle, with a potential range of −0.85 to −1.25 VHg/HgO at a scan rate of 1 mV s−1 under alkaline conditions (1 M KOH). Accelerated degradation tests (ADTs) for HER were conducted over 1000–5000 potential cycles at a scan rate of 100 mV s−1 over the same range. Additionally, constant current stability was assessed at −10 mA cm−2 for 24 h.
Electrochemical impedance spectroscopy was performed by applying a 30 mV DC overpotential with a 5 mV AC amplitude across a frequency range of 105–10−2 Hz in 1 M KOH using a potentiostat (Wuhan Corrtest Instruments Corp. Ltd, CS310). The obtained solution resistance (Rs) was used for iR correction. The double-layer capacitance (Cdl) was determined from linear sweep voltammetry (LSV) measurements, where the potential was first swept in the positive direction in the non-faradaic region and then reversed. The scan rate varied from 20 to 100 mV s−1, and the electrochemically active surface area (ECSA) was evaluated by plotting half the difference between the anodic and cathodic currents ([ia − ic]/2) at the midpoint of the potential range as a function of the scan rate.
2.4. AEMWE Operation
A membrane electrode assembly (MEA) was fabricated without hot pressing, incorporating a 1 × 1 cm2 cathode and anode on either side of a 20 μm-thick PiperION anion exchange membrane. The cathode utilized the Ni28Co62P10/CP electrode, the highest-performing catalyst developed in this study.
For anode fabrication, catalyst ink was prepared by dispersing Ni2Fe1 powder [40] and PiperION dispersion (5 wt%) in a solvent mixture of DI water, ethanol, and isopropyl alcohol at a 1:1:2 volume ratio. This was designed to achieve a solid content of 2 wt% with a Ni2Fe1 to ionomer mass ratio of 9:1. The resultant suspension was sonicated for 1 h to ensure homogeneous distribution. The ink was applied via handgun spraying onto a 600 μm-thick stainless steel (SS) fiber felt (Dioxide Materials), yielding a Ni2Fe1/SS anode with a catalyst loading of 3.0 mg cm−2.
To convert the ionically conductive components from the bicarbonate to the hydroxide (OH–) form, the anode and membrane were immersed in a 1.0 M KOH solution at room temperature for 12 h under sealed conditions, followed by thorough rinsing with DI water.
The AEMWE cell was assembled with a clamping torque of 80 IN∙LB, utilizing 215 μm-thick and 600 μm-thick polytetrafluoroethylene (PTFE) gaskets for the cathode and anode, respectively. A preheated 1.0 M KOH solution was supplied to the anode side at a flow rate of 3 mL min−1, maintaining the cell temperature at 80°C. The current–voltage (I–V) curve was recorded by averaging the current density over 1 min at each voltage step, at 50 mV increments within a voltage range of 1.35–2.1 Vcell. For durability assessment, the cell operated at a constant current density of 1.0 A cm−2 for 100 h.
3. Results and Discussion
The FE-SEM images in Figure 1 show the surface morphology of representative samples, along with their atomic compositions determined via EDS analysis. For Ni100, owing to its relatively positive reduction potential (−0.257 V vs. SHE), a significant amount of Ni ions is reduced during electrodeposition at –1.8 VSCE. The fibrous structure of the underlying carbon paper is then completely obscured, forming a thick electrodeposited layer with a cracked film structure. Porous aggregates are also observed on the noncracked regions of the deposited layer. In contrast, Co100, which has a more negative reduction potential and a lower precursor concentration compared to Ni100, forms agglomerated particles rather than the bulky deposition observed in Ni100. This allows for partial visibility of the carbon fibers beneath the deposited layer. The particulate deposition of Co is attributed to the use of CoCl2 as the Co precursor [41]. The morphology significantly transforms upon incorporating a small amount of P, as observed in the case of Ni92P8. A similar morphological change is observed in Co97P3, attributed to the inhibition of Niad [42] and Coad [43] diffusion by the introduced P. This leads to a more controlled deposition process, resulting in either fiber-aligned deposition along the carbon paper (NiP) or the formation of smaller agglomerated deposits (CoP). For Ni66Co34, anomalous deposition occurs, where the less noble metal (Co) is preferentially deposited. This phenomenon is attributed to pH variations near the electrode surface and the competitive adsorption of metal hydroxyl species [44, 45]. As a result, the bulk molar concentration ratio of the deposited catalyst (Ni:Co = 66:34) deviates considerably from the nominal electrolyte composition (Ni:Co = 0.5:0.15). Consequently, the preferential deposition of Co suppresses the overdeposition of Ni, preventing excessive deposition as observed in Ni100. This allows the deposition to follow the fibrous structure of the carbon paper. In Ni28Co62P10, the smooth morphology of NiP and the particulate morphology of CoP interact, resulting in the coexistence of both structural features (uniform particulate deposition on the carbon fibers). Despite using the same concentration of metal ions, the Ni-to-Co ratio in Ni28Co62P10 is reversed compared to Ni66Co34. This reversal is attributed to the presence of P, which enhances the anomalous deposition, further promoting the preferential deposition of the less noble metal, Co [46]. Consequently, the deposited layer predominantly exhibits a granular surface morphology characteristic of Co. EDS elemental mapping (Figure S1) further confirms that Ni, Co, and P are uniformly distributed across the surface without signs of phase separation. This supports the formation of a homogeneous NiCoP structure.
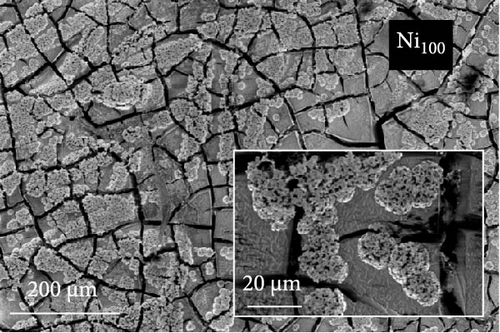
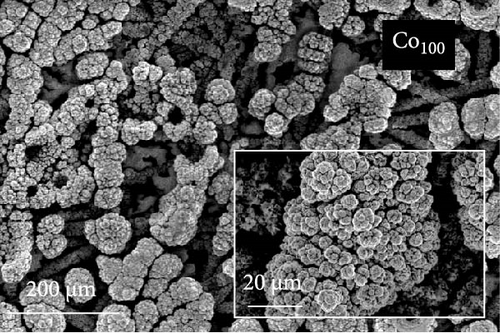
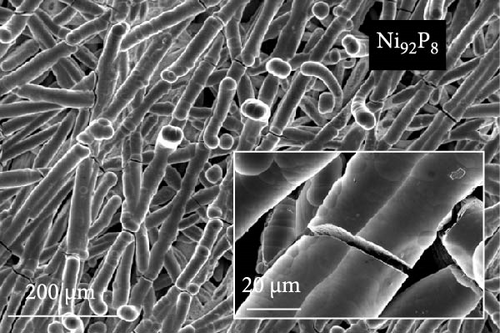
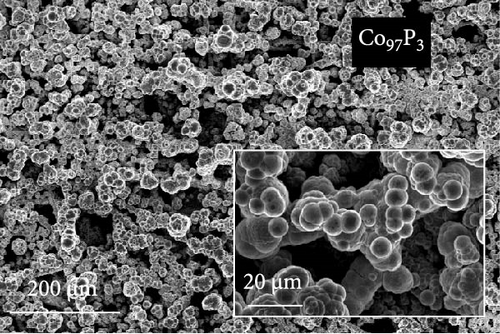
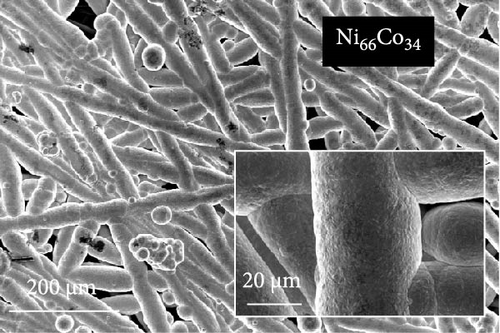

Figure 2 presents the XRD analysis results used to investigate the crystallinity of each catalyst. For the carbon paper, typical peaks are observed at 43.02°, 44.67°, and 54.79°. In the case of Ni100, the peaks at 44.51°, 51.85°, and 76.4° correspond to Ni (111), (200), and (220) (JCPDS #04−0850), respectively. For Co100, the peaks at 41.58°, 44.50°, and 47.44° correspond to Co (100), (002), and (101) (JCPDS 01-071-4239). However, the crystallinity of Co appears to be low. In Ni66Co34, due to the low Co content, only Ni peaks are observed at 44.41°, 51.79°, and 76.33°. Notably, the Ni (111) peak at 44.51° shifts by approximately 0.1° because of the alloying with Co. When phosphorus is introduced to the monometallic samples during deposition, the resulting XRD patterns differ significantly from those of the individual metals. In Ni92P8, a very broad peak is observed at 45.31°. The carbon peak at 44.67° is not visible, suggesting that this broad peak corresponds to that of Ni (111). The peak broadening suggests the underdevelopment of the crystalline structure due to the incorporation of phosphorus [42], as observed in the FE-SEM images in Figure 1. The grain sizes, calculated from the full width at half maximum of the Ni (111) peak, are 14.9 nm for Ni and 1.8 nm for Ni92P8. In contrast to Co100, which showed some crystallinity, Co97P3 exhibits a structure that is closer to amorphous, as does Ni28Co62P10. The excellent HER activity of amorphous catalysts has been widely documented in numerous studies [47, 48]. For most of the samples, the underlying carbon peak is not observed because of the thick deposition layer.
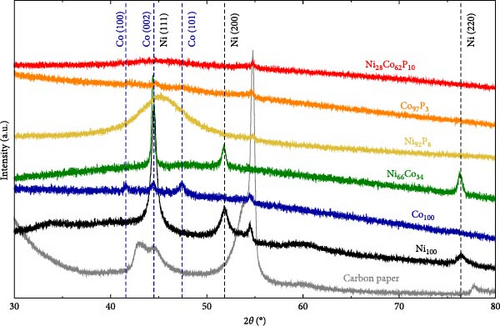
Figure 3a displays the iR-corrected HER polarization curves of several representative catalysts. The HER activity evaluations were carried out in an alkaline environment (1 M KOH). The catalysts evaluated include Ni100, Co100, Ni66Co34, Ni92P8, Co97P3, and Ni28Co62P10 (HER activity data for all synthesized catalysts are summarized in Table S1). The monometallic catalysts, Co100 and Ni100, exhibited overpotentials of approximately 310 mV and 226 mV at −10 mA cm–2, respectively, indicating very low HER activity. The Ni66Co34 alloy showed a slightly improved overpotential of 294 mV at −10 mA cm–2 compared to Co100. However, its activity remained inferior to that of Ni100 owing to the high Co content. This suggests that simple NiCo binary alloys are not optimal for effective alkaline HER catalysis. In contrast, the monometallic phosphorus compounds, Ni92P8 and Co97P3, exhibited significantly enhanced activity, with overpotentials of 84 mV and 87 mV at −10 mA cm–2, respectively. These values represent improvements of 142 and 223 mV compared to their respective monometallic counterparts, confirming that even a small incorporation of phosphorus can substantially boost alkaline HER performance. This enhancement has been widely observed in previous studies. For instance, a heat-treatment phosphorization method was used to synthesize Ni2P catalysts, which exhibited a 32 mV improvement in overpotential at −10 mA cm–2 compared to pure Ni [49]. Similarly, CoP2 catalysts synthesized via heat-treatment phosphorization showed a remarkable 238 mV enhancement in HER activity compared to monometallic Co under alkaline conditions [50]. A noteworthy finding of our study is that the NiCo alloy, which exhibited lower activity than monometallic Ni because of its high Co content, demonstrated exceptional performance upon phosphorus incorporation. Specifically, Ni28Co62P10 exhibited an overpotential of just 48 mV at −10 mA cm–2, surpassing the activity of both NiP and CoP. Thus, the formation mechanism of P-induced compounds in NiCo alloys differs fundamentally from that in monometallic Ni-based compounds, leading to superior catalytic performance.
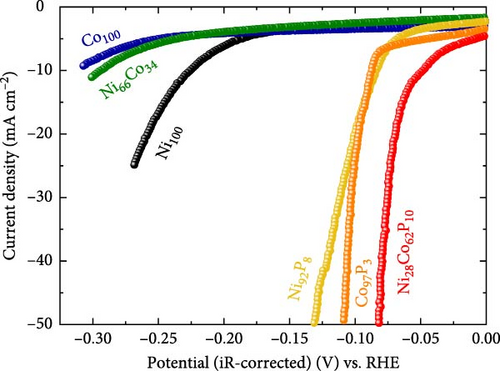
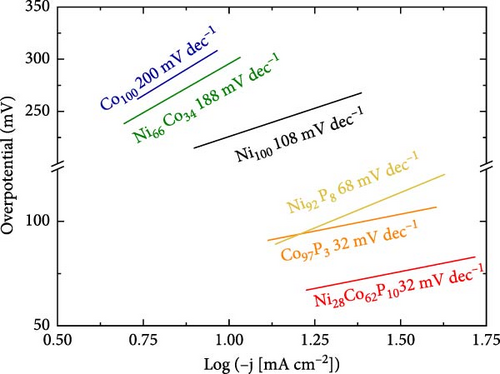
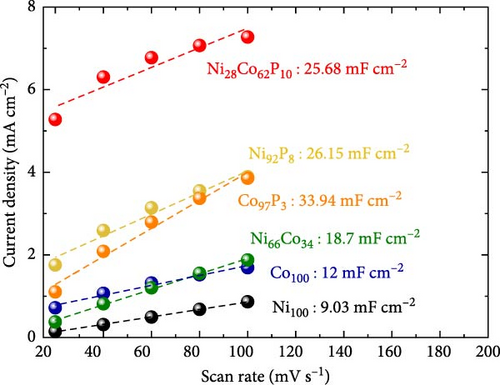
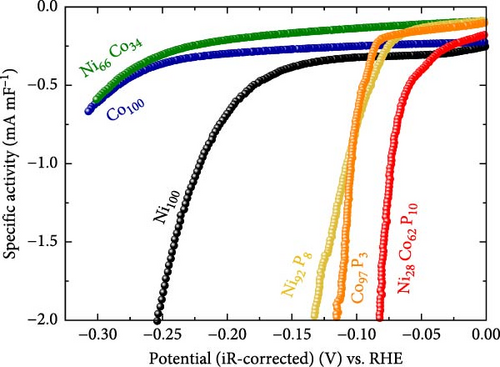
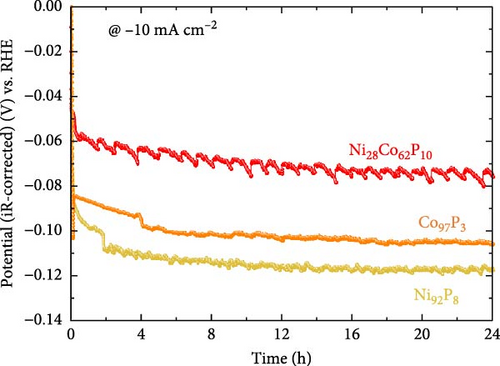
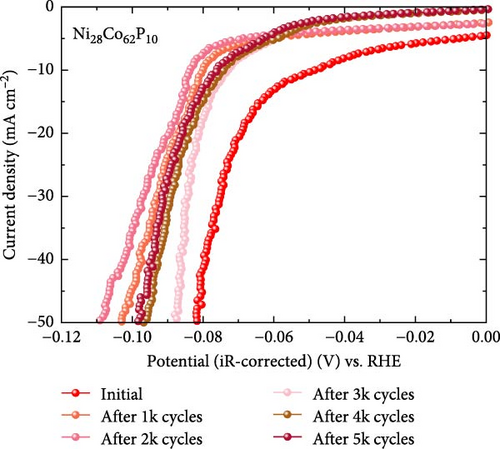
Figure 3b presents the Tafel slope values for each catalyst, derived from the polarization curves in Figure 3a. The Tafel slopes for Ni100, Co100, and Ni66Co34 are 108, 200, and 188 mV dec–1, respectively, indicating that the Volmer reaction is the rate-determining step. For Ni92P8, the Tafel slope is 68 mV dec–1, which is lower than that of Ni100, suggesting that the addition of a small amount of phosphorus shifts the HER mechanism from Volmer-limiting to Heyrovsky-limiting. In contrast, Co97P3 and Ni28Co62P10 exhibit Tafel slopes of 32 mV dec–1, indicating that the Tafel reaction is the rate-determining step [51]. Based on the HER polarization curves and Tafel slope values, the addition of phosphorus to a less active transition metal significantly accelerates the HER rate by altering the mechanism of water dissociation. This trend is further validated through comparative analysis with literature-reported NiCo-based phosphide/phosphate catalysts, as summarized in Table S2 and visualized in Figure S2. Furthermore, as previously discussed, the distinct compound formation between NiP and NiCoP plays a crucial role in influencing the HER reaction mechanism.
ECSA measurements were performed to explore the factors contributing to the varying HER activities of the catalysts. Figure 3c displays the Cdl values for each catalyst, derived from LSV conducted in the non-faradaic potential region of a 1 M KOH electrolyte (Figure S3). Ni100 and Co100 exhibit low Cdl values of 9.03 and 12 mF cm–2, respectively. In contrast, the Ni66Co34 alloy shows a higher Cdl of 18.7 mF cm–2 compared to the monometallic catalysts. These trends are consistent with the catalyst surface morphologies observed in the SEM images in Figure 1. Specifically, Ni100, with its plate-like deposition (Figure 1a), mitigates the area effect of the porous carbon paper. Meanwhile, Co100 (Figure 1b) shows a slight increase in surface area relative to Ni100 owing to its particulate morphology. Nevertheless, the bulky nature of its deposition prevents it from fully exploiting the structural advantages of the carbon fibers. In contrast, Ni66Co34, with limited deposition, maintains the carbon fiber structure, thus presenting a higher surface area.
For Ni92P8 and Co97P3, which incorporate phosphorus into the monometallic catalysts, there is a significant increase in Cdl. This is attributed to the well-developed pore structure of Ni92P8, which is deposited thinly along the carbon paper fibers (Figure 1c), and the high porosity of Co97P3, which forms smaller particle clusters (Figure 1d). Similarly, Ni28Co62P10 exhibits a considerable Cdl of 25.68 mF cm–2, and although its deposition is relatively thicker than that of Ni92P8 (Figure 1c) and Co97P3 (Figure 1d), resulting in a slightly lower Cdl than these catalysts, it still maintains a sufficiently high capacitance. Using the Cdl values from Figure 3c, the HER activity from Figure 3a was normalized to determine the specific activity, as shown in Figure 3d. While the specific activity of each catalyst follows a trend similar to that in Figure 3a. This statement suggests that metallic catalysts containing phosphorus not only have a relatively high ECSA but also possess other unique characteristics that enhance HER activity.
Figure 3e shows the results of the constant current durability test conducted for 24 h at −10 mA cm–2 on Ni92P8, Co97P3, and the most efficient HER catalyst, Ni28Co62P10. During the 24 h constant current durability evaluation, both Ni92P8 and Co97P3 demonstrated a sudden potential decay at an early stage of test (around 2–4 h), indicating poor constant current durability. In contrast, Ni28Co62P10 showed a stable potential with a decay rate of approximately 1.17 mV h–1, suggesting superior durability compared to the monometallic NiP and CoP. This performance is more favorable than previously reported decay rates for metal phosphides, such as approximately 2.88 mV h–1 for Rh2P [52] and 1.46 mV h–1 for O-NiCoP/Ni2P [53].
In addition to the constant current durability test, an ADT was conducted for Ni28Co62P10 within the potential range of −0.85 to −1.25 VHg/HgO at a scan rate of 100 mV s–1, as shown in Figure 3f. The Ni28Co62P10 catalyst performance declined up to 2000 cycles, followed by a slight recovery, ultimately stabilizing. After 5000 cycles, the catalyst showed a 28 mV increase in overpotential at −10 mA cm–2, with a decay rate of 5.6 μV per cycle.
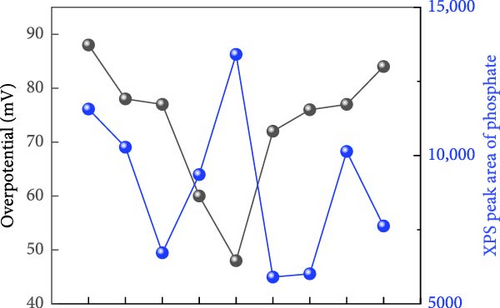
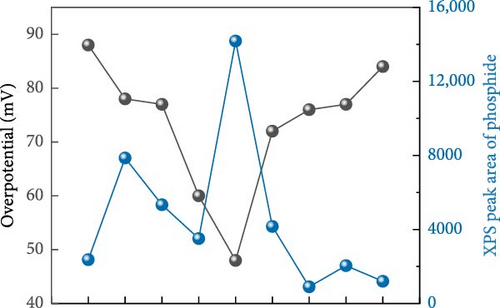
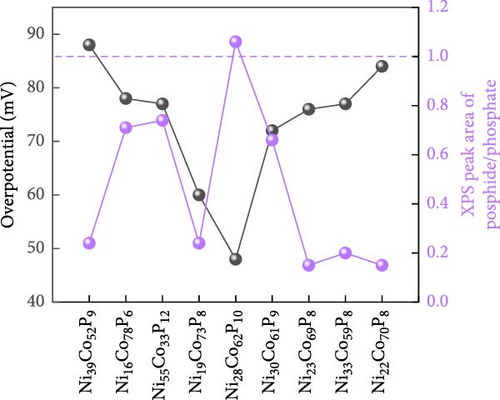
XPS analysis was performed to investigate the factors contributing to the excellent HER activity and durability of the Ni28Co62P10 catalyst. Figure 4 shows the XPS results of Co97P3, Ni92P8, and the most efficient HER catalyst, Ni28Co62P10, before and after 5000 cycles of ADT evaluation. Additional XPS data for various compositions of NiCoP catalysts, prepared with different precursor configurations in the electrolyte, are provided in Figure S4, and their HER activities are shown in Figure S5. The overpotentials at −10 mA cm–2 for these catalysts are summarized in Table S1. A key focus of the XPS analysis in Figure 4 and Figure S4 is the relative ratio of phosphate and phosphide species. In our previous study on NiP [54], the formation of phosphate and phosphide was found to be potential-dependent, with more negative step potentials favoring phosphate formation and more positive potentials promoting phosphide. In the present work, the coexistence of both species was achieved by applying potential cycles between −0.1 and −1.8 VSCE during electrodeposition. This behavior is attributed to local pH gradients generated by the HER, which create spatially distinct microenvironments across the electrode surface, favoring phosphide formation in more acidic regions and phosphate formation in more alkaline ones [55, 56]. Through XPS analyses of the various catalysts, we aim to correlate the presence ratios of phosphate and phosphide in each catalyst with their respective activity. The P 2p binding energy for Ni3(PO4)2 or Co3(PO4)2 is typically between 133–135 eV [57, 58], while for metal phosphides such as Ni2P, Ni5P4, Co2P, and CoP, the P 2p binding energy falls between 128–130 eV [59, 60]. Through these differences in characteristics, Figure 5 presents the relationships between the overpotential at −10 mA cm–2 and the phosphate peak area (Figure 5a), phosphide peak area (Figure 5b), and the phosphide/phosphate ratio (Figure 5c) based on the information obtained from Figure 4 and Figure S4. Several important conclusions can be drawn from Figure 5. First, HER activity shows no clear correlation with the EDS composition ratios of Ni, Co, and P in the catalysts. Second, HER activity tends to be proportional to the amount of phosphate, although there are several exceptions (Figure 5a). Third, HER activity exhibits a more clearly defined relationship with the amount of phosphide compared to phosphate, although there are some outlier samples (Ni55Co33P12, Ni19Co73P8) (Figure 5b). Lastly, HER activity shows a clear relationship with the phosphide/phosphate ratio, with only one exception (Ni19Co73P8) (Figure 5c). These findings suggest that the activity of metal-phosphorus compound catalysts is more strongly influenced by the constituent species (phosphate and phosphide) rather than the metal itself. Metal phosphides are recognized as active sites for HER catalysis, playing a positive role by enhancing the electrical conductivity and promoting electrode reactions [17, 26, 61]. Metals with low oxidation states are known to form phosphides [62]; however, further oxidation of phosphides (e.g., due to the presence of oxygen or moisture) can lead to the formation of phosphates [63]. Metal phosphates are stable compounds with relatively lower hydrogen evolution activity than phosphides because of their lower conductivity [64, 65]. However, recent studies have highlighted the potential of transition metal phosphates as HER catalysts. Along with the previously mentioned advantages of phosphate as an alkaline HER catalyst [30–32], Wijayanti et al. demonstrated that the (100) and (011) facets of nickel phosphate are the most stable surface facets, and the weaker hydrogen adsorption energies at the oxygen sites on these facets enhance product desorption, thereby improving HER performance [66]. Furthermore, Xing et al. reported that the amorphous Ni-Fe phosphate film/Ni foam enhances ionic conductivity, stabilizes catalyst performance by uniformly depositing on the Ni foam, and acts as a protective layer, increasing durability and achieving excellent performance with an 87 mV overpotential at −10 mA cm–2 in 1 M KOH [67]. Additionally, Yang et al. [68] showed that the amorphous FePO4 with a low d-band center energy level and low Gibbs free energy exhibits excellent electrocatalytic activity. These studies indicate that both TMPs and phosphates can contribute to HER activity. However, excessive surface phosphates are known to form a passive layer on the catalyst surface, negatively affecting catalytic activity [69], and an optimal ratio of phosphate to phosphide likely plays a crucial role in HER performance. As evident from Figure 5c, the Ni28Co62P10 catalyst, which shows the highest activity, has a phosphide/phosphate ratio close to 1. The Ni19Co73P8 catalyst, which is the only one deviating from the trend, appears to compensate for its low phosphide/phosphate ratio with a significantly high Cdl (Table S1, 38.2 mF cm–2) of the sample.
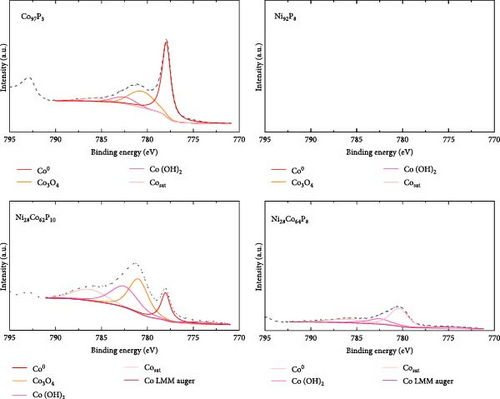
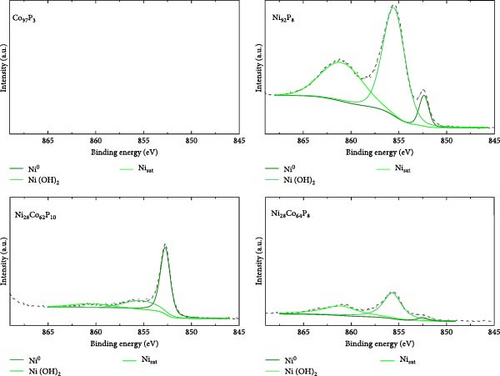
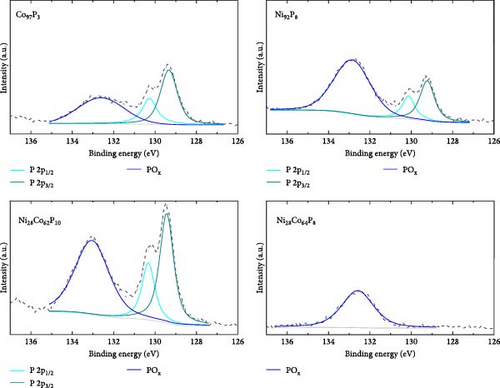
From the XPS analysis of CoP and NiP in Figure 4, which represent binary metal-phosphorus compounds, we can infer the preferentially formed species in each metal. This provides insights into why the formation of binary metal-phosphorus alloys is more beneficial and how these compounds influence catalyst durability. Considering that the oxidation state of metal in phosphate is higher than that in phosphide, the Co 2p3/2 spectrum of Co97P3 in Figure 4 reveals that metallic Co is dominant, with minor contributions from Co3O4 and Co(OH)2. Furthermore, the P 2p spectrum indicates that the P 2p peak of Co phosphide (128–130 eV) is more pronounced than that of Co3(PO4)2 (133–135 eV). In contrast, the Ni 2p3/2 spectrum of Ni92P8 consists of Ni(OH)2, with only a small fraction of metallic Ni. As a result, its P 2p spectrum shows that phosphate is more dominant than phosphide for Ni. These observations suggest that in catalysts synthesized via the co-electrodeposition of Co and phosphorus, the phosphide content is greater than that of phosphate (phosphide/phosphate >1). Conversely, in catalysts formed through the co-electrodeposition of Ni and phosphorus, the phosphide content is lower than that of phosphate (phosphide/phosphate <1). This implies that both catalysts deviate from the optimal composition. Given that phosphide exhibits a more direct correlation with HER activity compared to phosphate, this phenomenon provides a rationale for the superior HER performance of CoP over NiP (Figure 3a). Compared to these monometal-phosphorus compounds, Ni28Co62P10 exhibits a more balanced composition. In Figure 4, its Co 2p3/2 spectrum shows that metal oxides are more prevalent than metallic Co, whereas its Ni 2p3/2 spectrum reveals that metallic Ni is more dominant than Ni(OH)2. These complementary effects result in a nearly 1:1 ratio of phosphate to phosphide, as confirmed in the P 2p spectrum. Consequently, these findings suggest that an optimized combination of metals that preferentially form phosphate and that preferentially form phosphide can facilitate the design of more efficient transition metal-phosphorus compound catalysts.
The relationship between the ratio of phosphide to phosphate and HER activity provides a valuable framework for interpreting the performance degradation observed in the durability assessment of Ni28Co62P10. After 5000 cycles of ADT, EDS analysis confirmed a compositional shift to Ni28Co64P8. As illustrated in Figure 4, the metallic peaks of both Co and Ni decreased, accompanied by an increase in oxidation states. Additionally, the P 2p spectrum revealed the depletion of phosphide, with phosphate emerging as the dominant species. This indicates that the performance decline observed in Figure 3f over 5000 cycles is driven by the loss of phosphide, while the residual activity post-ADT is attributed to metal phosphate. These findings underscore the critical role of stabilizing metal phosphide, rather than relying solely on the inherently more stable metal phosphate, as a more effective strategy for enhancing long-term durability of metal-phosphorus compound HER catalyst.
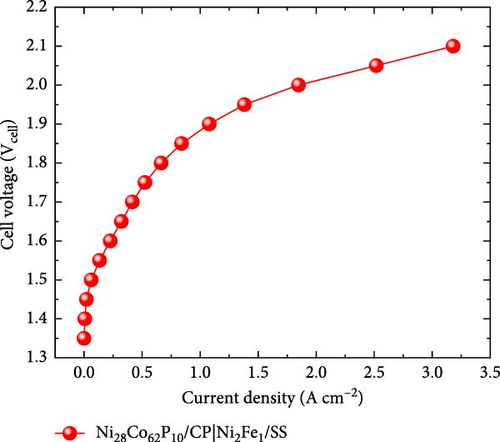
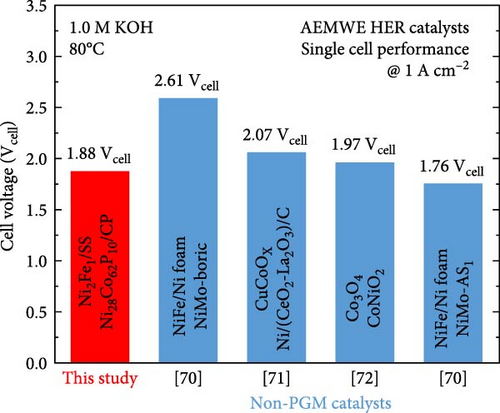
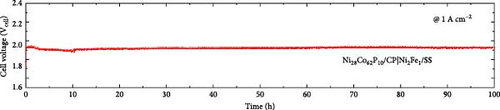
Figure 6a presents the AEMWE single-cell performance using Ni28Co62P10 as the cathode, which exhibited the highest HER activity in half-cell evaluations. The AEMWE system employing Ni28Co62P10 as the cathode achieved a cell voltage of 1.88 V at a current density of 1 A cm–2. Figure 6b compares the performance of the AEMWE system in this study with previously reported non-platinum-based electrodes (for both cathode and anode). The AEMWE single cell using Ni28Co62P10 as the cathode demonstrates performance that is comparable to or superior to existing nonplatinum-based AEMWE systems [70–72]. Figure 6c illustrates the long-term stability of the AEMWE single cell with Ni28Co62P10 as the cathode, operated at a constant current density of 1 A cm–2 for 100 h. The cell voltage exhibited a slight initial improvement during the first 10 h owing to activation, after which it stabilized at an average of 1.92 V. Over the 100 h operation, the degradation rate was measured at 0.21 mV h–1, confirming the outstanding durability of the system.
The exceptional activity and stability of this bimetallic metal-phosphorus catalyst highlight its potential for cost-effective AEMWE commercialization. Furthermore, this study provides critical insights into the respective roles of phosphide and phosphate in alkaline HER performance, offering valuable guidance for optimizing the activity and durability of metal-phosphorus catalysts.
4. Conclusions
This study systematically investigates the enhancement of the alkaline HER activity of transition metal-phosphorus compound catalysts, including Ni100, Co100, Ni66Co34, Ni92P8, Co97P3, and Ni28Co62P10. Based on its surface structure, crystallinity, electrochemical properties, and HER performance, Ni28Co62P10 exhibited the most outstanding HER performance because of the combination of Ni and Co and the addition of phosphorus. Ni28Co62P10 exhibited a low overpotential of 48 mV at −10 mA cm–2 and fast charge transfer kinetics with low Tafel slope of 32 mV dec–1, indicating a highly active surface and excellent electrochemical reactivity. The catalyst achieved a near 1:1 balance between phosphide and phosphate, which contributed to its high HER activity. Hence, maintaining an optimal balance between phosphide and phosphate is crucial for optimizing HER performance. Furthermore, the Ni28Co62P10 catalyst maintained its high performance after more than 5000 cycles of durability testing, which demonstrated the excellent durability of NiCoP catalysts. Finally, the Ni28Co62P10 catalyst showed outstanding performance in an AEMWE single-cell evaluation, achieving a cell voltage of 1.88 V at a current density of 1 A cm−2, which is significantly superior to previous studies. This confirms that NiCoP-based metal-phosphorus is a promising candidate as catalyst for HER in AEMWE. These findings clarify the correlation between the compositional characteristics and electrochemical performance of NiCoP catalysts and are expected to make a significant contribution to the future development of hydrogen evolution technologies.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Taeyoung Kim: conceptualization, formal analysis, investigation, methodology, writing – original draft. Kyeong-Rim Yeo: formal analysis, investigation. Hoyoung Kim: formal analysis, investigation. Jinwoo Lee: conceptualization, methodology, supervision, writing – review and editing. Soo-Kil Kim: conceptualization, funding acquisition, methodology, project administration, supervision, writing – review and editing.
Funding
This research was supported by the Chung-Ang University, Research Scholarship Grants in 2024 and the National Research Foundation of Korea (Grants RS-2023-00235596 and RS-2024-00413272).
Acknowledgments
This research was supported by the Chung-Ang University Research Scholarship Grants in 2024 and the National Research Foundation (NRF) of Korea grants funded by the Korean Government, MSIT (Grants RS-2023-00235596 and RS-2024-00413272).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




