Batch Optimization of Various Variables for Wastewater Treatment and Bioelectricity Generation Using Dual Chambered Microbial Fuel Cell
Abstract
Energy crisis and wastewater treatment are critical global issues. In this study, a novel separator was made by boiling cotton rope with solutions of various salts and their concentrations. It was then employed in a dual-chamber microbial fuel cell (MFC) to treat municipal wastewater collected from 25 Area Wah Cantt, Punjab, Pakistan. Batch scale experiments were carried out to evaluate the effect of various variables, that is, different salts (NaCl, KCl, and MgCl2) and their concentrations (0.2, 0.5, and 1 M) used in separator, wastewater volume (50, 500, and 1000 mL), and aluminum mesh thickness (0.6, 0.8, and 1 mm) on MFC performance in terms of current generation and wastewater treatment for 7 days. Analysis of collected wastewater showed that among the six studied physicochemical parameters, only two, that is, biological oxygen demand (BOD) and chemical oxygen demand (COD) were above the permissible limits of National Environmental Quality Standards (NEQS). Results after MFC experiments showed that separator containing 0.5 M NaCl produced a significantly (p < 0.05) high current of 68.16 µA as compared to the other studied salts and their concentrations, whereas COD and BOD were reduced up to 124.15 and 62.12 mg L−1, respectively. A wastewater volume of 1000 mL generated a significantly (p < 0.05) high current of 83.41 µA compared to the other studied volumes, where COD and BOD residual values were 123.25 and 59.56 mg L−1, respectively. An aluminum mesh thickness of 1 mm produced a significantly (p < 0.05) high current of 103 µA, while 120.89 and 68.93 mg L−1 were achieved COD and BOD values, respectively. It was concluded that MFC performance was enhanced with an increase in wastewater volume and mesh thickness. Therefore, it is recommended that further pilot-scale continuous studies be carried out to implement this research on a larger scale.
1. Introduction
Municipal wastewater is one of the most critical global environmental issues, as it is commonly released directly into the environment without any proper treatment. On a global scale, enormous volumes of municipal wastewater are generated annually, with an average of approximately 360,000 m3/year [1]. The energy demand is also increasing with the increase in population worldwide [2]. Excessive energy demands and environmental contaminants have accelerated the research and development of renewable and sustainable energy sources [3]. Due to the increasing demand for energy per year, it is necessary to find and use new, cost-effective, and renewable energy sources [4].
Among the 17 Sustainable Development Goals (SDGs) set forth by the United Nations, two important goals are for water (clean water and sanitation) and energy (affordable and clean energy). Therefore, to achieve these two main goals of the SDGs, a range of approaches and methods can be used to treat wastewater. Renewable energy source–based wastewater treatment is one of the recently developed techniques to overcome power generation and environmental contamination issues [5]. The microbial fuel cell (MFC) technology is the most important and suitable method for sustainable green energy production, along with wastewater treatment [6]. MFCs show distinct advantages compared to other conventional treatment methods, like direct generation of electricity, low carbon footprint, low sludge generation, and no requirement of an external electric source for aeration [7, 8]. Anaerobic fermentation and bioelectricity production by MFCs using several substrates, such as wastewater, are realistic for both wastewater treatment and energy production [9]. Wastewater is an important substrate in the MFC as it contains a high amount of organic material and is available free of charge [10]. The MFC is a modern technology that yields bioelectricity from the chemical energy found in wastewater. A MFC may also be called a reactor because it is a spontaneous reactor that primarily uses organisms present in the wastewater and degrades organic substances, resulting in the production of energy at that time without the need for energy to conduct the reaction [11]. MFC includes anode chamber that, under anaerobic conditions, operate under the electrochemical system through catalytic microorganism reactions. During the degradation of organic materials (biological substances) present in the wastewater by the electrogenic bacteria existing in the anode chamber, CO2, protons, and electrons are released [12]. The key difference between a single-chamber cell and a dual or double-chamber cell is the proton exchange membrane (PEM) or salt bridge [13]. There are various factors like temperature, pH, ionic strength of the medium, material of the anode or cathode, and membrane that affect the performance of MFCs and their energy production in wastewater treatment [14]. The basic function of the salt bridge is to maintain ion balance between both chambers [15].
A lot of research has been done on MFCs, as it is economical and technically possible for both wastewater treatment and the production of energy [16]. You, Greenman, and Ieropoulos [17] used various domestic wastewaters (bathroom, kitchen sink, dishwasher, laundry washing machine, and urinal) as substrate and found that urine-operated MFC removed chemical oxygen demand (COD) up to 38.9% ± 1.1% and produced a maximum power of 3.91 ± 0.27 mW. Said et al. [18] developed a prototype of MFC for the treatment of sewage water, having carbon and aluminum electrodes that generated a voltage of 1.73 V and a current of 0.07 mA. Khan et al. [19] also carried out batch MFC experiments to remove azo dye along with COD reduction and found 70% and 57% dye and COD removals, respectively. The effect of different catholyte (KMnO4 and K2Cr2O7) solutions on electricity generation showed that the KMnO4 solution had maximum electricity generation due to its higher standard reduction potential. Pugazhendi et al. [20] used an air cathode MFC with a carbon brush as anode with a twisted titanium wire and platinum-coated carbon cloth as cathode for the treatment of seafood industrial wastewater and removed 89% ± 1.4% of COD and 78% ± 1.5% of COD, respectively, and it also produced a power density of 530 ± 2.4 mW/m2. Agüero-Quiñones et al. [21] used MFC with graphite and aluminum electrodes, which produced an electric current of 0.08 mA with 70% COD removal from wastewater. Singh and Dharmendra [15] used dual-chambered MFC with graphite rod as anode and cathode, where the salt bridge was made of various salts (NaCl and KCl) and a variation in agar amount. It was concluded that a salt bridge having 3 M NaCl along with 12% agar removed 64.58% COD and produced a voltage of 337.25 mV. Literature shows that in most research, agar-salt bridges were used [22–24]. Traditional two-chamber MFC configurations that require a PEM can result in low internal resistance [25]. Reducing internal resistance is effective in increasing the current generation of MFCs. Despite the large amount of available work on MFC in the literature, there is still a need to develop a cost-effective MFC for municipal wastewater treatment and energy generation, as less literature is available on municipal wastewater treatment using economical MFC.
In the current study, a dual-chamber MFC was made using economical household materials. Various PEMs like Nafion, Flemion, and Hyflon that have already been used by many researchers are very costly [26, 27]. Therefore, in the current study, a novel separator was made by boiling cotton rope with solutions of various salts and their concentrations (details given in the methodology Section 2.3), while electrodes were made by using copper wire and aluminum mesh, as already used commercially available electrodes like platinum, titanium, etc. are very costly and corrosive [28]. Aluminum mesh has the properties of being nonreactive and noncorrosive [26] and can be used as an economical alternative to expensive electrodes. The effects of various variables like salts and their concentrations in separator, wastewater volume, and aluminum mesh thickness of electrodes were also analyzed in the current research on wastewater treatment and electricity generation in MFC. Up to the best of our knowledge, no study has been conducted to date using separator made from cotton rope boiled with solutions of various salts and their concentrations, and no research data is available to evaluate or optimize the effect of different variables like various salts and their concentration in separator, wastewater volume, and aluminum mesh thickness of electrode on municipal wastewater treatment and electricity generation using economical dual chamber MFC.
2. Materials and Methods
2.1. Sample Collection
A municipal wastewater sample (substrate) of about 8000 mL, which served as the substrate or feedstock for MFC, was collected from a sample site named 25 Area in the middle of the day in Wah Cantt, Punjab, Pakistan. The municipal wastewater sample was collected from the particular sample site used in the study as there is no wastewater treatment facility in the said area, and contaminated wastewater is affecting the environment as it enters the environment without any treatment. Therefore, this site was selected for the current study to address this issue.
The desired amount of substrate was separated in a plastic chamber at the time of each batch experiment. Different (six) physicochemical parameters like pH, temperature, total dissolved solids (TDS), biological oxygen demand (BOD), COD, and electrical conductivity (EC) of the wastewater were analyzed at the time of sample collection and after each MFC treatment of 7 days through batch experiments.
2.2. Physicochemical Analysis
pH and EC were measured by using a pH meter (ATC pH meter) and an EC meter (HANNA HI99300) by simply dipping the abovementioned meters in a water sample before and after each MFC experiment. TDS and temperature were determined by dipping the ATLAS TDS-3 m in a water sample before and after each MFC experiment. The colorimetric method (5220 D) was used to measure the COD [29], while the BOD was measured through the 5 days BOD (5210B standard method) method [29].
2.3. Novel Separator Formation and MFC Setup
In the current study, a dual-chamber MFC was made using economical household materials. First of all, an economical and novel separator (5 cm length) was made by boiling cotton rope with solutions of various salts and their concentrations. Different salts (KCl, NaCl, and MgCl2) and their solutions of various concentrations like 0.2, 0.5, and 1 M were used and boiled (at 100°C) with cotton rope separately until its absorbance capacity was finished (about 4–5 min) and then left in an open space until it got dried. Then a layer of tape was carefully wrapped around the dried cotton rope and fitted into a congested polypropylene random (PPR) copolymer pipe to improve the separator based on cotton rope in terms of sealing and durability. This additional security measure strengthened the system’s overall leak-proofing by adding another line of defense against potential leaks. The system was designed to prevent leakage between the anodic and cathodic chambers by firmly fitting the cotton rope into the PPR pipe and using epoxy hardener and resin (meridian adhesive group of company) to seal any micro-gaps and gap between the rope and the pipe’s inner surface. This resin served as a supplementary barrier against seepage.
Two plastic chambers were taken, and then a hole was made in these two chambers in which the prepared novel separator was placed to combine them. Epoxy hardener and resin made of meridian adhesive group of company was used to seal any remaining openings in the holes of both chambers and to prevent any leakage. In the first chamber (anode chamber), wastewater was taken (Figure 1) and aluminum mesh along with the copper wire was dipped in it as an anode, while in the second chamber (cathode chamber), fresh water (equal volume to wastewater) was taken, and aluminum mesh along with a copper wire for charge deposition was used. An aquarium pump was used to provide oxygen in the freshwater chamber [30].
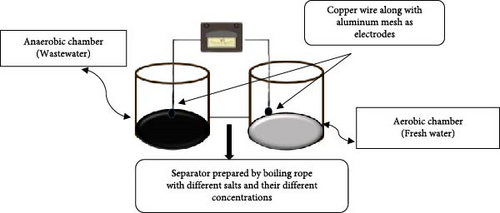
2.4. Variables
- •
Salts and their molar concentrations.
- •
Wastewater volume.
- •
Aluminum mesh thickness.
| S. No | Variables | Name/range | Constants |
|---|---|---|---|
| 1. | Salts/concentrations | NaCl, KCl, MgCl2/0.2, 0.5, 1 M | 500 mL wastewater, 0.6 mm aluminum mesh thickness |
| 2. | Volumes | 50, 500, 1000 mL | 0.5 M NaCl, 0.6 mm aluminum mesh thickness |
| 3. | Aluminum mesh thickness | 0.6, 0.8, 1 mm | 0.5 M NaCl, 1000 mL wastewater volume |
2.5. Batch Experiments of Different Variables
2.5.1. Effect of Salts and Their Concentration
To find out the effect of salts and their concentrations (in rope separator) on wastewater treatment and current generation for 7 days in this experiment, three salts (NaCl, KCl, and MgCl2) were used, and for each salt solution, three different concentrations, that is, 0.2, 0.5, and 1 M, were varied. While the volume of wastewater and mesh thickness were kept constant at 500 mL and 0.6 mm, respectively. The salt and the concentration giving the best results were then used in all other batch experiments.
2.5.2. Effect of Volume
To find out the effect of wastewater volume on wastewater treatment and current generation, the volume of wastewater was varied from 50, 500, and 1000 mL. The best salt, along with its optimum concentration, was selected from the first experiment as a constant value during this experiment. The mesh thickness of the electrode was constant at 0.6 mm.
2.5.3. Effect of Mesh Thickness
In this experiment, the aluminum mesh thickness was varied (0.6, 0.8, and 1 mm), while the best salt, its concentration, and volume were selected from the first and second batch MFC experiments as constant values.
All experiments with different variables were carried out in triplicate for seven consecutive days.
2.6. Measurements
The output of the MFC was expressed using current (μA). The voltmeter (TITAN DT-830D) was used for this purpose and was calibrated each time before use. Voltmeter readings were noted only after a steady and constant value was obtained, which took 3–4 h to achieve. Lag, log, exponential, and stationary phases were also measured through hourly base readings that expressed the growing and declining phases of current output in the case of the first variable, as the output power of a typical MFC is constantly changing depending on microbial activities and environmental conditions [31]. The electrical output of most MFCs follows the bacterial growth cycle [31]. Other readings for current and wastewater parameters after each variable in MFC batch experiments were noted after 24 h regular intervals for the next 7 days. MFC experiments were conducted for 7 days to evaluate the system’s long-term stability, long-term performance, and reaction to extended operating circumstances. This length offers insights into the MFC system’s capacity to sustain production trends over time, allowing for a comprehensive assessment of its sustainability and practical applicability.
The length of the wire was 1 m and the area of the wire was 2.5 m, so 1.2 Ω resistance was calculated and kept constant in all experiments. Physicochemical parameters of water were also measured after each experiment to check the experiment’s efficiency, including how well it treated the wastewater and how efficiently it generated electricity.
2.7. Statistical Analysis
One-way ANOVA was used to find out the statistical difference among the current production in 7 days by different variables, and two-way ANOVA was used to find out salt and concentration interactions and to determine the effect of continuous outcome variables on physicochemical parameters by using IBM SPSS Statistics 25. To determine the mean, standard deviation, etc., all the study data were statistically analyzed using SPSS and Microsoft Excel 2016. To generate graphs and charts, Microsoft Excel 2016 was used.
3. Results and Discussion
3.1. Physicochemical Analysis of Collected Municipal Wastewater Before Batch MFC Experiments
Six physicochemical parameters, that is, pH, temperature, TDS, BOD, COD, and EC, of the collected wastewater were analyzed before MFC experiments (Table 2), that is, at the time of collection. Results in Table 2 showed that the pH, EC, TDS, and temperature of the collected wastewater lie within permissible limits, while BOD and COD were above the permissible limits of National Environmental Quality Standards (NEQS). This is because the higher organic compounds are present in the wastewater that increased the BOD and COD values as residual food waste from bottles, cans, and emulsified oil increased in their concentrations along with natural plant degradation in wastewater [32].
| Sr. No | Parameters | Results | NEQS for municipal wastewater safe disposal standards (1999) |
|---|---|---|---|
| 1. | pH | 7.7 | 6–10 |
| 2. | EC | 1063 μS cm−1 | 2000 μS cm−1 |
| 3. | TDS | 510 mg L−1 | 3500 mg L−1 |
| 4. | Temperature | 18.5°C | 40°C |
| 5. | COD | 191 mg L−1 | 150 mg L−1 |
| 6. | BOD | 90 mg L−1 | 80 mg L−1 |
- Abbreviations: BOD, biological oxygen demand; COD, chemical oxygen demand; EC, electrical conductivity; NEQS, National Environmental Quality Standards; TDS, total dissolved solid.
BOD and COD values, as shown in Table 2, are higher than the permissible limits of NEQS, but are very low as generally observed in municipal wastewater, which may be because of the settling of organic particles from municipal wastewater in an open canal, due to stagnation or sluggish flow conditions as wastewater was flowing slowly at the time of sample collection in the canal, or it may be due to the dilution of collected wastewater from any unidentified pollution source in the canal before collection.
3.2. Effect of Various Variables on Electricity Generation and Wastewater Treatment in MFC
3.2.1. Effect of Salts and Their Solution Concentrations on Electricity Generation
Salt type and concentration play an important role in current production because the presence of salt in the PEM helps in the movement of ions from the anode to the cathode side. To find out the effect of various salts and their concentrations (in rope separator) on wastewater treatment and current generation, in this experiment, three salts (NaCl, KCl, and MgCl2) were used, and for each salt, the solution concentration was varied as 0.2, 0.5, and 1 M. While the volume of wastewater and mesh thickness were kept constant at 500 mL and 0.6 mm, respectively. The output was measured through a voltmeter in voltage (mV), and a resistance of 1.2 Ω was applied and kept constant in all the experiments.
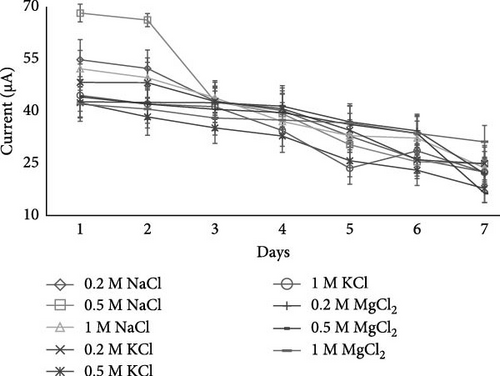
Results in Figure 2 showed that in case of 0.2 M NaCl salt in the separator of the dual-chambered MFC on day 1, the current was 54.75 µA, which was significantly higher (p < 0.05) than the current generated on the last (7th) day, which was 22.5 µA. The same results were observed with the increase in concentration of NaCl salt at 0.5 and 1 M in separator, as their first readings (day 1) were significantly (p < 0.05) higher, that is, 68.16 and 52.16 µA, than their last readings (day 7), that is, 24.91 and 32.25 µA, respectively.
Organic substrates undergo microbial oxidation at the anode to produce an electric current [33]. The microbial activity of the substrate in the anode chamber and the electrolyte in the cathode chamber determines the reduction in current, voltage, COD, and power production. The voltage generated in an MFC increases with the levels of microbiological activity in the anode chamber and electrolyte liberation activity in the cathode chamber [34].
With time, the current generation began to decrease due to nutrient depletion. After a few days, a current drop was observed which might be due to the death of microorganisms in the substrate [35]. Rahmani et al. [36] also confirmed the reduction in current and voltage which may be linked with the death of microorganisms in a substrate (wastewater).
The literature reveals that increasing the salt concentration in the salt bridge solution significantly impacts the voltage output. In addition to reducing activation loss, increasing the concentration of salt facilitated the transfer of more protons from the anode to the cathodic chamber [24]. In research carried out by Sevda and Sreekrishnan [24], various salt solutions (5% NaCl, 1 M NaCl, and saturated potassium) were utilized in salt bridges, and it was observed that larger voltages and currents are correlated with higher salt concentrations.
However, in the current study, 0.5 M NaCl produced a significantly (p < 0.05) higher current (68.16 µA) as compared to the other salts and their concentrations, as shown in Figure 2, because when there is less concentration of salt, there is less movement of ions. On the other side, excessive salt concentration is also not good for maximum output, as excessive salt concentration may produce internal resistance in separator that will decrease the movement of the ions from the anode to the cathode through separator. Our results showed that a moderate amount of salt concentration is best for electricity generation, whereas an excessive amount of salt concentration may produce internal resistance in the separator or salt bridge. 1 M of NaCl salt produced a current of 52.16 µA, and it is because of the larger amount of salt that internal resistance in the salt bridge (separator) could be produced, which led to less movement of ions to the cathode side. While comparing the current results, it was noticed that NaCl produced significantly (p < 0.05) more current than KCl and MgCl2, as given in Figure 2. This may be attributed to differences in ionic radii and hydration energies. The ionic radius of Na+ is approximately 102 pm, while Mg2+ is around 72 pm, making Na+ larger. Additionally, Na+ has a lower hydration energy than Mg2+, which influences its mobility and conductivity in solution, resulting in a higher current generation. Singh and Dharmendra [15] also used various salts along with different percentages of agar in a salt bridge having graphite rods as electrodes and revealed that the NaCl salt bridge showed better results than the KCl salt bridge, which had an optimum agar of 12% and produced a maximum voltage of 319 mV. Another study found that the semisolid agar salt bridge (112.2 µA) did not work as well as the liquid-phase saturated NaCl solution as a salt bridge (185.5 µA) because the agar salt bridge was less conductive and could not exchange ions as well [37]. In research carried out by Muralidharan et al. [38], a current of 256 µA was produced by using 1 M NaCl in a salt bridge having carbon electrodes, where the current was reduced up to 62 µA when the molar solution of NaCl was increased up to 9 M.
3.2.1.1. Biological Cycles
Unlike most electrical and chemical energy systems, which have a constant output power due to stable physical or chemical reactions, the output power of MFC is determined by the dynamic microbial activity in the system [39]. Therefore, the output power of a typical MFC is constantly changing depending on microbial activities and environmental conditions [31]. The microbes in the wastewater samples are native ones, but inside MFC, their environmental conditions differ from those in natural wastewater.
The growth cycle of a bacterial culture generally has four distinct phases: the lag phase, in which bacteria are metabolically active but not dividing; the exponential phase, in which growth is exponential; the stationary phase, in which growth reaches a plateau as the number of dying cells equals the number of dividing cells; and the death phase, characterized by an exponential decline in the number of living cells [40, 41]. The electrical output of most MFCs follows the bacterial growth cycle [31].
So, in this study, biological cycle means lag (initial phase), log (exponential phase), stationary, and decline phases, which were obtained by using current measurements for the next 24 h. As in the case of NaCl, 0.5 M concentration produced maximum current; so in the next 24 h, current production in the case of NaCl salt bridge (separator) with 0.5 M concentration was observed, and the current production curve with NaCl salt showed that NaCl salt took 9 h to yield maximum current production that was 116 µA (Figure 3) and then after 9 h it started to decline, and the last current reading was 66 µA after 24 h. At the start, there was no movement of ions, so after some time, when microbes started decomposition of the organic or inorganic substrate, energy was generated, and when microbes started mobility, it tended to increase the current output.
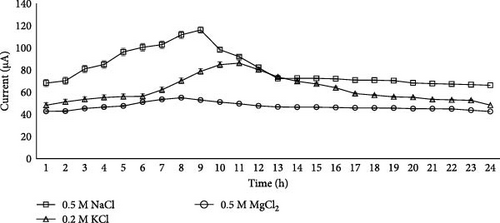
In the case of KCl, a 0.2 M concentration produced maximum current, so in the next 24 h, current production of KCl salt separator having 0.2 M concentration was observed, and the current production curve with KCl salt showed that KCl salt took 11 h to yield maximum current production, and then after 11 h, it started to decline, and the last current reading was 48 µA after 24 h. In the case of MgCl2, separator having a 0.5 M concentration produced maximum current, so in the next 24 h, current production of MgCl2 having 0.5 M concentration was observed, and the current production curve with MgCl2 salt showed that MgCl2 salt took 8 h to yield maximum current production, and then after 8 h it started to decline, but MgCl2 produced steady current as compared to the KCl, and the last current reading was 42.5 µA after 24 h.
3.2.2. Physicochemical Parameters Analysis
After MFC treatment in the case of salts and their concentrations, different physicochemical parameters of water were analyzed.
3.2.2.1. pH
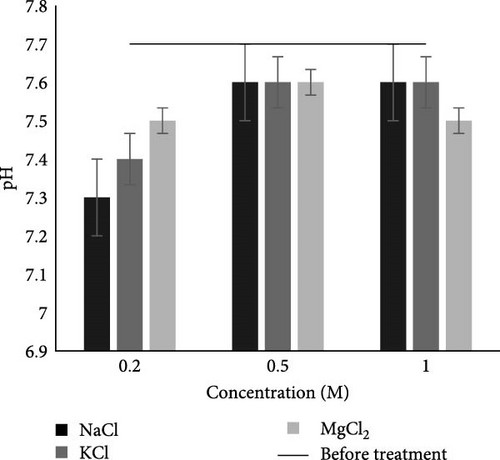
Before the MFC experiment, the pH of the collected wastewater was 7.7, as shown in Table 2, and that was within the permissible limits of NEQS. After the salts and their concentration (separator) MFC experiment as shown in Figure 4A, the pH of all salts and their concentrations lies within range of 7.3–7.6. Results showed that after MFC salt and its concentrations variable experiments, the pH of water slightly decreased in the case of all salts and their concentrations. While in all salts and their concentrations in separator, pH was within the permissible limit of NEQS after the experiment. A similar result in the slight drop of pH from 7.5 to 7.3 was observed in municipal wastewater after MFC treatment by Parkash [42]. Another study by Gil et al. [43] also found that neutral pH 7 led to higher current production. Neutral pH is regarded as beneficial for biofilm growth on the surface of the anode electrode [44]. The optimal metabolic activity of microorganisms not only requires a low concentration of ions but also a pH close to neutral [9]. According to Puig et al. [45], higher pH results in a negative effect on bacteria in the anodic chamber and also affects electricity generation.
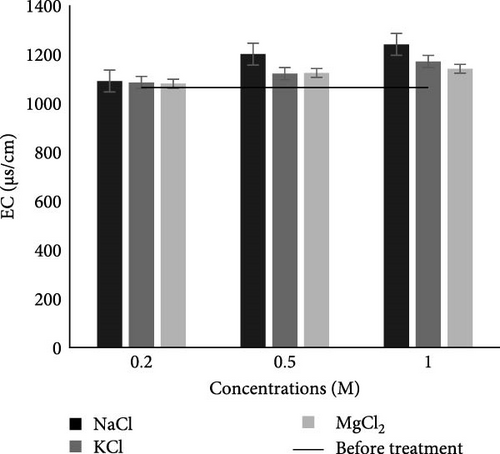
3.2.2.2. EC
The EC of collected municipal wastewater before the MFC experiment was 1063 μS/cm (Table 2), which was within the permissible limit of NEQS. However, after the MFC experiment, as shown in Figure 4B, in the case of all studied salts and their concentrations the EC lies with in range of 1090–1240 μS/cm. It is also noticed that in the case of all three salts at 1 M concentration, NaCl showed significantly (p < 0.05) higher EC as compared to the other studied salts. All EC values were also within the permissible limits of NEQs after the MFC experiment. The significant increase in EC following the MFC treatment can be linked to the breakdown of complex organic compounds, which produced smaller charged molecules and may have raised the conductivity of the solution [46]. According to Singh and Dharmendra [15], the increase in EC after MFC treatment is because of free electrons present in the anodic chamber. Similar results were observed by Singh and Dharmendra [15], where the EC value of domestic wastewater increased from 1038 to 4200 µS when treated through MFC with a salt bridge made from salt and 12% agar.
In this research, we have used only salts as an electrolyte in separator rope, which may be leaching from it, and this leakage can be reduced by using some inert material like agarose (inert material) with salt to control the leaching of salts if such a setup is scaled up. However, through literature, it is also found that generally, raw domestic and industrial wastewaters lack the necessary ionic conductivity to support high energy production in MFCs. As a result, they often require the addition of inorganic salts, such as sodium chloride and phosphate buffer salts, to maintain an adequate solution’s ionic strength [47, 48]. According to Aghababaie et al. [14] increased salinity and ionic strength can improve MFC performance by increasing the substrate’s conductivity. Since higher conductivity has been associated with higher power outputs, it is crucial to achieve and sustain higher conductivity levels in the MFC reactors [48, 49].
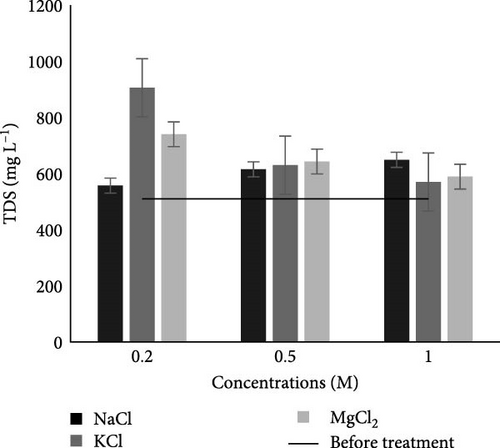
3.2.2.3. TDS
Before the experiment, the TDS of the collected wastewater was 510 mg L−1 (Table 2), which was within the permissible limit of NEQS. Results in Figure 4C showed that after the MFC experiment in the case of various studied salts and their concentrations TDS values in water were within the permissible limit of NEQS.
The TDS value increased (Figure 4C) in all studied salts and their concentrations, which is due to the increase in the number of microorganisms after MFC treatment [42]. An increase in the number of microorganisms will result in an increase in the degradation of pollutants in the wastewater, which ultimately increases TDS in the water. Although the salts were not given to the anodic chamber directly, their presence in the cotton rope treated with salts indirectly affects the MFCs’ degrading processes by facilitating electron and ion transfer, both of which are necessary for microbial activity and current production. Another reason for the increase in TDS values with increase in concentration in the case of KCl and MgCl2 could be the leakage of these salts from separator which could be controlled by using some agarose material in separator along with salts. However, through literature, it is also found that generally, raw domestic and industrial wastewaters lack the necessary ionic conductivity to support high energy production in MFCs. As a result, they often require the addition of inorganic salts [47, 48], therefore, increase in TDS within permissible limits of NEQS has no disadvantages, but it is beneficial for MFC.
Our result of TDS is similar to research carried out by Parkash [42], in which TDS was increased after 20 days of MFC experiment in a dairy, agro, distillery, and municipal wastewater from 86,000, 80,000, 606,000, and 1400 mg L−1 to 112,000, 120,000, 718,000, and 324,000 mg L−1, respectively. TDS was increased from 1010 ± 45.1 to 1350 ± 17.3 mg L−1 from piggery wastewater using dual-chambered MFC [50].
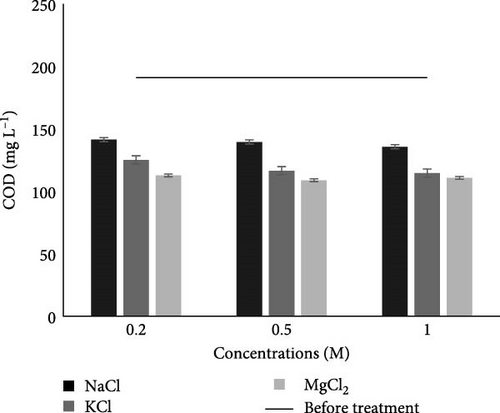
3.2.2.4. COD
Before the experiment (Table 2), COD (191 mg L−1) was not within the permissible limit of NEQS, but after the MFC experiment, all readings for all salts and their concentrations showed COD values within the permissible limit of NEQS. From the results (Figure 4D), it is clear that in the case of all three salts, the 1 M concentration of KCl showed a significantly (p < 0.05) lower COD value, which might be due to the more degradation of soluble organic compounds by microbes. Sivakumar [51] also noticed a decrease in the COD value in dairy industry wastewater from 4321.4 ± 95 to 591.9 ± 6 mg L−1 after treatment with MFC having a salt bridge made of 1 M NaCl concentration and 10% agar concentration. In another study, similar results were observed after the MFC experiment, where the COD value of wastewater was reduced from 416 to 124 mg L−1 [21]. Our results are consistent with those of Al-Saned, Kitafa, and Badday [52] and Ge et al. [25]. Based on the data, the COD values appear to be reduced most by 1 M concentrations of NaCl, KCl, and MgCl2, and of these, 1 M KCl showed the biggest percentage reduction of about 42.93%. The increased microbial activity has led to a larger reduction in COD and more effective organic matter breakdown.
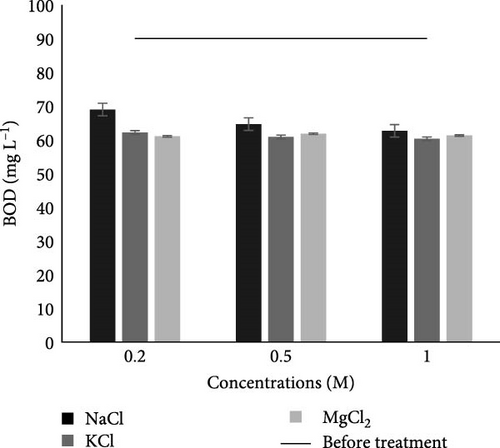
3.2.2.5. BOD
BOD generally reflects how much oxygen is needed to break down the organic matter in water. Before the MFC experiment, the BOD in municipal wastewater was 90 mg L−1 which was more than the permissible limit of NEQS as given in Table 2. However, BOD after the experiment, in case of studied salts and their concentration lies with in range of 68.93–60.81 mg L−1, which is within the permissible limits of NEQS as shown in Figure 4E. A similar result was observed by Sivakumar [51] in research where BOD was reduced from 2850.5 ± 56 to 333.4 ± 8 mg L−1 from dairy industry wastewater after treatment with MFC having a salt bridge made of 1 M NaCl concentration and 10% agar concentration. In another study, BOD was also reduced from 5430 to 1356 mg L−1 after 6 h of MFC treatment of dairy wastewater using a Zn electrode [53]. Our results are consistent with those of Ihenacho et al. [50], Obasi and Onukwuli [54], Tale et al. [55], Aswin, Begum, and Sikkandar [56], and Ge et al. [25].
3.2.2.6. Temperature
The temperature of MFC-treated wastewater was same that was 15°C after the MFC experiment, as shown in Table 1, and at the initial, it was 18.5°C. Experiments were carried out at 25°C, and there will be no external rise in temperature. The temperature was within the permissible limit of NEQS after the MFC experiment.
3.3. Effect of Wastewater Volume on Electricity Generation in MFC
To find out the effect of wastewater volume on wastewater treatment and current generation, the volume of wastewater was varied from 50, 500, and 1000 mL. The best salt and best concentration (i.e., NaCl and 0.5 M) were selected from the first batch experiment as they produced the maximum current. The aluminum mesh thickness of the electrode was kept constant at 0.6 mm. Results in Figure 5 showed that a 1000 mL volume of wastewater produced more current as compared to other studied volumes (500 and 50 mL) on both days 1 and 7. This might be due to various MFC configurations [57, 58], like salt bridge and aluminum mesh. More current generation in the case of a 1000 mL wastewater volume (substrate volume) shows more degradation of organic materials in the anodic chamber of a 1000 mL volume, which results in more free electrons in the anode [15].
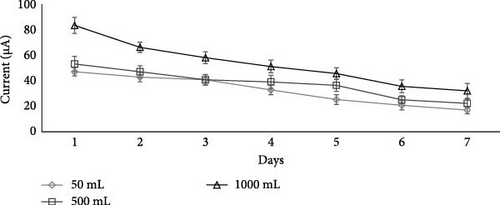
Whereas in the case of 50 mL of wastewater, the current output (Figure 5) was significantly (p < 0.05) high, that is, 47.08 µA on day 1 as compared to day 7 (17 µA). One thousand milliliter of wastewater produced more current (Figure 5) as compared to the others and was then chosen as the volume of wastewater for the next batch of MFC experiments.
3.3.1. Effect of Wastewater Volume on Various Physicochemical Parameters of Wastewater in MFC
3.3.1.1. pH
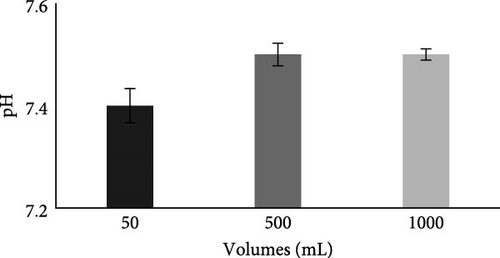
The pH of the collected wastewater was 7.7, as shown in Table 2, and that was within the permissible limits of NEQS. Results in Figure 6A show that after the MFC batch experiment, the pH of all studied water volumes (50−1000 mL) lies within range of 7.4 ± 0.1–7.5 ± 0.1, which is within permissible limit of NEQS.

3.3.1.2. EC
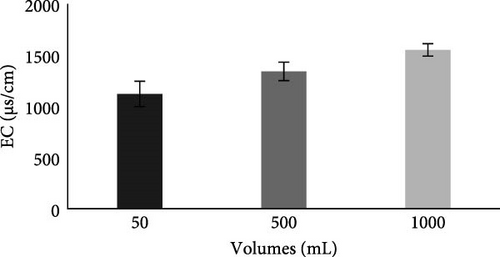
Before the MFC experiment, the EC of collected municipal wastewater was 1063 μS/cm, which was within the permissible limit of NEQS as given in Table 2, and after the experiment (Figure 6B), the EC of all studied wastewater volumes lies within the permissible limit of NEQS. No work in the literature is available to evaluate the effect of various substrate volumes on current generation and water parameters in MFC; only the effect of the concentration of substrate on MFC is present in the literature. The significant increase in EC following the MFC treatment can be linked to the breakdown of complex organic compounds, which produced smaller charged molecules and may have raised the conductivity of the solution [46]. The more current generation in the case of a 1000 mL wastewater volume also shows more degradation of organic materials in the anodic chamber of a 1000 mL volume, which results in more EC in the water.
Our results are consistent with Singh and Dharmendra [15], where the EC value of domestic wastewater was increased from 1038 to 4200 µS when treated through MFC. This leads to an increase in TDS levels and a corresponding increase in EC values.
3.3.1.3. TDS
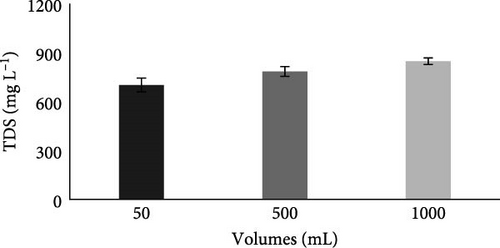
Before the experiment, results (Table 2) showed that the TDS of the collected wastewater was 510 mg L−1, which was within the permissible limit of NEQS.
Results in Figure 6C show that after the MFC experiment, the TDS value increased in all water volumes but these values were also within the permissible limit of the NEQS standard, as its permissible limit is 3500 mg L−1. The increase in TDS after MFc experiment might be due to the increase in the number of microorganisms after MFC treatment [42]. An increase in the number of microorganisms will result in an increase in the degradation of pollutants in the wastewater, which ultimately increases TDS in the water
3.3.1.4. COD
Before the MFC experiment, COD was 191 mg L−1 which was above the permissible limit of NEQS as given in Table 2. Results in Figure 6D showed that after the volume variable MFC batch experiment, all COD readings (Figure 6D) lie within in range of 123−129 mg L−1 and are within the permissible limit of NEQS. Our result is similar to that of Al-Saned, Kitafa, and Badday [52], they used MFC to treat dairy wastewater and reduced COD from 2610 to 210 mg L−1, where the maximum current was found to be 28 µA. In another study, COD was reduced from 7380 to 1654 mg L−1 after 6 h of MFC treatment of dairy wastewater using a Zn electrode [53]. Obasi and Onukwuli [54] also observed a reduction in COD from 2052 to 134 mg L−1 in poultry wastewater using MFC. In another research, COD was reduced from 5311.67 ± 313.2 to 4699.7 ± 57.5 mg L−1 from piggery wastewater using dual-chambered MFC [50]. Our results are consistent with those of Wang et al. [59], Agüero-Quiñones et al. [21], Sivakumar [51], Tale et al. [55], Aswin, Begum, and Sikkandar [56], and Ge et al. [25].
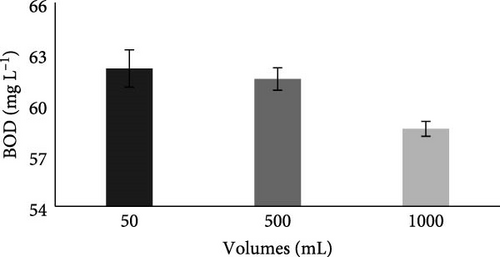
3.3.1.5. BOD
Carbonaceous material, or BOD, in wastewater is used as a food source by bacteria present in the anode chamber, which converts it to carbon dioxide, and as a result, BOD will decrease [9]. Results in Table 2 showed that initially, before the MFC experiment, the BOD of collected municipal wastewater was 90 mg L−1, which was above the permissible limit of NEQS. It was noticed (Figure 6E) that after the experiment, all BOD readings were within the permissible limit of NEQS as compared to the collected wastewater. A similar result was observed by Ihenacho et al. [50], where BOD was reduced from 1705.33 ± 255.8 to 1383.3 ± 76.4 mg L−1 from piggery wastewater using dual-chambered MFC. In another study, BOD was also reduced from 493 to 134 mg L−1 in poultry wastewater using MFC [54]. Our results are consistent with those of Sivakumar [51], Sahana and Manjunath [53], Tale et al. [55], Aswin, Begum, and Sikkandar [56], and Ge et al. [25].
3.3.1.6. Temperature
The temperature of water was the same in all volumes, which was 15°C after the MFC experiment, and initially it was 18.5°C, that is, before and after the experiment, it was within the permissible limits of NEQS. The temperature was not too much affected by the MFC experiment.
3.4. Effect of Aluminum Mesh Thickness on Electricity Generation in MFC
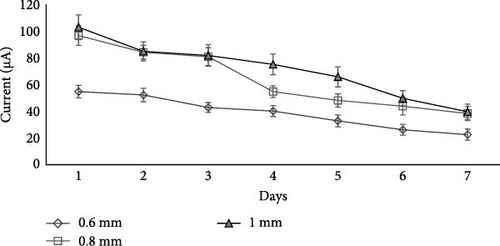
In this experiment, the aluminum mesh thickness was varied as 0.6, 0.8, and 1 mm, having a surface area of 8, 9, and 10 cm2, while the best salt (NaCl), its concentration (0.5 M), and volume (1000 mL) were selected from the first and second experiments as a constant value to check out the aluminum mesh thickness effect on the wastewater treatment and electricity production in 7 days.
Results (Figure 7) showed that aluminum mesh thickness affects wastewater treatment and electricity production as 0.8 and 1 mm mesh thickness produced significantly (p < 0.05) more current, that is, 96.75 and 103 µA, respectively, at day 1 and 38.33 and 39.66 µA, respectively, at day 7 as compared to the 0.6 mm aluminum mesh thickness. It is because by increasing the thickness of aluminum mesh, there is an increase in the surface area of the cathode and anode [60]. Ahn and Logan [60] compared the current generation in an MFC experiment using carbon felt anodes having three different thicknesses, that is, 0.32, 0.64, and 1.27 cm, and noted that the thickest anode of 1.27 cm resulted in the highest current of 0.46 mA compared to 0.64 cm (0.39 mA) and 0.32 cm (0.32 mA). Similar results were observed by Logan et al. [61] and Hays, Zhang, and Logan [62].
3.4.1. Effect of Mesh Thickness on Various Physicochemical Parameters of Wastewater in MFC
3.4.1.1. pH
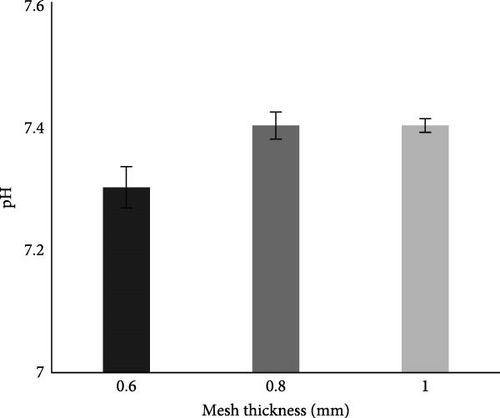
The pH of the collected wastewater was 7.7, as shown in Table 2, and that was within the permissible limits of NEQS. Results after the MFC batch experiment (Table 2 and Figure 8A) show that the pH of the water in all studied mesh thickness lies with in range of 7.3–7.4 which is within permissible limits of NEQS.
3.4.1.2. EC
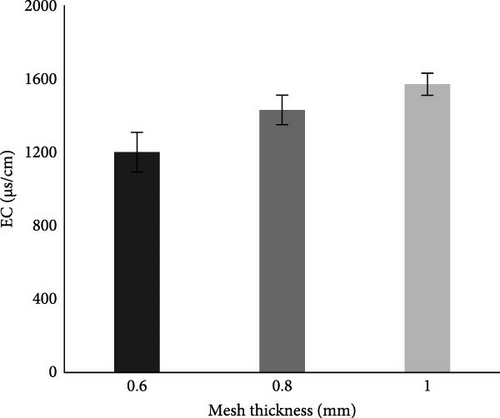
Before the MFC experiment, the EC of the collected municipal wastewater was 1063 μS/cm, which was within the permissible limit of NEQS as given in Table 2. Whereas after the batch experiment (Figure 8B), it was noticed that in all aluminum mesh thicknesses, 1 mm showed significantly (p < 0.05) higher EC. This is because the MFC system experienced an increase in microbial activity and pollutant breakdown as a bigger mesh may provide more surface area for the colonization of microorganisms which could lead to higher microbial activity and pollutant breakdown [60]. It was also noticed that after the MFC experiment in the case of all aluminum mesh thickness, the EC of water was within permissible limits of NEQS.
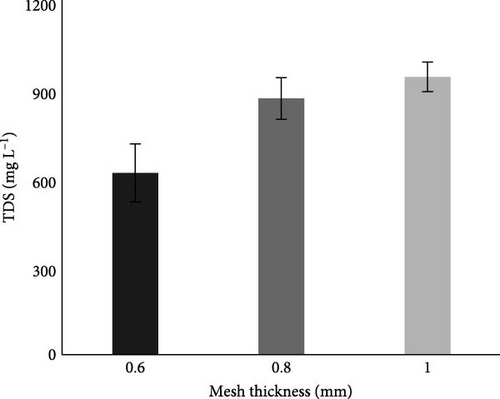
3.4.1.3. TDS
Before the experiment, Table 2 showed that the TDS of the collected wastewater was 510 mg L−1, which was within the permissible limit of NEQS. Whereas after the MFC experiment, TDS of wastewater was increased with increase in mesh thickness, but it was observed (Figure 8C) that TDS values were also within the permissible limit of NEQS, as its permissible limit is 3500 mg L−1 (Table 2).
3.4.1.4. COD
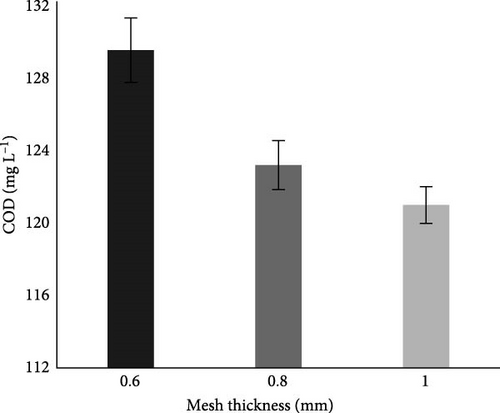
Before the MFC experiment, the COD of wastewater was 191 mg L−1, which was more than the permissible limit of NEQS as given in Table 2. But after the MFC experiment in the case of all aluminum mesh thickness (Figure 8D), the COD of the wastewater was within the permissible limit of NEQS. Another study carried out by Tale et al. [55] showed a similar result where COD was reduced from 328 to 192 mg L−1 in wastewater through the MFC experiment. Aswin, Begum, and Sikkandar [56] noted that the COD of leather industry wastewater was reduced from 2754 to 882 mg L−1 after the MFC experiment, while in the case of dairy wastewater, it was decreased from 1711 to 388 mg L−1. Ge et al. [25] also examined the working of MFC in series for 100 mL municipal wastewater treatment and observed a decrease in COD from 156 ± 42 to 33 ± 15 mg L−1. Our results are consistent with those of Wang et al. [59], Agüero-Quiñones et al. [21], Al-Saned, Kitafa, and Badday [52], Sahana and Manjunath [53], Obasi and Onukwuli [54], and Sivakumar [51].
3.4.1.5. BOD
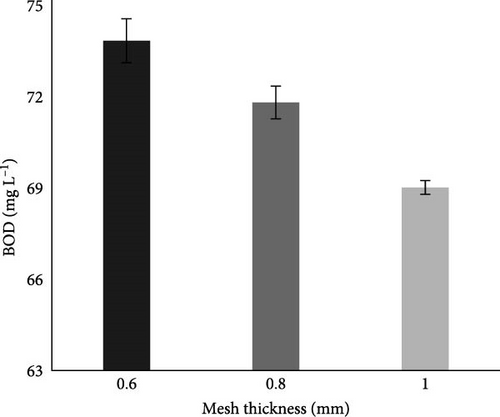
Results in Table 2 showed that initially, before the MFC experiment, the BOD of the collected municipal wastewater was 90 mg L−1, which was above the permissible limit of NEQS. Whereas after the MFC experiment in case of aluminum mesh thickess variable, as shown in Figure 8E, the BOD of the water was within the permissible limit.
3.4.1.6. Temperature
The temperature of the collected municipal wastewater was the same in all aluminum mesh thicknesses, which was 15°C after the MFC experiment and at an initial temperature was 18.5°C. The temperature was within the permissible limit of NEQS both before and after the MFC experiment. Another study carried out by Tale et al. [55] showed that BOD was reduced from 180 to 15 mg L−1 in wastewater through the MFC experiment. Similar results were noted by Aswin, Begum, and Sikkandar [56], where the BOD of leather industry wastewater was reduced from 920 to 340 mg L−1 after the MFC experiment, while in the case of dairy wastewater, it was decreased from 562 to 226 mg L−1. In another study, Ge et al. [25] examined the working of MFC in series for 100 mL municipal wastewater treatment and observed a decrease in BOD from 67 ± 26 to 27 ± 8 mg L−1. Our results are consistent with those of Sivakumar [51], Sahana and Manjunath [53], Ihenacho et al. [50], and Obasi and Onukwuli [54].
4. Conclusions
The municipal wastewater generation rate is increasing continuously, and it is not being recycled or treated. The energy crisis is also increasing, and we need alternative sources of energy. The current study was designed to evaluate the effect of various variables on municipal wastewater treatment and electricity generation using a dual-chamber MFC with a novel separator. Collected municipal wastewater from 25 Area of Wah Cantt City, Punjab, Pakistan, showed that out of six studied parameters, only two, that is, BOD (90 mg L−1) and COD (191 mg L−1) were above the permissible limits. The maximum current of 68.16 µA was produced by NaCl having a 0.5 M concentration, which was significantly (p < 0.05) higher as compared to other salts and concentrations (KCl and MgCl2) with 70% and 35% removal of COD and BOD, respectively. While in the biological cycle of all salts, the maximum current obtained from the NaCl salt bridge was significantly (p < 0.05) higher, that is, 116.33 µA, as compared to KCl (80.25 µA) and MgCl2 (54.91 µA). While in volume variation, a maximum current of 83.41 µA was produced from 1000 mL of NaCl, which was significantly (p < 0.05) higher as compared to day 7 with 80% and 40% removal of COD and BOD, respectively. Aluminum mesh size variation also leads to the current production of about 103 µA, which was significantly (p < 0.05) higher at day 1 as compared to day 7 from 1 mm aluminum mesh thickness having NaCl salt in salt bridge with 85% and 45% removal of COD and BOD, respectively. The efficiency of the salts used in the separator in the current study is in the order of NaCl > KCl > MgCl2. All six studied water parameters, including BOD and COD, after MFC batch experiments were within the permissible limits of NEQS in all studied variables. The compliance of treated water with NEQS emphasizes the system’s practical ability for the treatment of municipal wastewater and green energy production. Optamization of various other parameters like pH, microorganisms, separator length, etc. are still required to be carried out in batch experiments before the pilot study. However, the main limitation of current research is its batch experimentation mode which limits its application to larger-scale continuous system. Therefore, the optimization of various operational conditions for pilot scale such as scaling reactor design, hydraulic retention time, analyzing feed characteristics, and automatic monitoring system should need to be explored for efficiency and adaptability of this system at a larger scale. However, it is also recommended that in the future, further studies related to load and OCV should be carried out. This work provides a strong foundation for the development of cost-effective and green wastewater treatment technologies while also providing crucial knowledge to bridge the gap between laboratory innovation and industrial-scale application.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research received no external funding.
Open Research
Data Availability Statement
The data used to support the findings of this study will be provided by the corresponding author upon request.




